Water Extract of Notopterygium Incisum Alleviates Neuropathic Pain by Regulating TRPV1
-, -, , -, -, -
(School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, 210023, China)
ABSTRACT: OBJECTIVE To explore the effect of water extract of notopterygium incisum (WN) on neuropathic pain and its molecular biological mechanism. METHODS Pain behavior test to detect the effects of WN on thermal hyperalgesia and mechanical hyperalgesia in acute pain and chronic constriction injury (CCI) induced neuropathic pain model. Immunohistochemistry and qPCR were used to detect the expression of TRPV1. Calcium imaging was used to detect calcium influx of capsaicin (TRPV1 agonist) in CCI mice after oral administration of WN. RESULTS WN significantly reduced the thermal hyperalgesia and mechanical hyperalgesia in acute pain model and CCI model (P<0.001). The expression of TRPV1 in protein level and mRNA level in DRG neurons were significantly inhibited by administration of WN (P<0.05). The response of DRG neurons to capsaicin was significantly inhibited by the treatment of WN (P<0.001). CONCLUSION WN alleviated CCI-induced mechanical allodynia and thermal hypersensitivity via modulating TRPV1. Our results revealed a new molecular biological mechanism of analgesia effect to traditional Chinese medicine.
KEYWORDS: notopterygium incisum; neuropathic pain; DRG; TRPV1
Neuropathic pain (NP) is usually caused by nervous system diseases or dysfunction. Pain can be initiated or caused by a primary lesion or dysfunction in the nervous system[1-2]. According to a European study, the prevalence of neuropathic pain in the general population is as high as 8.0%[3]. NP is a kind of chronic pain that greatly affects the quality of life of patients. It has been reported that the mean total all-cause direct medical costs were estimated to be 10 002 RMB per year for each patient[4]. Because of its complicated pathogenesis, high incidence and low cure rate, more and more attention has been paid by researchers. Currently, antidepressants and anticonvulsants (gabapentin and pregabalin) are recommended as first-line treatment[5-6]. However, in some cases, drug therapy alone cannot adequately control chronic pain and is accompanied by some side effect. Traditional Chinese medicine is famous for its widely-accepted efficacy. Notopterygium is one of thousands of analgesic Chinese medicine, which is often used to treat cervical pain, lumbar pain, and rheumatic pain. However, the analgesic mechanism of WN is still unknown.
Recently, sensory physiology and pain research has led to the discovery of multiple receptors within nociceptor for neuropathic pain, among which is TRPV1[7]. Transient receptor potential V1 (TRPV1) is a non-selective cation channels involved in inflammatory and nociceptive pain[8]. When the nerve is damaged, the activity and expression of TRPV1 is up-regulated, indicating that TRPV1 is closely related to the neuropathic pain[9]. Increased responsiveness to heat stimuli could be due to the observed increases in the mRNA and protein expression of TRPV1 DRGs[10]. The researchers targeted TRPV1 to find more pain-killers[11]. Therefore, TRPV1-targeted drugs could rapidly become available if we found more Chinese herbs to regulate TRPV1.
In this paper, we obtained water extract of notopterygium (WN) by decoctingNotopterygiirhizomaet radix. We found WN could alleviate the mechanical allodynia and heat allodynia in the established murine models of acute pain and chronic nerve compression injury. In our results, we found WN reduced the expression of TRPV1 and inhibited the calcium influx induce by capsaicin (TRPV1 agonist) in the CCI induced neuropathic pain model.
1 Materials and Methods
1.1 WN extraction
Notopterygium was purchased from Jiangsu Hospital of Traditional Chinese Medicine (Jiangsu, China). 200 g of Notopterygium were refluxed twice with water (1∶10 for the first extraction and 1∶8 for the second extraction,w/v) for 2 h and filtered. The filtrates were concentrated and evaporated under vacuum to yield about 34.9 g of semi-solid.
1.2 Animals
This study was approved by the Animal Care and Use Committee of Nanjing University of Chinese Medicine (Nanjing, China). Experiments were conducted according to the animal research ethical guidelines of the International Association for the Study of Pain. Male C57BL/6 mice were housed in groups of four per cage in our animal center, with free access to food and water. Only healthy animals weighing 15-20 g and displaying normal water and food intake were included in the study. All behavioral tests were performed with an experimenter blind to the groups.
1.3 Tail flicking test
Set the temperature of the water bath to 43 ℃, and measure the temperature of the water bath with a thermometer before the experiment to ensure that the temperature is constant at 43 ℃ (KEXI Instrument, China, HH-1). Fix the mouse with a fixator to expose the tail. Face the nose of the mouse to the fixed plug hole to ensure the mouse can breathe freely after oral administration of WN (WN, 195 mg/kg, 0.1 mL). The control mice were treated with saline (oral administration of saline, 0.1 mL). After 2 minutes of acclimation, put the tail in the water bath with constant temperature and start the stopwatch timer. When the mouse tail appears obvious swing, stop timing immediately and record the time. Measure three times for each mouse and take the average value as the threshold value of the mouse.
1.4 Capsaicin induced pain test
Place the transparent cylindrical long tube on the plexiglass plate.Then the camera was prepared for recording. After the mice were acclimated for 30 min, 10 μL capsaicin (500 μmol/L, Sigma, USA) was injected subcutaneously into the soles of hind paw. Then the behavior was recorded in one hour. The number of licking feet (model side) was counted after the video recording. There were two groups in this experiment, sham group (oral administration of saline, 0.1 mL) and WN group (oral administration of WN, 195 mg/kg mice, 0.1 mL).
1.5 Chronic constriction injury (CCI)
In the CCI model, the mice were randomly divided into 4 groups: Sham group (exposed the sciatic nerve, but without sciatic nerve ligation), CCI group (sciatic nerve ligation), CCI treated with WN group (WN, 195 mg/kg mice, 0.1 mL) and CCI treated with GBPT (gabapentin, positive control, 200 mg/kg mice, 0.1 mL) group. Mice were anaesthetized with isoflurane (Yuyan instrument, Shanghai, China) and restrained in a lateral position as before[12]. A surgical incision was made at the thigh root midline, and the right sciatic nerve was exposed in the middle of the thigh. Three ligatures were tied around the nerve by using 8-0 silk braided cord (Shanghai Pudong Jinhuan Medical Products Co., Ltd) with 1 mm space. The force of ligation was based on leg reflex. The length of the affected nerve was 4-5 mm. After the operation, the wounds were sutured and the animals were positioned supine and kept that position until they recovered from anesthesia. A sham operation was performed in the same manner except for sciatic nerve ligation. All surgical procedures were performed under sterile conditions.
1.6 Von-Frey test
Animals were acclimated to the testing environment for 30 min before the initiation of behavior tests. Animal behaviors were analyzed by an investigator who was blind to the grouping. Mechanical allodynia was assessed by measuring the paw withdraw threshold with a set of Von Frey filaments (0.04-2 g) (Aesthesio, UGO, Italy). Mice were placed on an elevated metal grid (100 cm × 50 cm). The filament was applied to the plantar surface at a vertical angle for up to 3 s from the bottom. Fifty percent MWT values were determined using the up-down method as before[13].
1.7 Thermal radiation test
Thermal hyperalgesia was assessed by measuring the paw withdraw latency to radiant heat stimuli after the Von-Frey tests. Mice were placed in a transparent plastic box (4.5 cm×3 cm×10 cm) on a glass platform (Plantar Test Apparatus, IITC Life Science,USA) and were acclimated to the testing environment for 30 min before the experiments. Radiant heat was adjusted to 18% of maximal output and shone on the center of the paws. The radiant heat source (UGO Basile plantar test. 37370) was applied to the center of the plantar surface of the hind paw with at least 3 min intervals. Each mouse was tested more than 3 times, with each test performed 20 min apart. The average thermal withdrawal latency (TWL) of the trials was recorded as the response latency.
1.8 Immunofluorescent staining in dorsal root ganglia
Dorsal root ganglia tissues were collected from L4-L5 DRGs. For immunostaining of transient receptor potential cation channel V1 (TRPV1), sections were incubated with blocking solution (containing 3% fetal bovine serum, 0.1% Triton X-100, and 0.02% sodium azide in PBS) for 2 h at room temperature followed by rabbit anti-TRPV1 (1∶100, Abcam, ab62053) at 4 ℃ overnight. Next, the sections were incubated in Alexa Fluor-conjugated goat anti-rabbit IgG secondary antibody (1∶100, Beyotime, A0453) at room temperature for 2 h. For immunostaining of neuron, the DRG sections were incubated in mouse anti-NeuN (1∶800, Abcam, ab27266) at 4 ℃ overnight. Next, the sections were incubated in Alexa Fluor-conjugated goat anti-mouse IgG secondary antibody (1∶200, Beyotime, A0460) at room temperature for 2 h. The number of fluorescence-positive DRG neurons in L4 and L5 was counted and calculated. After stained pictures were captured, they were merged into an image. Three mice from each group were analyzed. Quantification of immunoreactivity was finished as before[9].
1.9 qPCR
Total RNA was extracted from freshly isolated DRGs (L4-L5, S1-S2) using Trizol reagent (Vazyme, China, R401-01) and was treated with RQ1 DNase (Promega) as before. Reverse transcription was performed using the Transcriptor First Strand cDNA Synthesis kit (Roche, Basel, Switzerland). For qPCR, Light Cycler 480 SYBR Green I Master (Roche, Basel, Switzerland) was used. The reaction was run in a Light Cycler 480 Ⅱ qPCR instrument (Roche, Basel, Switzerland) using 1 μL of the cDNA in a 20 μL reaction according to the manufacturer's instructions. Calibrations and normalizations were done using the following 2-ΔΔCTmethod. GAPDH was used as the reference gene for qPCR experiments. Primers for TRPV1, forward primer: ATCATCAACGAGGACCCAGG, reverse primer: TGCTATGCCTATCTCGAGTGC. Primers for GADPH, forward primer: TGGATTTGGACGCATTGGTC, reverse primer: TTTGCACTGGTACGTGTTGAT.
1.10 Dorsal root ganglia neuron culture
The mice were anesthetized with 1% sodium pentobarbital. The DRGs were harvested as we described previously[12]and immediately transferred to a cold DH10 medium (DMEM/F-12, 10% FBS, and 1% penicillin-streptomycin-glutamine; Invitrogen). DRGs were washed 2-3 times in warm DH10 and then treated with enzyme solution (5 mg/mL dispase and 1 mg/mL collagenase type Ⅰ in Hanks Balanced Salt Solution without Ca2+and Mg2+; Invitrogen) at 37 ℃ until the cells were dissociated. Cell suspensions were then filtered through a 100 μm cell strainer (BD, Franklin Lakes, NJ, USA). After being centrifuged at 1 000 r/min for 5 min, DRG neurons were re-suspended in DH10, and glial cell-derived neurotrophic factor (GDNF, 50 ng/mL, Millipore, Billerica, MA, USA) was added. Fifty microliters of suspended cells in solution was plated onto pre-sterilized glass coverslips that had been coated with 0.5 mg/mL poly-D-lysine (Biomedical Technologies Inc., Stoughton, MA, USA) and 10 μg/mL laminin (Invitrogen). Plated neurons were cultured in an incubator (95% O2and 5% CO2) at 37 ℃ and used for calcium imaging studies within 48 h.
1.11 Calcium imaging
The DRG neurons were loaded with fura-2-acetomethoxyl ester (Molecular Probes, Eugene, OR, USA) for 30 min at 37 ℃ in the dark in accord with previous studies[14]. After being washed 3 times with PBS, the glass coverslips were placed into a chamber and perfused with a solution containing 137 mmol/L NaCl, 5.4 mmol/L KCl, 1.2 mmol/L MgCl2, 1.2 mmol/L NaH2PO4, 1 mmol/L CaCl2, 10 mmol/L glucose, and 20 mmol/L HEPES (pH 7.4) (SCR, Shanghai,20201127). A high-speed continuously scanning monochromatic light source (Polychrome V, TILL Photonics, Graeling, Germany) was used for excitation at 340 nm and 380 nm to detect changes in intracellular free calcium concentration.
1.12 Statistical analyses
2 Results
2.1 The concentrations of WN in the experiments
The normal dose of WN treatment used in mice is 195 mg/kg. To explore the analgesic effect of different concentrations of WN, we administrated 1/6 of normal dose (32.5 mg/kg), normal dose (195 mg/kg), 1/2.45 of normal dose (79.59 mg/kg) and 6 times of normal dose (1 170 mg/kg) for oral treatment.
2.2 WN alleviated acute pain behavior induced by capsaicin in mice
To explore the analgesic effect of WN, we administrated different concentrations of WN to the mice before the tail flicking test (43 ℃). We found the 1/6 normal dose had no analgesic effect in the tail flicking test, the normal dose, 1/2.45 times of normal dose and 6 times of normal dose had similar analgesic effect (Figure 1a). Therefore, we used the normal dose (195 mg/kg mice) in the subsequent experiments. Then we focused on the time of the best analgesic effect of WN treatment. We found that the best analgesic effect WN is one hour after oral administration (Figure 1b). TRPV1 is important in the heat allodynia and mechanical allodynia in neuropathic pain[15]. To explore the molecular biological mechanism of analgesia effect of WN, we injected capsaicin (10 μL, 500 μmol/L, Sigma) to the hind paw of mice one hour post WN administration. We found that WN alleviated the number of lifts after injecting capsaicin compared with sham group (Oral administration of water) (Figure 1c,***P<0.001).According to the behavior tests, we speculate that WN may affect TRPV1 to alleviate the acute pain.

Note: (a) Analgesic effects of WN in the tail-flick assay (43 ℃) in different concentrations. Multiple of normal dose: 1 times (195 mg/kg), 1/2.45 (79.59 mg/kg), 1/6 (32.5 mg/kg), 6 times (1 170 mg/kg). (b) The time course of the hot tail-flick latencies was observed after WN treatment (n=8). (c) Effects of WN in the capsaicin assay. WT mice were used in this assay (n=8). Two-way ANOVA (a, b), t-test assay (c). *P<0.05, **P<0.01, ***P<0.001.
2.3 WN relieved pain behavior in CCI mice model
To make sure whether WN could relieve the neuropathic pain, we established the CCI model and orally administrated mice with WN daily for 5 days when pain behavior appeared obviously in CCI model (Figure 2a). We then recorded the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) on the 1st, 3rd, 5th, 7th, 9th, 11thand 13thday after operation. Our results showed that WN enhanced MWT compared with the CCI group (Figure 2b, c, two-way repeated measures ANOVA, factorial design). We did not observe any statistical differences in the MWT values following CCI operation between WN group and GBPT group (Figure 2c). These results suggested that WN relieved mechanical pain in the neuropathic pain. Furthermore, WN relieved the TWL compared with the CCI group (Figure 2e, f, two-way repeated measures ANOVA, factorial design). The above results suggested that WN relieved the mechanical allodynia and heat allodynia in neuropathic pain.
2.4 WN alleviated TRPV1 expression in CCI model
In order to explore the molecular mechanism of the inhibitory effect of WN, the DRG neurons of the same side of L4 and L5 of the four groups of mice were sampled after the 14thday. We detected the expression of TRPV1 in DRG neurons. As shown in Figure 3a and Figure 3b, in comparison with DRGs from CCI group, the percentage of TRPV1-positive neurons was obviously reduced in DRGs from the WN group (Figure. 3a, b,P<0.05,n=3). Moreover, the mRNA of TRPV1 was detected by Realtime-PCR. As expected, TRPV1 expression was reduced in DRG neurons in WN treated group (Figure. 3c,P<0.05,n=3). These results indicated that the expression level of TRPV1 is related to WN alleviating neuropathic pain in the CCI model.
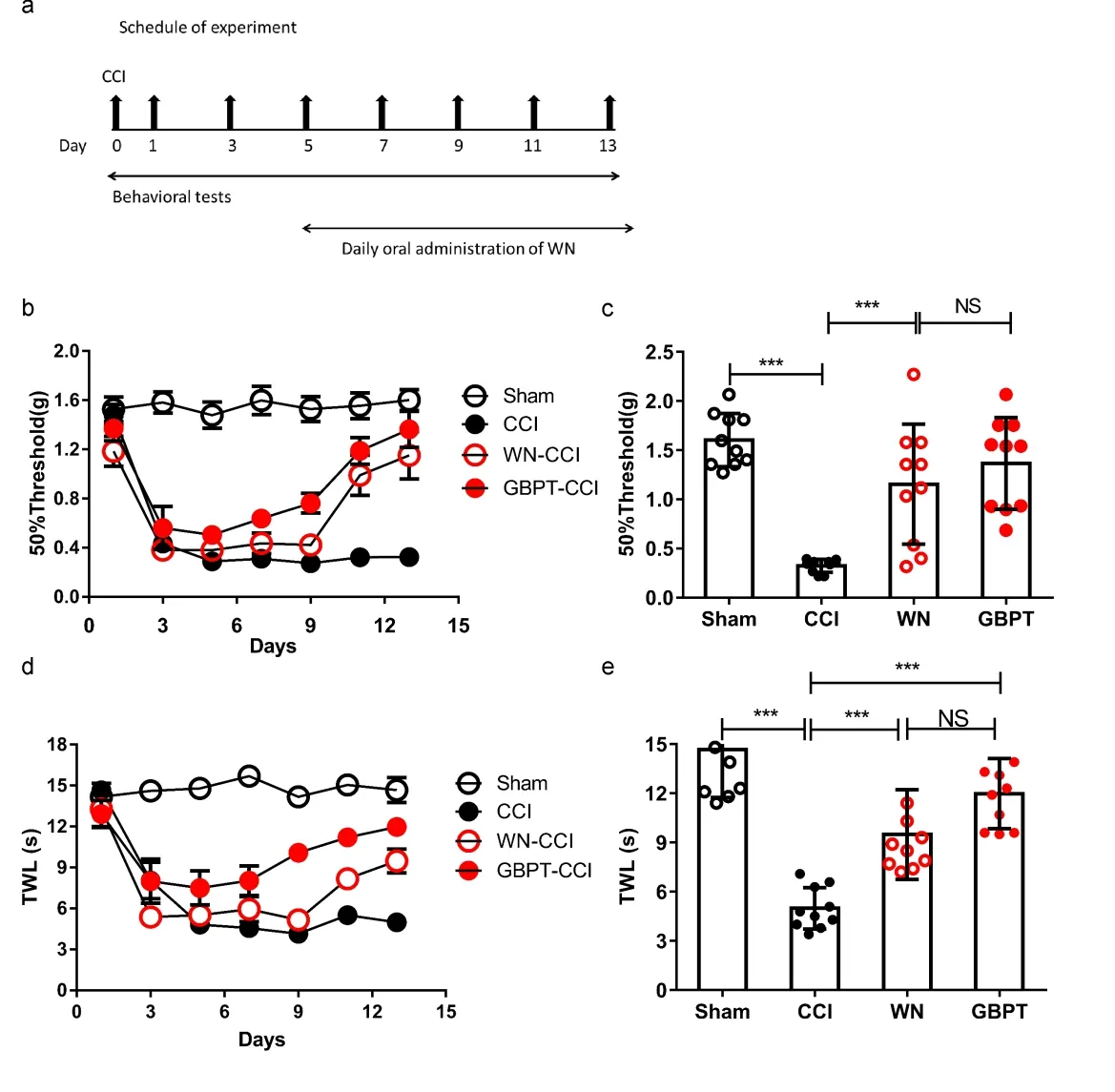
Note:(a) Schedule of WN and GBPT treatment. (b) Von-Frey test over 14 days after treating with WN and GBPT in CCI model. Values represent the changes from the baseline MWT values that were recorded on day 1 after operation. (c) The MWT values on the 14th day after the operation were compared (n=10). (d) Heat radiant testing over 14 days after treating with WN and GBPT in CCI model. Values represent the change from the baseline TWL values that were recorded on day 1 after operation. (e) The TWL values on the 14th day after the operation were compared (n=10). Two-way ANOVA and t-test assay. ***P<0.001.
2.5 WN reduced the calcium influx induced by capsaicin in CCI group
We further studied the sensitivity of DRG neurons from CCI model mice to capsaicin. The DRG neurons from CCI ipsilateral on the same segment of the Sham group, CCI group, WN treated CCI group and GBPT treated CCI group were cultured in different culture dishes. TRPV1 agonist (Capsaicin, 500 nmol/L) was used to activate these DRG neurons (Figure 4a). The results indicated that the ratio of responsive neurons significantly increased in the CCI group compared with sham group, while WN reduced the ratio of response neurons significantly in the CCI group (Figure 4b,P<0.05,n=3). Moreover, the amplitude of response DRG neurons was calculated. WN reduced the amplitude of response DRG neurons induced by capsaicin in the CCI group (Figure 4c,P<0.001,n=3). The attenuated effect of WN was almost the same as that of GBPT in our experiment.
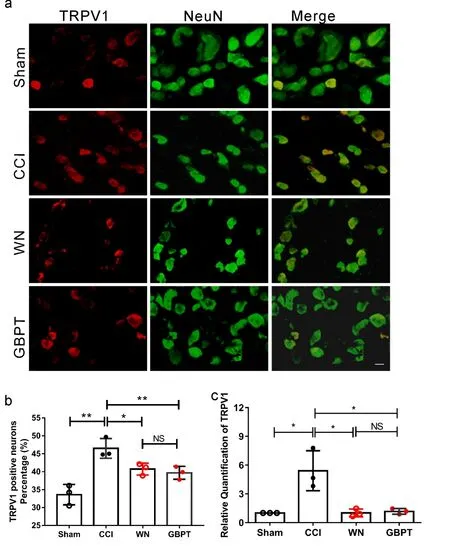
Note:(a) Immunofluorescence staining shows representative image of TRPV1 expression co-expressed with NeuN (markers of neurons) in L4, L5 DRG neurons in sham group, CCI group, CCI treated with WN, CCI treated with GBPT. Scale Bar: 20 μm. (b) The percentage of TRPV1 positive neurons in L4, L5 DRG (n=3). (c) The results of real-time PCR show the transcriptional expression of TRPV1 in four groups(n=3). Two-way ANOVA and t-test assay, *P< 0.05.
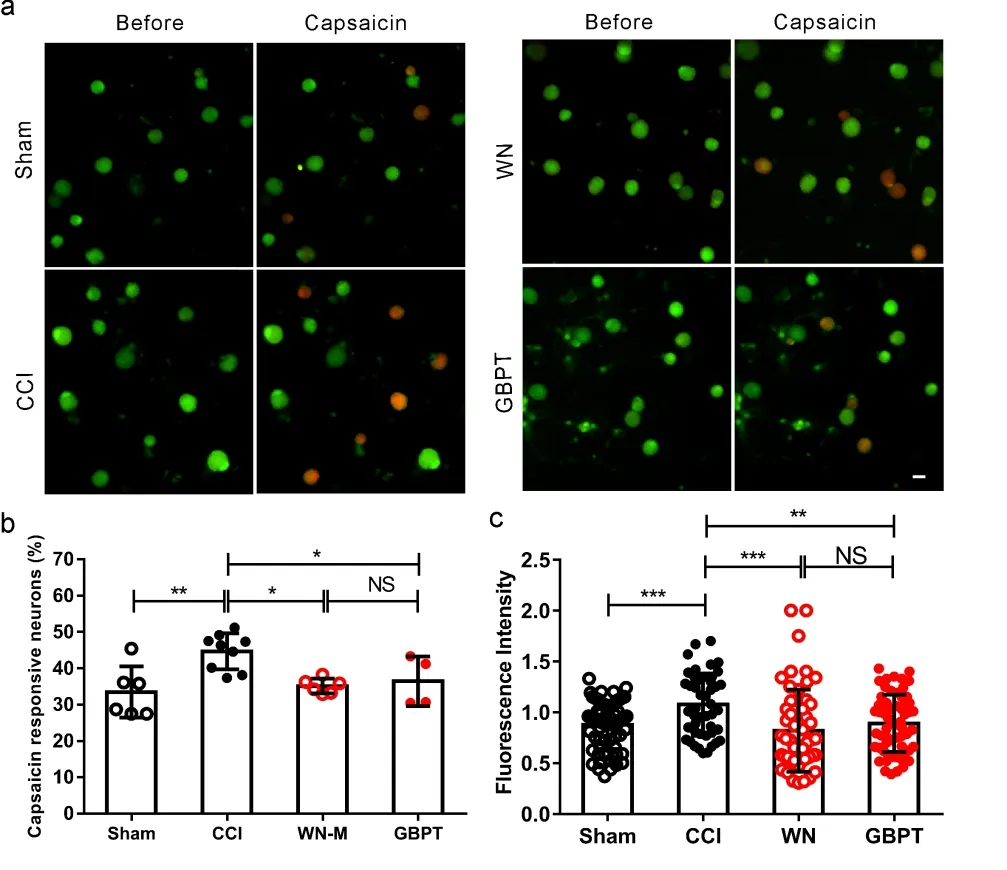
Note: (a) Representative calcium images in cultured L4, L5 DRG neurons in sham group, CCI group, CCI treated with WN group and CCI treated with GBPT group. Scale Bar: 20 μm. (b) The percentage of capsaicin-responsive neurons at the level of L4, L5 DRG in the four groups (% of cultured neurons, n=3). (c) The fluorescence intensities of capsaicin-induced calcium influx in DRG neurons in the four groups (n=3). Two-way ANOVA and t-test assay.*P<0.05,**P< 0.01,***P<0.001.
3 Discussion
The International Association for the study of pain (IASP) defines neuropathic pain (NP) as "pain caused by injury or disease directly affecting the somatosensory system"[2]. 10%-20% of patients are clinically diagnosed with neuropathic pain[3]. Neuropathic pain is one of the most difficult chronic pain to control. The cost of treating neuropathic pain in our country is about 1.4 billion yuan per year[4]. Neuropathic pain seriously affects the quality of life of patients and brings heavy burden to the society. The mechanism of neuropathic pain is not clear and the therapeutic effect is limited. Here, we present a Chinese herb WN which is effective to treat neuropathic pain. For its higher efficiency and lower side effect, WN is usually used in the treatment of pain in traditional Chinese Medicine. The treatment of WN reduced the mechanical allodynia and heat allodynia after 5 days of CCI operation (Figure 2). Our findings highlight WN as a promising drug for the treatment of neuropathic pain.
TRPV1 is a none-selective cation channel, which was successfully cloned by David Julius in 1997[8]. It is expressed in unmyelinated class C afferent fibers and can be activated by capsaicin, heat stimulation and acid environment, resulting in the sensitization of primary afferent neurons[8,16]. It is reported that TRPV1 is vital for neuropathic pain[15,17].The silence of spinal TRPV1 could attenuate neuropathic pain in rat[18]. The different factors such as pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in DRG could regulate TRPV1 expression and sensitivity during the development of neuropathic pain[15]. Recent studies targeted TRPV1 to look for analgesics[11]. TRPV1 is a key factor involved in the development of neuropathic pain. We found that WN inhibited TRPV1 to alleviate the mechanical allodynia and heat allodynia in CCI model. In the subsequent experiment, we found that WN inhibited the expression of TRPV1 in the neuropathic pain model. WN is the water extract of notopterygium. The components of WN may directly target TRPV1 to inhibit the CCI induced pain.
Notopterygium grows naturally in the west provinces of China[19]. The plant is mainly used for headache, cervical pain, joint pain, muscle pain. In clinic, it is often used in combination, for the treatment of wind cold, dampness, severe body pain, and unsmooth joint pain. Compared with western medicine, it has fewer side effects (such as adverse reactions, deterioration rate) and lower recurrence rate, which can reduce the mental burden of patients and improve the quality of life. In our study, the attenuated effect of WN was almost the same as that of GBPT, one of the classical anti-pain drugs[20]. Our results indicate that WN may play an important role in the treatment of neuropathic pain in the future.
Nevertheless, the anti-nociceptive effect of WN is from the multiple elements in its water extraction, such as isoimperatorin (0.38%) and nodakenetin (0.04%)[21]. However, whether the monomers within the WN have the same effect on TRPV1 modulation is still unknown. Further studies will be focused to solve these problems.
In conclusion, we found that the water extract of notopterygium incisum (WN) could alleviate mechanical allodynia and heat allodynia in neuropathic pain. The mRNA and protein levels of TRPV1 were reduced after WN treatment, as detected by qPCR and immunofluorescence methods. Furthermore, the calcium influx induced by TRPV1 agonist capsaicin was alleviated by the treatment of WN. In summary, WN alleviates neuropathic pain by regulating TRPV1.

