GC-MS Analysis of Volatile Components in ‘Changping 8’ Apple Fruit
Jie LI Yu WANG Huan LIU Jicheng HAN
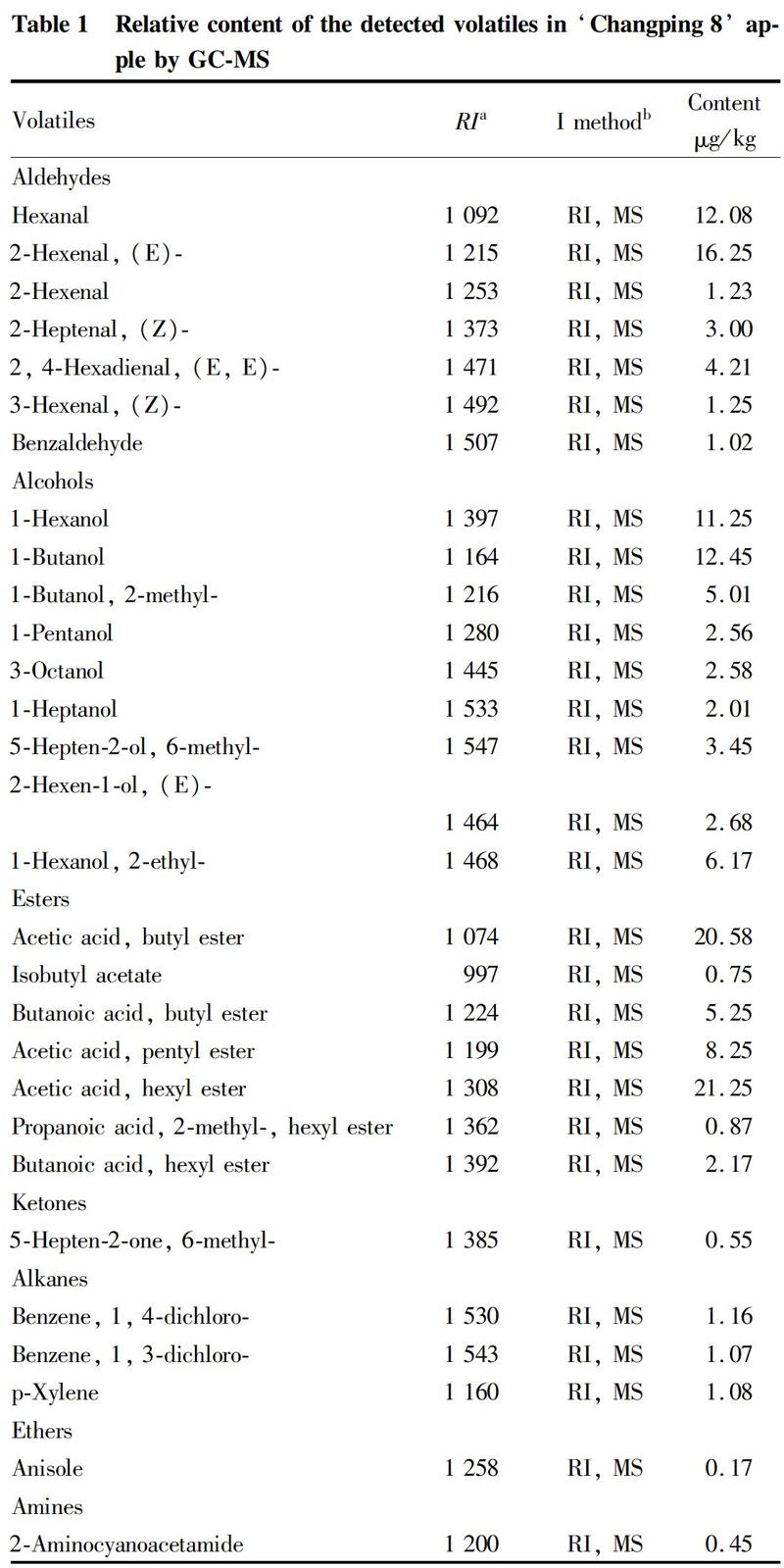

Abstract Solid-phase and micro-extraction combined with GC/MS (SPME-GC-MS) were employed to detect main aroma components in ‘Changping 8 apple fruit. The results showed that, 29 kinds of compounds were identified from the aroma components in ‘Changping 8 apple fruit, and they were aldehydes, alcohols, esters, ketones, etc. The main aroma components of apple fruits were acetic acid, hexyl ester, acetic acid, butyl ester, (E)-2-hexenal, 1-butanol, hexanal, and 1-hexanol.
Key words ‘Changping 8 apple; Aroma volatile compounds; SPME; GC-MS
Aroma composition is an important fruit quality which is mainly derived from various trace volatile substances. Although they only account for 0.001%-0.01% of fresh weight of fruit, they play an important role in the flavor of fruits. The formation of aroma components in fruits is influenced by many factors, and the same variety will produce different flavors in different climates and topographies[1].
More than 300 volatile molecules have been reported in fresh apples[2]. Esters are the most abundant volatile compounds emitted by apple, and have been proposed for cultivar classification[3]. Acetic acid, hexyl ester, ethyl butanoate, butanoic acid, and hexyl ester were prominent within the blend of volatiles produced by fruit throughout maturation[4-5].
Material and Methods
Materials
The ‘Changping 8 apple trees were planted on the base of Changli Fruit Research Institute, Hebei Academy of Agriculture and Forestry Sciences, Northeast of Hebei Province, China. Fuji apple fruit was collected in late October. These samples were smooth and uniform in size. After peeling, 50 g of pulp sample was frozen with liquid nitrogen, added with 1 g of PVPP (to remove polyphenols and prevent samples from oxidating) and 0.5 g of D-gluconic acid lactone (to inhibit the activity of glucoside enzymes), and then rapidly crushed into powder. These powdery samples were stored in a refrigerator at -80 ℃ for the determination of aromatic substances.
The fiber of divinylbenzene/carboxen/polydimethylsi-loxane (DVB/CAR/PDMS, 50/30 μm thick, gray color) was preconditioned prior to the analysis in the injection port of the gas chromatograph according to the instructions suggested by the manufacturer.
C7-C30 normal alkanes for calculating the retention indices (RI) were purchased from Aldrich Chemical Co. Authentic reference aroma compounds were obtained from Beijing Peking University Zoteq Co., Ltd.
Methods
SPME sampling: First, the samples that stored at -80 ℃ were taken out and thawed quickly. Second, they were centrifuged at 8 000 rpm and 4 ℃ for 10 min. Third, 6 ml of each supernatant was transferred to a 20 ml vial (special for SPME). Fourth, 10 μl of 4-methyl-2-amyl alcohol (4M2P) aqueous solution (1.038 8 g/L) was added as internal standard. Fifth, before the SPME fiber was inserted into the vial, the vial was sealed with one Teflon cover and equilibrated for 30 min at a 40 ℃ magnetic stirrer. Finally, the fiber was exposed in the upper space of the sealed vial to ex-tract compounds for 30 min.
Analysis by GC-MS
A GCMS-QP2010 equipment was used. The GC was equipped with an HP-INNOWAX capillary column (60 m×0.25 mm×0.25 μm, Agilent Technologies). Helium was the carrier gas with a constant flow of 1 ml/min to the column. The injection port temperature was at 250 ℃. It was parsed for 5 min at the injection port. The initial oven temperature was at 50 ℃, which was held for 5 min and then increased at 3 ℃/min to 120 ℃, which was held for 5 min and finally increased at 6 ℃/min to 250 ℃, which was held for 5 min. The injection port was in the splitless mode. Electron impact ionisation was used at 70 eV (EI). The acquisition of mass spectra was performed in a mass range of 30-500 m/z. The ion source temperature was at 230 ℃. MS was detected with 2 min solvent delay. The analysis of the sample at each condition was repeated 3 times. C7-C30 n-alkanes were run under the same chromatographic conditions used in the separation of the compounds in our samples in order to calculate the retention indices (RIs) of detected compounds. The system of AMDIS (Automatic Mass Spectral Deconvolution and Identification System) was used to analyze the mass spectrogram. Compounds were identified by comparing their mass spectra with those included in the NIST11 database, and confirmed by comparison of the retention time of the separated constituents with those of the authentic samples and by comparison of retention indexes of the separated constituents with the RIs reported in literatures.
As shown from Table 1, it could be seen that a total of 29 volatile compounds were detected in ‘Changping 8 apple, including 7 esters, 9 alcohols, 7 aldehydes, 3 alkenes, 1 amine, 1 ketone, and 1 ether. The predominant volatile compounds in ‘Changping 8 apple were acetic acid, hexyl ester, acetic acid, butyl ester, (E)-2-hexenal, 1-butanol, hexanal, and 1-hexanol. Thus, it can be seen that the main aroma components were esters.
Discussion
Among the 29 kinds of aroma components obtained, the compounds of acetic acid, hexyl ester, acetic acid, butyl ester, (E)-2-hexenal, 1-butanol, hexanal and 1-hexanol were also appeared in apples[6-7], pears[8], grapes[9], raspberries[10], blueberries[11], kiwifruit[12], apricots[13]and peaches[14-16], it suggested that these compounds are the building blocks of fruit flavor. Besides, compared with Fuji, Ruiyang, Ruixue and Xinhongxing, the common components of apple aroma can be found: hexanal, 2-hexenal, 1-butanol, acetic acid, hexyl ester, 1-butanol, 2-methyl-, 1-hexanol, butanoic acid, and butyl ester.
References
[1]SANZ C, OLIAS JM, PEREZ AG. Aroma biochemistry of fruits and vegetables. In phyto chemistry of fruit and vegetables[J]. New York: Oxford University Press Inc., 1997.
[2]NIJSSEN LM, VAN INGEN-VISSCHER CA, DONDERS JJH. VCF volatile compounds in Food: Database (Version 13.1.)[J]. Zeist (The Netherlands): TNO Triskelion Recuperato da, 2011.
[3]HOLLAND D, LARKOV O, BAR-YAKOV I, et al. Developmental and varietal differences in volatile ester formation and acetyl-CoA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit[J]. J. Agric. Food Chem., 2005(53): 7198-7203.
[4]BERGER RG. Flavours and Fragrances-Chemistry, Bioprocessing and Sustainability[D]. Springer-Verlag: Berlin, Germany, 2007.
[5]VILLATORO C, ALTISENT R, ECHEVERRIA G, et al. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of "Pink Lady" apples[J]. Postharvest Biol. Technol., 2008(47): 286-295.
[6]DENG R. Effects of bagging on fruit quality of‘Ruiyang&‘Ruixue apple cultivars[D]. Yangling: Shanxi. 2017.
[7]NIE LC, SUN JS, CHEN HJ, et al. Study on fruit aroma of different apple cultivars[J]. Scientia Agricultura Sinica. 2006, 39(3): 641-646
[8]RAPPARINI F, PREDIERI S. Pear fruit volatiles[J]. In Horticultural Reviews; Janick, J., Ed.;John Wiley & Sons: Hoboken, NJ, USA, 2003: 237-324.
[9]KALUA CM, BOSS PK. Comparison of major volatile compounds from riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest[J]. Austra. J. Grape Wine Res., 2010(16): 337-348.
[10]KLESK K, QIAN M, MARTIN R. Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington[J]. J. Agric. Food Chem. 2004(52): 5155-5161.
[11]DU XF, PLOTTO A, SONG M, et al. Blueberry volatile composition of four southern highbush cultivars and effect of growing location and harvest date[J]. J. Agric. Food Chem. 2011(59): 8347-8357.
[12]CARCIA CV, STEVENSON RJ, ATKINSOn RG, et al. Changes in the bound aroma profiles of "Hayward" and "Hort16A" kiwifruit (Actinidia spp.) during ripening and GC-olfactometry analysis[J]. Food Chem., 2013(137): 45-54.
[13]GONZLEZ-AGERO M, TRONCOSO S, GUDENSCHWAGER O, et al. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.)[J]. Plant Physiol. Biochem., 2009(47): 435-440.
[14]EDUARDO I, CHIETERA G, BASSI D, et al. Identification of key odor volatile compounds in the essential oil of nine peach accessions[J]. J. Sci. Food Agric., 2010(90): 1146-1154.
[15]WANG YJ, YANG CX, LI SH, et al. Volatile characteristics of 50 peaches and nectarines evaluatedby HP-SPME with GC-MS[J]. Food Chemistry, 2009(116): 356-364.
[16]ZHANG B, SHEN JY, WEI WW, et al. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening[J]. Agric. Food Chem, 2010(58): 6157-6165.
- 农业生物技术(英文版)的其它文章
- Review on Effects of Sunlight on the Internal Quality of Peach Fruit
- Research Progress on Genetic Breeding of Sweet Sorghum Related to Sugar Traits
- Screening of Red-flesh Small Watermelon Varieties for Substrate Cultivation in Spring Greenhouses
- Planting Techniques of Pennisetum giganteum in Huanghuai Area
- Bibliometric Analysis of Status Quo and Trend of the Research on Duck Based on the Web of Science Database
- Preparation and Insecticidal Activity of Sea Anemone Peptide AP-GI from Aiptasia pallida

