Fabrication of pH-Responsive Hydrogels Composed of Chitosan-Fulvic Acid Microparticles and Konjac Glucomannan
CHEN Xiaohan, PANG Jie,3,4,*
(1.College of Food Science, Fujian Agriculture and Forestry University, Fuzhou 350002, China; 2.Engineering Research Centre of Fujian-Taiwan Special Marine Food Processing and Nutrition, Ministry of Education, Fuzhou 350002, China;3.State Key Laboratory of Food Safety Technology for Meat Products, Xiamen 361100, China;4.Key Laboratory of Marine Biotechnology of Fujian Province, Institute of Oceanology, Fujian Agriculture and Forestry University,Fuzhou 350002, China)
Abstract: A series of konjac glucomannan (KGM) hydrogels were prepared using chitosan-fulvic acid microparticles(CFMPs) as the reinforcing agents.CFMPs possessed high surface density and oxygen-containing functional groups,which could induce hydrogen-bonding interaction between KGM and CFMPs to form a more dense network structure.The effects of CFMPs on the network structure and releaseing behavior of KGM hydrogels were evaluated.The rheological results revealed that the regular change in the steady shear viscosity and dynamic viscoelasticity of all hydrogel-forming solutions was attributed to the reinforced network structure with strong interaction.Fourier transform infrared spectral analysis indicated that the carboxyl groups (–COOH) in CFMPs successfully formed hydrogen bonds with the hydroxyl groups (–OH) in the KGM polymer chains.Scanning electron microscopic images revealed that the structure of hydrogels was dense with interconnected pores.In addition, the in vitro drug-releasing behavior of KGM/CFMP hydrogels in artificial intestinal and gastric fluids was investigated.KGM/CFMP composite hydrogels possessed excellent pH-responsive property due to the protonated amine groups (–NH3+).Therefore, KGM/CFMP composite hydrogels have a huge potential as a pH-responsive drug delivery system.
Keywords: konjac glucomannan; chitosan-fulvic microparticles; hydrogel; pH-controlled release
Hydrogels are composed of hydrophilic polymer chains with three-dimensional network, which are cross-linked by hydrogen bonding, physical entanglements or covalent bonds[1].Hydrogels have been tremendously used in the development of controlled-release and sustained-release drug delivery systems[2-3]since they could not only protect the drug from hostile environments for the slow release by diffusion or erosion, but also exhibit large reversible deformations triggered by environmental changes in pH, temperature, electric and magnetic fields[1].Nevertheless, one major challenge of hydrogels is fabricating compact network hydrogels without compromising their swelling characteristics[4].
Konjac glucomannan (KGM) is a high molecular-weight neutral polysaccharide extracted from the tubers of theAmorphaphalluskonjacplants[5].It is mainly composed ofβ-1,4 linkedD-mannose andD-glucose with acetyl groups attached randomly to C6 position[6].KGM has demonstrated great potential in hydrogel application due to excellent biocompatibility, biodegradability, gelling, and swelling properties[5,7].It has been found that KGM could only be hydrolyzed by colonic bacterial enzymes while not being degraded in the gastrointestinal tract, thus it has attracted much attention for promising application in drug delivery[5,8].However, the application of neat KGM hydrogel in drugs release has been seriously restricted due to its weak network structure[9-10].Consequently, many strategies are being explored to improve the efficiency of KGM hydrogels in drugs delivery systems, such as physical treatment[11-12],chemical cross-linking[13]and reinforcing agents[13-14].
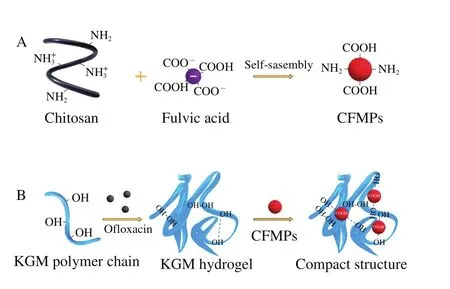
Recently, microparticles (MPs) with distinctive characterizations (such as high surface area, nano-scaled size,and surface morphology) are served as the reinforcing agents which is an emerging strategy to fabricate compact network of polysaccharides-based hydrogels[15-16].However, with the increasing demand of intelligent hydrogels, there is still a challenge to control the swelling volume of MPs-reinforced hydrogels through varying environmental factors (eg.pH).Chitosan (CS) have attracted a great deal of interests in the field of biomedical engineering, due to its pH-responsive behavior caused by a large amount of amine groups(—NH3+) on their active functional surface[17].Fulvic acid(FA), as an anionic organic compound, is economical and ubiquitous[18].It is produced by organic material (i.e.plants and microorganisms) degradation, and has been considered to be stable under alkaline and acidic environment[19].The chitosan-fulvic acid microparticles (CFMPs) were fabricated via the electrostatic self-assembly approach.It could be used as a natural reinforcing agent of biopolymer with higher surface site density and oxygen-containing functional groups,such as hydroxyl (—OH), carboxyl (—COOH) and phenolic(—Ph—OH)[20-21].To the best of our knowledge, no study has been conducted on the KGM composite hydrogel using CFMPs as a reinforcing agent.
Herein, KGM/CFMPs composite hydrogel were fabricated using CFMPs as the reinforcing agent.The influences of CFMPs on the network structure of KGM/CFMPs composite hydrogels were studied.Ofloxacin(model drug) loading efficiencies (DLEs) and encapsulation efficiencies (EEs) of the hydrogels, and ofloxacin release behavior in different mediums were also evaluated.It is presumed that CFMPs could not only improve the structure of KGM hydrogels, but also improve their pH-responsive release characteristic.
1 Materials and Methods
1.1 Materials and reagents
KGM (purity ≥ 95%; viscosity: 35 000 Pa·s at 25 ℃,1% solution (m/m)) was purchased from Sanai Konjac Development Co.(Yunnan, China).CS was purchased from Sinopharm Chemical Reagent Co.Ltd.(Shanghai, China)with a molecular weight of 450 kDa and with a deacetylation degree of 85%.FA was purchased from Shanghai Macklin Biochemical Co.Ltd.(Shanghai, China) with a molecular weight of 308 Da.Ofloxacin (95%), Pepsin (250 U/mg), and trypsin (250 U/mg) were purchased from Beijing Solarbio Science & Technology Co.Ltd.(Beijing, China).Other reagents of analytical grades were purchased from Sinopharm Chemical Reagent Co.Ltd.(Shanghai, China).Deionized water was used in all experiments.
1.2 Instruments and equipments
Nicolette 6700 spectrophotometer (Thermo Fisher Scientific Co.Ltd., MA, USA); Zeta-sizer Nano ZS90(Malvern Instruments, UK); SU8010 scanning electron microscopy (SEM) (Hitachi, Tokyo, Japan); MCR 301 rheometer (Anton Paar Instruments Inc., Austria).
1.3 Methods
1.3.1 Preparation of CFMPs
2 g CS powder was dissolved in 100 mL 2% (V/V)aqueous acetic acid to gain the CS solution, which was stirred overnight until dissolved.Then, FA (5 mg/mL) was dissolved into CS solution (pH 4, 0.5 mol/L NaCl, 40 ℃).The mixed solution was centrifuged at 4 000 r/min under 4 ℃ for 30 min to obtain CFMPs.CFMPs were re-suspended in distilled water, sonicated and freezing dried for further use.
1.3.2 Preparation of KGM/CFMPs composite hydrogels
The KGM/CFMPs composite hydrogels were obtained by mixing 1.5 g KGM and different CFMPs concentration of 1.4%, 2.8%, and 4.3% (m/m) denoted as KGM/CFMPs-1,KGM/CFMPs-2, and KGM/CFMPs-3, respectively.Then, the pH value was adjusted to 9.0 and incubated in the water bath at 90 ℃ for 60 min.After cooling down to room temperature,the KGM/CFMPs composite hydrogels was obtained.As a control group, 1.5 g of KGM was used to prepare KGM hydrogel through the above steps, and denoted as KGM.
1.3.3 Characterization of hydrogels
1.3.3.1 Fourier transform infrared (FT-IR) analysis
The FT-IR spectrum of samples were detected in the range of 400-4 000 cm-1at a resolution of 4 cm-1.Before testing, the samples were mixed with potassium bromide and then compressed.
1.3.3.2 Size distribution and zeta potential
The particle size, polydispersity index (PDI) and zeta potential of the MPs were measured.
1.3.3.3 SEM
The hydrogel samples were cut into rectangular strips of 1 cm × 5 cm, which were frozen in solution nitrogen and then separated.They were carried out to obtain the cross-sectional microstructures, and the detection voltage was 3.0 kV.Before testing, the samples were sputtered with about 20 nm thick gold for 60 s.
1.3.3.4 Rheology
The rheological properties of the solutions were determined.The parallel-plates (PP50, 50 mm diameter and 1 mm gap) were used in all rheological tests.The shear rate was set to 0.1-100.0 s-1, and the temperature to 25 ℃.
Meanwhile, for the process of dynamic test, the angular frequency ranged from 0.1 to 100 rad/s at 25 ℃ to search for the dynamic change of storage modulus (G’), the loss modulus (G’).
1.3.3.5 Drug encapsulation efficiency and loading efficiency determination
For the determination of drug loading and drug content,drug loaded hydrogels was prepared by adding ofloxacin(60 mg) in 100 mL water and shaken at 70 r/min for 1 h before the addition of KGM and CFMPs mixtures.Ofloxacin concentration in simulated intestinal fluid (SIF, pH 7.4)and simulated gastric fluid (SGF, pH 1.2) solutions was determined using UV-2600 spectrophotometer at characteristic wavelength (294 nm).
All the tests were conducted in triplicate.The EEs and DLEs of ofloxacin were calculated according to the following equations respectively[7]:

WheremAandmTrepresent the actual and theoretical contents of ofloxacin of the hydrogels/mg, respectively;mMrepresents the weight of dried hydrogels/mg.
1.3.3.6 Drug release studies
To study the influences of CFMPs on thein vitrorelease behavior of KGM hydrogels, two different buffer solutions,named SGF (pH 1.2) and SIF (pH 6.8), were prepared to study the ofloxacin release of the hydrogels.125 mg dried hydrogels containing ofloxacin was taken in 50 mL SGF and SIF at 37 ℃ under constant shaking at 60 r/min.After a pre-determined time interval, 5.0 mL of the medium was removed, then 5 mL of the fresh solution was added to the mediums every time to keep a total volume of 50 mL.All the tests were conducted in triplicate.The cumulative release (CR)of ofloxacin was calculated in terms of the equation (3)[5]:
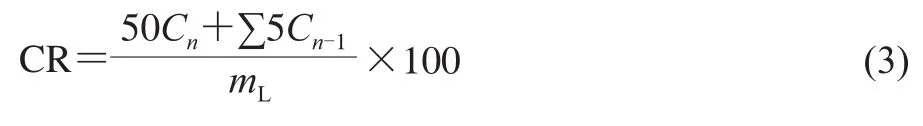
WhereCnandCn-1represent the ofloxacin concentration in the release medium atnandn-1 times, respectively;ndenots the times of taking out of the solution;mLrepresents the initial amount of ofloxacin loaded.
1.3.3.7 Ritger-Peppas model
Ritger-Peppas model[22]was used to investigate thekinetics of ofloxacin release from KGM/CFMPs composite hydrogel in SGF and SIF.

WhereQtdenoted the CR (%) of ofloxacin at timet;nwas the release exponent;nandKwere the release exponent and the kinetic constant, respectively.
1.4 Statistical analysis
All experiments were carried out with replicate.The data from all studies were reported as±s.The data analysis was conducted using SPSS version 25.0 and OriginPro 9.1 to assess the statistical significance of final results.Statistical significance was accepted atP< 0.05.
2 Results and Analysis
2.1 Characterization of CFMPs
2.1.1 FT-IR analysis
The FT-IR spectrum of CS, FA, and freeze dried CFMPs were depicted in Fig.1.The FT-IR curves of CFMPs showed the absorption peaks characteristic of a functional group and the interaction between CS and FA.For the curve of native FA, the bands at 1 028 cm−1were ascribed to C—O bending vibration from the CH3O or CH2OH groups of FA backbone[19].And the bands at around 1 429 cm−1were related to the carboxylate stretching vibration, the bands at 1 607 cm−1were attributed to aromatic C—H and C—O stretching of conjugated carbonyl groups.The bands at 2 840 and 2 944 cm−1were attributed to C—H stretching vibration of—CH3and —CH2groups, respectively.The broad peaks observed around 3 464 cm-1were assigned to stretching vibration of O—H from phenol groups and carboxyl groups,as reported in previous literature[19].The spectra of CS exhibited the characteristic bands at 1 661 and 1 600 cm-1could be assigned to amide-I and amide-II stretch, respectively.A wide adsorption band appeared in the range of 3 200 to 3 600 cm−1were assigned to the overlapping of symmetric and asymmetric—NH2and vibrational —OH stretches[23], respectively.
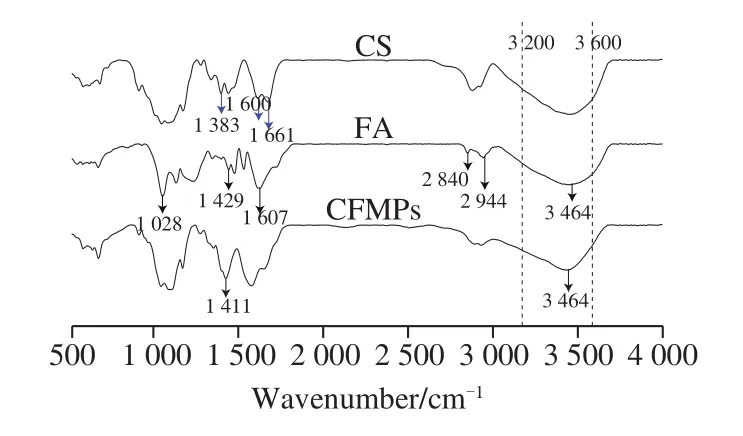
Fig.1 FT-IR spectra of CS, FA, and freeze-dried CFMPs
After adding FA, the CFMPs showed an inconsistent characteristic spectrum, and the absorption peak at 1 383 cm-1basically disappeared, possibly due to carboxyl groups(—COO-) in FA and amine groups (—NH3+) in CS had an electrostatic self-assembly reaction, thus generating the new ionic bonds[24-25].Meanwhile, with FA addition, the absorption peak representing N—H stretching at 3 464 cm-1was significantly increased, indicating the improvement of ionic gelation strength.And the new narrow peak at 1 411 cm−1appeared, which may be related to the appearance of more free carboxyl groups (—COOH) in the system.The change of FT-IR spectra indicated that the incorporation of FA could indeed reinforce ionic bonds[24-25].
2.1.2 Size distribution and zeta potential
The average size and PDI of MPs in water were measured using Zeta-sizer Nano-ZS90 equipment (Fig.2A).For the CFMPs, a predominant particle size of (50 ± 5) nm(8.25% intensity) in a narrow size distribution (PDI=0.416)was observed, it indicated that CFMPs possessed excellent dispersity[26].These results was further confirmed by using SEM analysis (Fig.3).
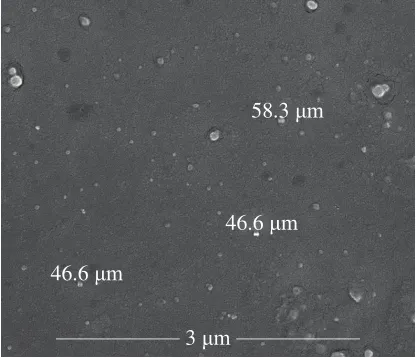
Fig.3 SEM image of CFMPs
The zeta potential was a vital value that represented the electrostatic repulsion between adjacent MPs (Fig.2B)[27].The determination yielded zeta potential value of CFMPs was +52 mV, probably due to amine groups(—NH2) of CS was on the surface of MPs, thus forming highly stable suspensions[28].

Fig.2 Particle size distribution (A) and zeta potential distribution (B) of CFMPs determined by Zeta-sizer Nano-ZS90
2.2 Characterization of CFMPs
2.2.1 Rheological analysis
Fig.4 showed the shear rheological curves of hydrogelforming solutions.It was found that all solutions emerged in similar trend.As the shear rate increased, the viscosity tended to decrease and eventually tended to be flat.This phenomenon proved that all the hydrogel-forming solutions were a typical pseudoplastic fluid[29-30].The viscosity of all hydrogel-forming solutions increased with increasing shear rate at low shear rates, and showed slight shear-thickening behavior when the shear rate was 0.2 s-1.Several studies had reported the similar behavior of polysaccharide solution, which might be explained by the opened entanglement structure that could not be promptly restored[31].Thus, it was reasonable to infer that the entanglement structure was formed between KGM and CFMPs.Meanwhile, it could be clearly seen from Fig.4 that CFMPs had a significant influence on the rheological properties of the hydrogel-forming solutions.With the addition of CFMPs, the viscosity curve showed an upward trend.It indicated that a new molecular interaction was generated between CFMPs and KGM, thus strengthening the viscosity of the hydrogel-forming solutions[32].However,when CFMPs was added in excess with a volumetric ratio weight ratio of 4.3%, the viscosity of the hydrogel-forming solution was distinctly reduced, possibly due to the original ordered microstructure being destroyed and the aggregation of CFMPs by excessive concentration[33].

Fig.4 Steady-shear rheological properties of KGM and KGM/CFMP hydrogel-forming solutions
TheG’ andG’ represented the elastic and viscous response of a given material, respectively[34].It was an important index that could indicate investigation of interaction in polymer molecules.Based on the changes of the rheological properties of the hydrogel-forming solutions,the effect of CFMPs on the KGM hydrogel solutions would be studied.Fig.5 indicated that theG’ for KGM, KGM/CFMPs-1, KGM/CFMPs-2 and KGM/CFMPs-3 was higher than theG’, showing that the elastic modulus dominated the dynamic process in the system.However, at a lower angular frequency, theG’ was a little higher than theG’.Fig.5 showed that the intersection point moved from 7.10 rad/s to 10.05 rad/s with the increase in CFMPs concentration resulting in the formation of entanglement structure.

Fig.5 Angular frequency of KGM and KGM/CFMP hydrogel-forming solutions
2.2.2 FT-IR analysis
In order to investigate the interactions between KGM and CFMPs, the functional groups of CFMPs, KGMhydrogel, and KGM/CFMPs composite hydrogels were determined by FT-IR and results shown in Fig.6.FT-IR spectra of the pure KGM hydrogel showed that the absorption bands at 870 cm-1and 2 896 cm-1were attributed to mannose unit stretching vibrations and C—H stretching, respectively.The stretching vibrations around 1 652 cm−1were overlapped by the intra-molecular hydrogen bonds.The wide broad absorption peak around 3 200-3 600 cm−1could be ascribed to the stretching vibration of O—H[5].
The FT-IR spectra of the KGM/CFMPs composite hydrogels were a similar pattern in comparison to the KGM hydrogel, indicating that no chemical bond was created between CFMPs and KGM in the hydrogels.After adding CFMPs, the KGM/CFMPs-2 composite hydrogels showed that the absorption peak at 1 411 cm-1almost disappeared,possibly due to —COOH in CFMPs and —OH in KGM had an interaction, thus generating new hydrogen bonds[30].Meanwhile, with the continuous increase of CFMPs addition,the absorption peak representing O—H stretching at 3 344 cm-1was continuously increased, indicating the improvement of hydrogen bonding strength.The change of FT-IR spectra indicated that the incorporation of CFMPs could indeed reinforce hydrogen bonds.However, the absorption peak of the curves of KGM/CFMPs-3 composite hydrogel was also increased at 1 411 cm-1, which might be due to the increase of free —COOH caused by excessive CFMPs.The excessive use of CFMPs might cause selective interaction, leading to a decrease in the overall degree of interaction.As shown in Fig.6, the addition of CFMPs to the KGM matrix generated the interaction forces such as hydrogen bonds, thus causing the change of characteristic peaks in the infrared spectrum.

Fig.6 FT-IR spectra of CFMPs, KGM and KGM/CFMP composite hydrogels
2.2.3 Morphological characterization
To further confirm the microstructure of hydrogels.The network structure of the lyophilized KGM hydrogel and KGM/CFMPs composite hydrogels was depicted by SEM analysis.As shown in Fig.7A, discontinuous and disordered network structure was observed in the KGM hydrogel.It meant that the polysaccharide system of KGM was weak[5].On the other hand, the denser structures and interconnected pores had emerged after the CFMPs were added, as shown in Fig.7B, C.This phenomenon was related to the formation of inter-molecular hydrogen bonds between carboxyl groups in CFMPs and hydroxyl groups in KGM polymer chains,suggesting that the compact network structure was generated through acting CFMPs as a reinforcing agent[35-36].However,it was noted that the excessive CFMPs on sample of KGM/CFMPs-3 composite hydrogel, promoting porous network structure.It meant that the excessive CFMPs might affect compact network structure of the hydrogels (Fig.7D).From all the data of characterizing the chemical structure, it was confirmed that CFMPs produced the hydrogen bonding interaction in the KGM/CFMPs system.Meanwhile, the effect also showed a regular change with the increase of CFMPs concentration in the hydrogels.Therefore, KGM/CFMPs composite hydrogels were consistent with the expectation that the KGM/CFMPs composite hydrogels possessed the compact network structure.
2.3 pH-Controlled release behavior
2.3.1 Drug entrapment efficiency
To explore the influences of CFMPs proportion on the drug release behavior of the KGM/CFMPs composite hydrogels, the EEs and DLEs were studied for acquiring ofloxacin content in hydrogels.The EEs and DLEs of KGM/CFMPs composite hydrogels were recorded in Table 1.Compared with KGM hydrogel, KGM/CFMPs composite hydrogels showed that the value of EEs and DLEs were significantly higher (P< 0.05).These phenomena may be due to a larger specific surface area for ofloxacin storage possessed by KGM/CFMPs composite hydrogels[7,37-38].However, The EEs and DLEs of KGM/CFMPs composite hydrogel slightly reduced with further increasing the CFMPscontent.It verified that compact structure contributed to encapsulation efficiency.
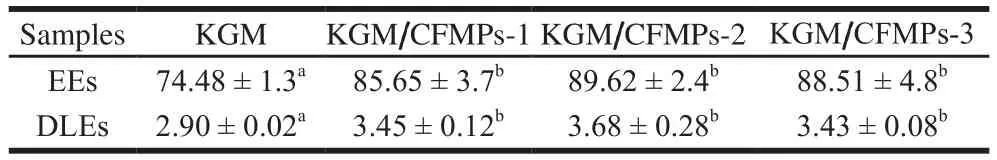
Table 1EEs and DLEs of KGM hydrogel and different KGM/CFMP composite hydrogels%
2.3.2In vitrodrug release
In order to evaluate the influences of CFMPs on the drug delivery properties of obtained KGM/CFMPs composite hydrogels, the release profile of ofloxacin released from KGM hydrogel and KGM/CFMPs composite hydrogels in buffer solutions of SIF and SGF at 37 ℃ was studied.As displayed in Fig.8A and B, more than 95% of ofloxacin was released from KGM hydrogel in the first 1 h, it indicated that KGM hydrogel exhibited serious initial burst release behavior in two buffer solutions, which was ascribed to KGM hydrogel possessed poor network structure and excellent swelling property[39].
Meanwhile, it could be clearly seen from Fig.8A and B those CFMPs had a significant impact on the drug release behavior of the KGM/CFMPs composite hydrogels.With the addition of CFMPs, the cumulative release curves showed a slowly increase trend.It indicated that the contact between high ofloxacin concentration gradient and the medium was retarded[40], probably due to the compact porous network structure prevented ofloxacin from spreading into medium.Yuan Yi et al.[7]have reported the ciprofloxacin diffusing through a compact structure of AG-KGM hydrogel, leading to the cumulative release of ciprofloxacin, which was approximately 80% after 5 h.However, when CFMPs was added in excess (4.3%), the speed of drug release behavior was distinctly accelerated, possibly due to the original compact network microstructure destroyed by excessive addition, Fattahpour et al.[41]have reported the decreasing of hydrogel pore sizes resulted from the nanoparticles reinforcement on the inter and intra-molecular structure.
As Fig.8A and B displayed, it was worth pointing out that the amount of ofloxacin released from KGM/CFMPs-2 composite hydrogel at SGF was higher than that at SIF.This may be attributed to the expansion of porous network structure of hydrogels and thus facilitate the diffusion of ofloxacin from the KGM/CFMPs composite hydrogels[42].This result indicated that the electrostatic repulsion caused by the protonation of amine groups (—NH3+) of CFMPs in SGF, leading to a decrease in the overall degree of hydrogel bonds interaction.However, there was no obvious pH-responsive ability of KGM/CFMPs-1 and KGM/CFMPs-3 composite hydrogel in the drug release behavior.A possible reason for the lack of responsiveness of hydrogel was the lower degree of compact network structure.The change of drug release behavior confirmed that the incorporation of CFMPs could indeed reinforce network structure.Therefore,it was consistent with the expectation that the KGM/CFMPs composite hydrogels were outstanding drugs delivery materials with pH-controlled release behavior.The possible mechanism of KGM/CFMPs hydrogel as shown in Fig.9.
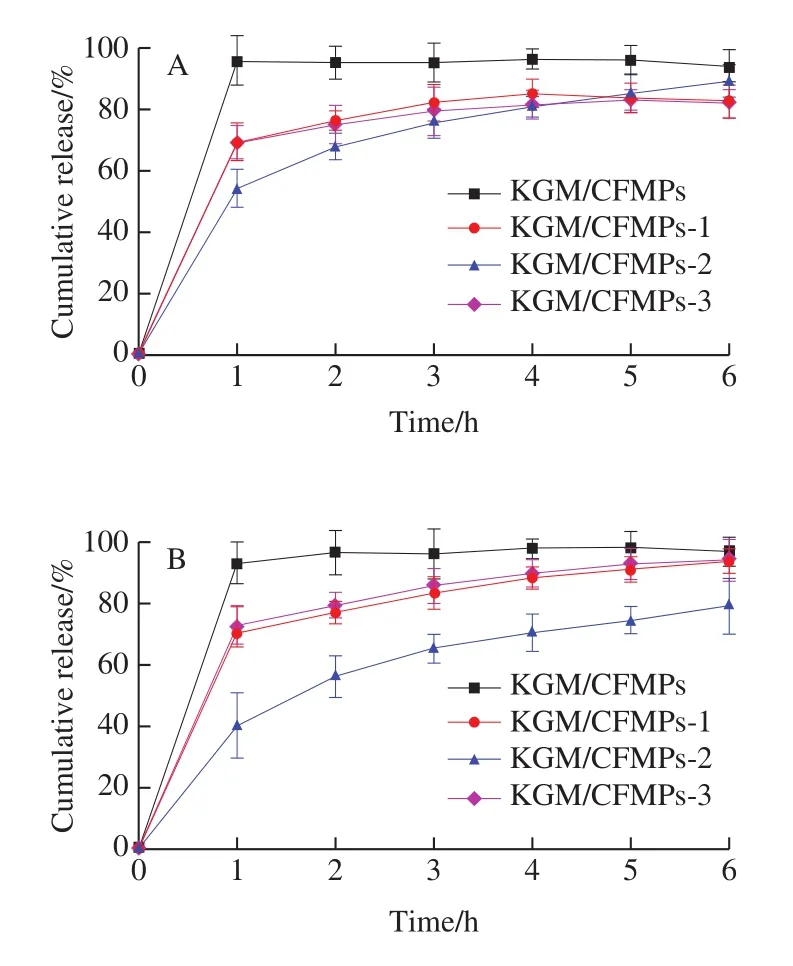
Fig.8 Cumulative release curves of KGM and KGM/CFMP composite hydrogels in SGF (A) and SIF (B)
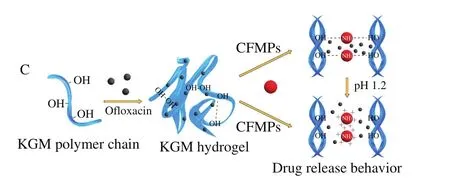
Fig.9 Possible formation mechanism of CFMPs (A), possible mechanism of CFMPs effect on the structure (B) and drug release behavior of KGM/CFMPs hydrogel (C)
2.3.3 Ritger-Peppas model
To further explain the drug release characteristics of the KGM/CFMPs composite hydrogels, the frequently used drug release model, namely, Ritger-Peppas model was applied.Results from Table 2 suggested that equations were used to describe the kinetics of ofloxacin released from KGM/CFMPs-2 composite hydrogel, whereR2was 0.999 3, and 0.995 5 in two different environments, respectively.When thenvalue of Ritger-Peppas model was below 0.45,it suggested that Fickian diffusion took the dominant role[43].These results indicated that the ofloxacin in KGM/CFMPs composite hydrogels were released by diffusion through the porous and channels in the compact network structure[44].

Table 2Results of ofloxacin release curve fitting to Ritger-Peppas model
3 Conclusion
In summary, the hydrogels were developed by incorporating KGM with the different contents of CFMPs.The influences of CFMPs on the structure and drug release characteristics of KGM hydrogels were systematically investigated.The rheological analysis illustrated that a new molecular interaction was generated between CFMPs and KGM.FT-IR spectra confirmed that the hydrogen bonds formed due to the interaction between —COOH groups of CFMPs and —OH groups of KGM polymer chains.SEM results demonstrated that the compact structure in porous network was fabricated due to interaction between CFMPs and KGM.In vitroassay showed that KGM/CFMPs composite hydrogels avoided the burst release behavior in the form of Fickian diffusion.It is highlighted that KGM/CFMPs composite hydrogels possessed prominent pH-controlled release behavior, which was ascribed to the protonated amine groups (—) in CFMPs.Therefore, KGM/CFMPs composite hydrogels provided valuable contribution to the development of pH-responsive drug delivery systems.

