Stem cell therapy in ocular pathologies in the past 20 years
Giovanni Miotti,Pier Camillo Parodi,Marco Zeppieri
Giovanni Miotti,Pier Camillo Parodi,Department of Plastic Surgery,University Hospital of Udine,Udine 33100,Italy
Marco Zeppieri,Department of Ophthalmology,University Hospital of Udine,Udine 33100,Italy
Abstract Stem cell therapies are successfully used in various fields of medicine.This new approach of research is also expanding in ophthalmology.Huge investments,resources and important clinical trials have been performed in stem cell research and in potential therapies.In recent years,great strides have been made in genetic research,which permitted and enhanced the differentiation of stem cells.Moreover,the possibility of exploiting stem cells from other districts (such as adipose,dental pulp,bone marrow stem cells,etc.) for the treatment of ophthalmic diseases,renders this topic fascinating.Furthermore,great strides have been made in biomedical engineering,which have proposed new materials and threedimensional structures useful for cell therapy of the eye.The encouraging results obtained on clinical trials conducted on animals have given a significant boost in the creation of study protocols also in humans.Results are limited to date,but clinical trials continue to evolve.Our attention is centered on the literature reported over the past 20 years,considering animal (the most represented in literature) and human clinical trials,which are limiting.The aim of our review is to present a brief overview of the main types of treatments based on stem cells in the field of ophthalmic pathologies.
Key Words:Stem cells;Multipotent mesenchymal cells;Adipose stem cells;Novel therapies;Eye pathology;Cornea;Cell therapies;New materials
INTRODUCTION
Numerous studies in scientific medical literature continually show that cell therapy tends to be an effective alternative and innovative method to regenerate damaged tissue[1].In the last decades,studies have shown that stem cell therapies can bring substantial benefits to patients suffering from a wide range of diseases and injuries[2].For this reason,huge investments,resources and important clinical trials have been performed in stem cell research and potential therapies based on stem cells.In these past years,there has been a continuing expansion in the number and types of stem cells assessed for potential treatment use.Stem cells are now being used in various fields of medicine,ranging from hematology to cardiology,in addition to neurology,plastic surgery,dentistry,etc.[3-5].Although literature has reported limited success to date,clinical trials continue to evolve as our understanding enhances regarding the physiology and mechanisms underlying potential healing benefits behind stem cells.Recent advancements in regenerative medicine have also considered the use of stem cells for cellular repair and regeneration[6].
Stem cell therapies have shown great potential in numerous studies reported in literature concerning injuries and diseases of the eye[3].This progress is a result of several factors,including the relatively small numbers of cells required,easy accessibility for surgery and straightforward assessment and visualization of grafts.Numerous types of cells have been used in clinical trials for the eye[2].Research on stem cell therapies has been applied to almost all parts of the eye.
Based on our preliminary animal experiments regarding stem cells therapies in corneal healing[3,7],and the growing number of studies that show great clinical potentials of stem cells for this therapeutic approach,we decided to assess the literature reported in the last 20 years.Considering that the number of studies reported in literature since 2000 is immense,and our experience in this field is limiting,we do not intend to provide an exhaustive meta-analysis,but a quick overview of the use of stem cells for ophthalmology treatment;thus,we apologize in advance if opinion leaders and experts in this field of study have not been cited in our paper.The aim of our review is to present a brief overview of the main types of treatments based on stem cells in the field of ophthalmic pathologies,by briefly addressing the what,why,which,how,when and where of this issue.We have tried to concentrate or review on pertinent human studies;however,considering most of the literature to date tends to be based on animal experimentation,mention has also been made regarding this vast body of literature.
MATERIALS AND METHODS
We conducted a search of the literature published between January 1,2000,to December 20,2020,using MEDLINE (PubMed).The database was first searched using the key words “stem,cell and eye”,in which 7486 studies were found.We considered only studies in English and those referring to humans,thus reducing the count to 3941.The reference lists of all retrieved articles were scanned to identify additional relevant studies.We then considered only articles based on “stem cells therapy” (2194 papers),and we excluded “case reports”,“case series”,“conference papers”,“letters” and “in vitro” (1179 articles).Results were then divided and sorted by when/where stem cells were used,which included:Ocular surface;corneal epithelium,stroma,endothelium,limbus;trabecular meshwork;lens;optic nerve;retina.The details regarding the selection of papers considered in the manuscript are listed in Figure 1.

Figure 1 The selection of PubMed literature published from 2000 to 2020,which was considered in the manuscript.
Only articles with abstracts were considered (1148 articles).After a selection by title and abstract,185 articles were analyzed.A quality score was calculated for each article using a checklist from the American Society of Plastic Surgeons guidelines for therapeutic studies[7].Each study was appraised by at least two reviewers (GM and MZ),and rating decisions were based on the consensus of the reviewing authors.A summary of the most significant studies and conclusions is reported in Tables 1 and 2.
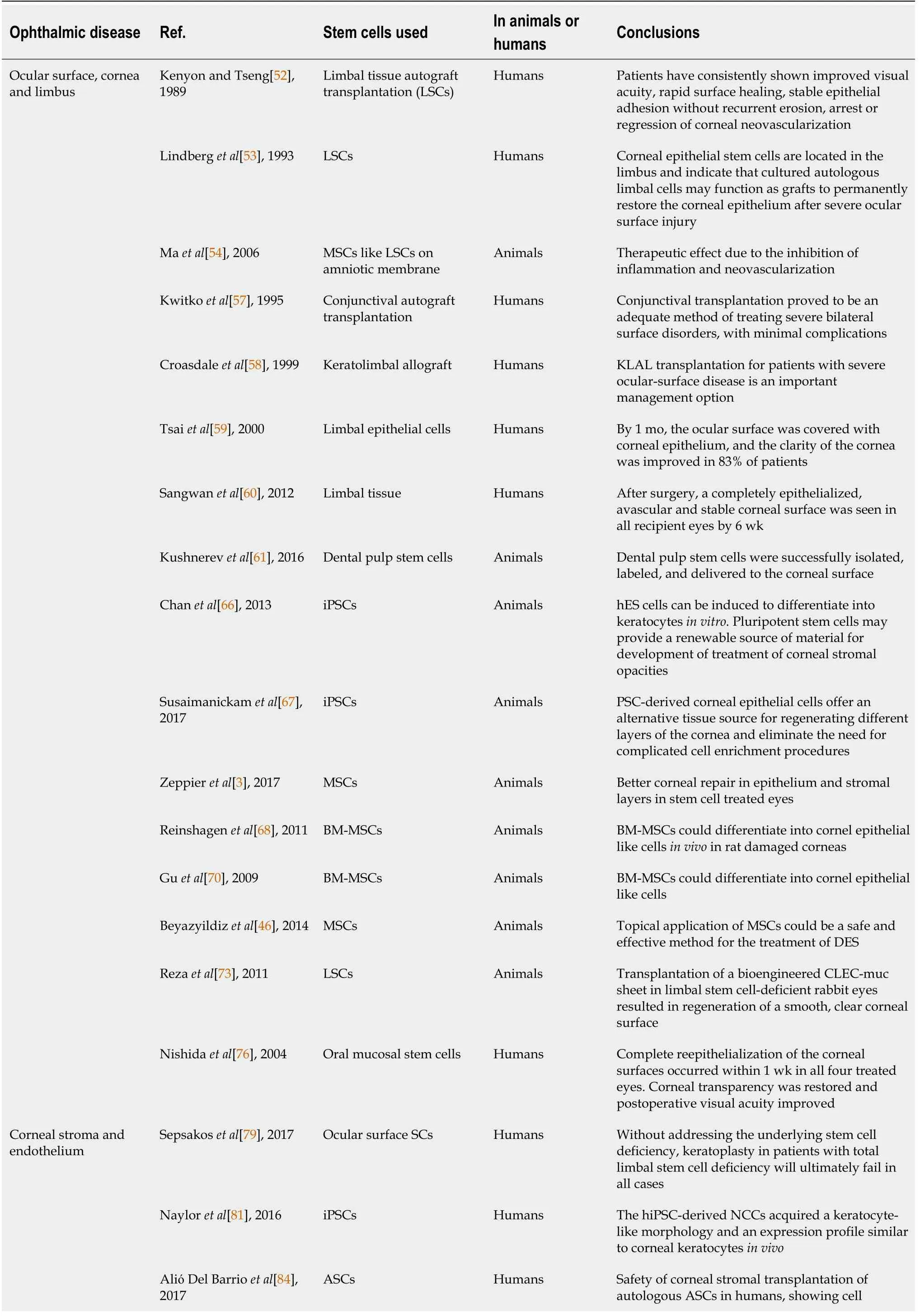
Table 1 Clinical trials in ocular diseases

ASC:Adipose-derived stem cell;BM-MSC:Bone marrow-mesenchymal stem cell;CLEs:Cutaneous lupus erythematosus;CM:Ciliary margin;DES:Discrete event simulation;HCEC:Human corneal endothelial cell;hESCs:Human embryonic stem cells;hiPSC:Human-induced pluripotent stem cells;iPSC:induced pluripotent stem cell;KLAL:Keratolimbal allograft;LEC:Lens epithelial stem/progenitor cell;LSC:Limbal stem cell;MSC:Mesenchymal stem cell;NCC:Neurocysticercosis;ON:Optic nerve;PDL:Periodontal ligament;PSC:Pluripotent stem cell;RGC:Retinal ganglion cell;TM:Trabecular meshwork;UC-MSCs:Umbilical cord-mesenchymal stem cell;UCB:Umbilical cord blood.
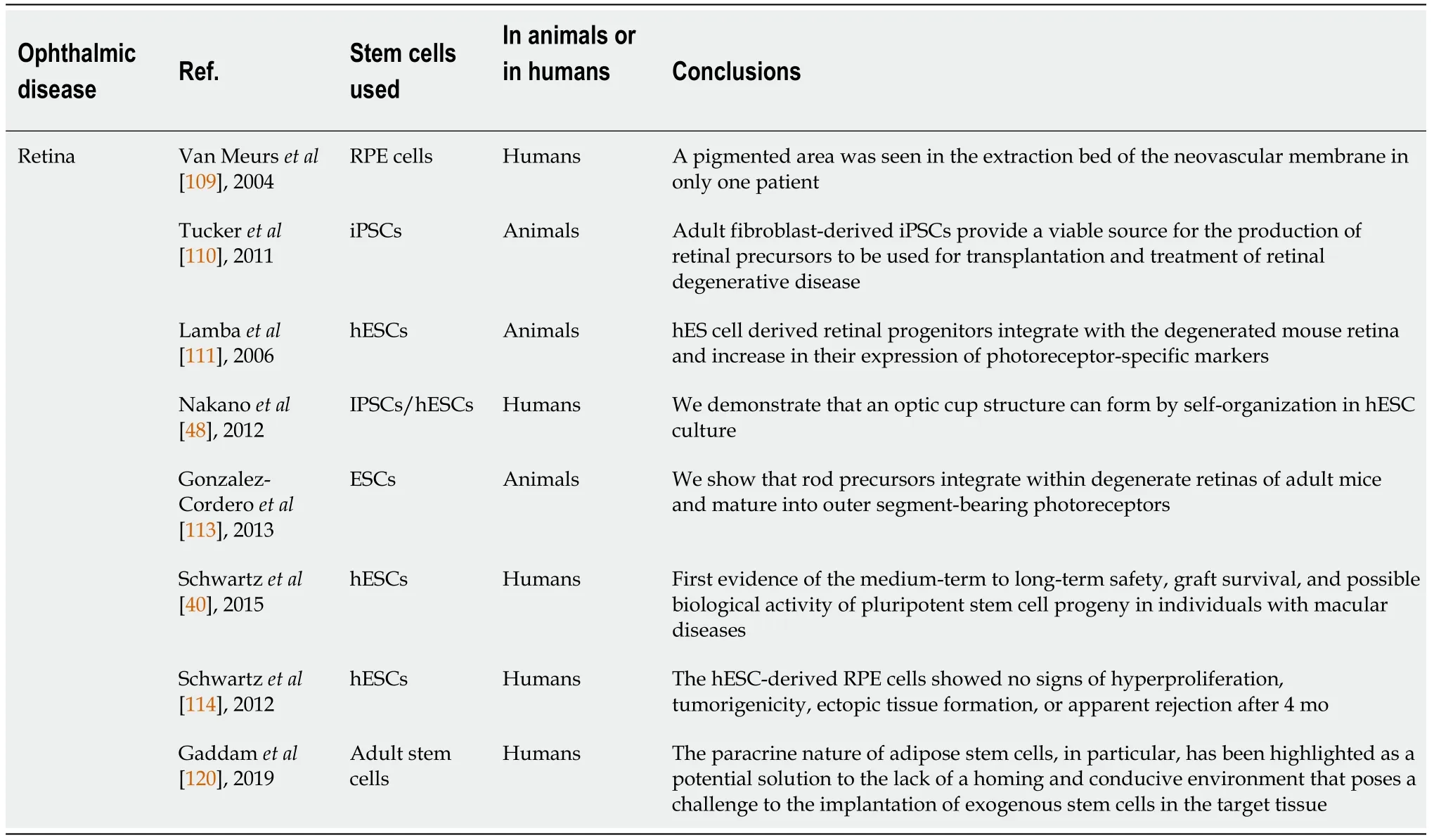
Table 2 Retinal diseases and therapies
What are stem cells
In brief,stem cells are undifferentiated cells that are present in the embryonic,fetal and adult stages of life and give rise to differentiated cells,which are the building blocks of tissue and organs.In the post-natal and adult stages of life,tissue-specific stem cells are found in differentiated organs and are instrumental in repair following injury to the organ.The main characteristics of stem cells are self-renewal (the ability to proliferate extensively),clonality (usually arising from a single cell) and potency(the ability to differentiate into different cell types)[8].
Stem cells can be classified as totipotent (zygote),pluripotent [embryonic stem cells(ESCs) and induced pluripotent stem cells (iPSCs)],multipotent [mesenchymal stem cells (MSCs)] and oligopotent.Totipotent cells form embryonic and extra-embryonic tissues.Pluripotent cells form all three germ layers,while multipotent cells generate cells limited to one germ layer.
The human body develops from the zygote and blastocyst from which ESCs are derived into germ layers endoderm,mesoderm and ectoderm.Specific organs arise from germ layers.Some of the progenitor cells that have contributed to organ formation do not terminally differentiate but are retained as tissue stem cells and can be found in bone marrow,bone,blood,muscle,liver,brain,adipose tissue,skin and gastrointestinal tract[9].The tissue stem cells may be called progenitor cells since they give rise to terminally differentiated and specialized cells of the tissue or organ.These cells may be dormant within tissues;however,have the ability to proliferate under circumstances of injury and repair[10].The dynamics of tissue stem cells or progenitor cells varies from tissue to tissue.In bone marrow,liver,lung and gut,for example,stem cells regularly proliferate to supplement cells during normal turnover of cells or tissue injury.In other organs,however,like pancreas,heart or nervous system,stem cells proliferate to replace damaged cells following injury[11,12].
Why use stem cells in ocular diseases
The human eye is a remarkable structure produced from the coordinated development of multiple tissues.It is the result of a combination of tissues deriving from neuroectodermal (e.g.,retina),ectodermal (e.g.,lens and cornea) and mesodermal lines.Diseases and injuries that compromise the function of any of these major ocular tissues can lead to blindness[13,14].Considering the fundamental importance and influence on quality of life of sight,research has invested enormous resources and studies towards the search for new therapies to prevent and treat ocular disorders.There is a notable history of trail-blazing work in ocular medicine,exemplified by tissue transplantation,the use of laser therapy,the recent gene-therapy and cell therapy[14].Due to burden of eye disease,and its relative ease of accessibility,the eye is a prime target for stem cell transplantation therapies,in which complications tend to be rare (e.g.,overgrowth and tumor formation)[15].In addition,advanced methods currently exist to examine easily and thoroughly assess the clinical results of eye transplant therapies.Modern technology is readily available to provide a non-invasive quantifiable visualization of most structures of the eye,and visual functions can routinely be assessed rapidly,quantitatively and accurately.
Which stem cells can be considered for treatment in ophthalmology
Human pluripotent stem cells:ESCs and iPSCs:Human pluripotent stem cells(hPSCs) have been considered promising sources for regenerating damaged tissues and organs because of their ability to differentiate into cells from three embryonic germ layers[16].These cells can also be maintained in an undifferentiated state for a prolonged period in culture[8].There are numerous scientific studies regarding therapy with hPSCs,specifically,in the form of iPSCs.Several studies have reported the use of iPSCs in retinal degenerative pathologies[2,17,18].hPSCs have the greatest potential for cell replacement and can be successfully pre-differentiated prior to transplantation in the eye.iPSCs are pluripotent stem cells generated from somatic cells by cellular genetic reprogramming using defined transcription factors[8].First described in 2007 by Takahashiet al[19],iPSCs used in the experiments were derived from skin fibroblasts produced using retroviral technology[19].iPSCs provide a uniquein vitromodel that allows the generation of retinal progenitor cells.An advantage of iPSCs made from a patient’s own cells could reduce the need for immunoprotective regimens post-transplantation[15].It has been demonstrated that these iPSCs have similar characteristics features of PSCs,conserving the possibility to generate tissues from each of the three germ layers[20].The greatest problem reported regarding iPSCs was genomic instability,which may induce teratoma.The aim of research in recent years has been to create safety protocols capable of guaranteeing genomic stability[21].The greatest success of hPSCs has been in retinal pigment epithelial cell (RPE)/photoreceptor replacement for aged-related macular degeneration (AMD),but studies have also reported the potential successful use in almost all structures of the eye[22,23].
Adult stem cells:The use of adult stem cells represents an easier route to regenerativecell therapies.The ability of some adult tissues (i.e.skin,haemopoietic system,bone,liver,etc.) to repair or renew,indicates the presence of stem or progenitor cells[9].Numerous clinical trials based on the use of adult stem cells have also been developed in the field of ophthalmology.
Considering that the component of the eye,mainly the retina,is directly derived from the central nervous system (CNS),studies regarding cell therapy have shown to be difficult,limiting,yet potentially fascinating.The nervous system was thought to be rigidly constructed,with no capacity for physical repair.However,studies of nerve development have led to isolation of cells from the developing and mature mammalian CNS that shows numerous properties of stem cells [neural stem cells(NSCs)][9].NSCs have been studied with success in spinal cord injury and traumatic brain injury[24].NSCs secrete trophic factors,and therapies involving these cells may have potential for the neuroprotection of photoreceptors rather than replacement of retinal neurons,including retinal ganglion cells (RGC)[22,25].
Bone marrow stem cells (BMSCs) are progenitors of bone,cartilage and skeletal tissues,in addition to the hematopoiesis-supporting stroma and adipocyte cells.These cells can differentiate into mesenchymal cells,visceral mesoderm,neuroectoderm and endoderm characteristicsin vitro[26].In ophthalmology,BMSCs can be used to prevent graftvshost disease in corneal transplantation.These cells have also been studied in retinal diseases.The rationale for exploring the use of BMSCs as a potential therapy is a paracrine trophic effect on degenerating ischemic retina[22,27].
The previous view of adult stem cells has been that the differentiation potential was strictly limited to cell lineages found within the tissue of origin studied in tissues such as skin and bone marrow.During the past few years,this view has changed.Several studies have shown apparent plasticity of adult stem cells,like the ability to differentiate to cell types other than the tissue of origin[9].This remarkable finding challenged the long-held assumption that truly multipotent or pluripotent stem cells did not persist beyond early stages in embryogenesis.
Multipotent stem cells can be inserted in this context because they express markers of pluripotency previously seen only in ESCs or pregastrulation embryos[9].The most commonly studied multipotent SCs in ophthalmopathies are MSCs.MSCs are classically the “post-natal,self-renewing and multipotent stem cells giving rise to all skeletal tissues”[2].They have been found in different fetal tissues,in extraembryonic tissues [placenta,umbilical cord (UC) and amniotic fluid] and in adult tissues (bone marrow,peripheral blood,adipose tissue,dermis,synovium,periosteoum,cartilage,skeletal muscle,fallopian tubes,menstrual blood,gingiva and dental tissue and eye)[28].Placental and adipose-derived MSCs are,by far,considered in the largest number of clinical trials.
Fetal MSCs are scarcely considered in reported studies probably due to the difficulties in achieving sufficient cell numbers after expansion[29].
UC-MSCs have excellent proliferation and differentiation properties.These cells are readily available in large quantities and cultured rapidly.Moreover,UC incorporates both mesenchymal and epithelial stem cells with anti-inflammatory and immuneprivilege properties that can be differentiated into corneal epithelial,stromal and endothelial cells[29].
Human adipose-derived stem cells (ASCs) are an easily accessible autologous stem cell source.They have been shown to support the growth of many types of stem cells including human embryonic stem cells (hESCs),iPSCs and limbal stem cells (LSCs)[30].ASCs resemble BMSCs in terms of morphology,proliferation and multipotency[31].These cells have been considered for various ocular disorders,from retinal degeneration (neuroprotection and neurogenesis)[22,31] to corneal dise-ases[1,3,32].
Dental pulp stem cells (DPSCs) were the first human dental stem cells isolated from the dental pulp of permanent teeth[33].These cells show the unique characteristic of the potential to differentiate into not only typical mesodermal cell lineages but also ectodermal and endodermal cell lineages.DPSCs have exhibited the potential to differentiate into active neurons,cardiomyocytes,myocytes,melanocytes and hepatocytelike cells.These cells have shown higher angiogenic,neurogenic and regenerative potential when compared to BMSCs.In the field of ophthalmology,these cells have been studied for corneal repair[34],treatment of glaucoma and retinal diseases[35].
Going even more specifically,let us analyze the eye structure.Within the healthy eye,different types of adult stem cells can be found,which can be considered as eye stem cells.Retinal pigment epithelium stem cells (RPESCs) represent one of the latest discoveries in terms of stem cells of the eye.Retinal epithelium arises from the CNS neuro-epithelium.Interestingly,studies have shown how these cells can proliferate in some animal species and in certain conditions (e.g.,injury).Saleroet al[36] identified a subpopulation of RPE cells that can be activated to self-renew and that exhibit multipotentially,producing either stable RPE progeny or neural,osteo,chondro or adipo-lineage mesenchymal progeny[36].Studies have demonstrated how RPESCs,to date unknown,can proliferate pathologically in specific conditions,giving rise to retinal disorders.RPESCs are considered as a new type of SC capable of producing both CNS and mesoderm-associated lineages[36].Autologous RPE cell replacement has provided proof-of-concept evidence that cell therapy may have promising applications for retinal degenerative diseases.RPE cell source,however,is restricted for applications in RPE cell replacement due to the limited number of donor eyes and ethical issues concerning this type of treatment[37].
Hallmark features of the corneal epithelium include the high regenerative potential and the capacity for rapid ocular surface repair through proliferation and centripetal migration of progenitor cell populations residing at the border of the cornea and the sclera in a location called limbus.LSCs represent a quiescent cell population with high proliferative potential,which enables efficient corneal regeneration and repair[38].LSCs are fundamental for corneal homeostasis.A loss or deficiency [LSC deficiency(LSCD)] of these cells causes the disruption of homeostasis[39].LSCs can be collected using biopsy and expanded in culture,and then they can be transplanted in the diseased eye[1].
Criteria for selecting stem cells type:As explained above,stem cells for the treatment of ocular disorders can be obtained from the same eye (or fellow eye),from other tissues of the patient,or from a donor.As with all treatments,clinicians seek safe,widely available and economically viable therapies.Obtaining SCs from the patient’s own tissues have changed the therapeutic perspectives.This option has the advantage of not requiring immunosuppressive therapies and guarantees no immune reactions[40].Another element that guides the choice of SCs is the availability of cells.Alternatives to pluripotent stem and eye tissue cells have been considered in literature,however,these options tend to be limiting due to ethical constraints,possible unknown adverse effects,little availability and unsustainable costs[21,37].Autologous sources,such as MSCs (UC and adipose stem cells in particular),have been widely studied in recent years because of the large availability and the confirmed safety in other medical uses[3,4],and seem at the moment to preferential options for the future.
How can stem cells be used in clinical research
Multiple techniques to approach stem cell therapy for ophthalmic disorders have been described and studied over time.
Transplantation is the most used technique for stem cell therapy in the eye.SCs can be transplanted either as a cell suspension[40] or on different substrates,like autologous sheets of cells,biomaterial-based patches or three-dimensional (3D)structures[41,42].SC transplantation is a technique substantially used in all eye structures.The key sites currently targeted include:The cornea,mainly the clear tissue covering the front of the eye that helps focus the incoming light;the neural retina,which contains the photoreceptors;and the RPE,a single layer of pigmented cells that plays a key role in maintaining the photoreceptor cells and the blood-retina barrier[15,41,42].
Stem cells injection (pluripotent,MSCs or hematopoietic stem cells)[27,40] is a procedure still under study today,considered especially for the treatment of AMD.To date,these therapies are not yet proven to be safe and effective in ophthalmic use,however,have shown to be widely validated in other body districts[43].Several clinical experiments have proposed the injection of SCs into the intravitreal space(exploiting the paracrine effect of SCs) or in the subretinal space;however,results were not as expected,and several collateral effects have been reported in literature[44]that have partially “extinguished” the enthusiasm for results obtained in animal clinical trials.
Topical stem cells therapies (eye drops) have been extensively studied to treat ocular surface diseases.Hematopoietic stem cells from UC blood serum[45] have been proposed for topical treatment of ocular surface diseases.The rationale for applying serum to the ocular surface is that,compared to conventional lubricant treatments,it more closely resembles natural tears due to several of its biochemical constituents.It has produced satisfactory results in terms of efficacy and safety.Moreover,MSCs (in particular BMSCs[46] and ASCs[3]) have been used topically for dry eye syndrome or other corneal injuries or diseases with promising results.The rationale for MSCs eye drops is the wide-ranging differentiation potential,anti-inflammatory and immunomodulatory effects of MSCs[46-49].
In what ocular disorders can stem cells be used
Ocular surface,cornea and limbus:The ocular surface is the interface between the functioning eye and the environment.This surface provides anatomic,physiologic and immunologic protection and comprises the palpebral and bulbar conjunctival epithelium,the corneoscleral limbus,the corneal epithelium and the tear film[50].The functions of ocular surface include maintaining optical clarity of the cornea and protection of the structures of the eye from microbes,trauma and toxins.Disorders of the ocular surface include a variety of conditions.The most common are dry-eye disease,blepharitis,ocular allergies and pterygia.Less common diseases are LSCD and ocular surface diseases due to systemic diseases.The narrow zone between cornea and bulbar conjunctiva is defined as limbus,which contains layers of cells populated by Langerhans cells,melanocytes and LSCs (the stem cells that generate corneal epithelium).If a patient has an extensive destruction of the limbus,a functional corneal epithelium can no longer be formed,and the cornea reacquires an epithelium by invasion of bulbar conjunctival cells.The only way to prevent the corneal conjunctivation is indeed to restore the limbus.Keratoplasty has been considered the conventional gold standard treatment before the introducing of regenerative medicine[51].
The first attempt to restore limbus,using free limbal tissue grafts from the uninjured eye,was published by Kenyon and Tseng in 1989[52].The potential of limbus grafts promoted additional studies in this field,which brought the discovery of LSCs[38,53].In 2006,Maet al[54] showed how transplantation of human MSCs on amniotic membrane,like LSCs on amniotic membrane,could reconstruct severely damaged rat corneal surface.They demonstrated how the therapeutic effect did not come from epithelial differentiation of MSCs but was probably due to the inhibition of inflammation and neovascularization.
Since then,cultures of LSCs have been used to treat corneal diseases and injuries,initially engrafting the injured eye from the uninjured one[55],then treating bilateral pathologies with expanded cultures of LSCs[56].The fragility and limited number of active SCs of the corneal epithelium in culture,in addition to the induction of iatrogenic damage to the healthy fellow eye,however,tend to slow-down and limit the clinical application of LSCs.For these reasons,several materials have been proposed to enhance results,which include fibrin glue,amniotic membrane,polymers,collagen sponges or strips and devitalized membrane or polymers[57].
LSCD is the most challenging disease of the ocular surface for ophthalmologists.Corneal LSC grafting procedures have been utilized.This procedure involves either direct transplantation of limbal tissue or transplantation ofin vitroexpanded cells on a variety of biological or synthetic carrier materials[29].These techniques include conjunctival limbal autograft,living-related conjunctival allograft[57],keratolimbal allograft[58],autologousex vivocultivated limbal epithelial transplantation[59],simple limbal epithelial transplantation[60],cultivated oral mucosal epithelial transplantation[61] and transplan-tation of peripheral corneal cells[62].Clinical studies using conjunctival limbal autografts have shown excellent short-term and good longterm results,however,with high risks of complications,which mainly include induced damage to the donor eye[63].For these reason,other types of stem cells have been proposed to produce LSCs and corneal epithelium cells.
In 2012,an efficient protocol,with positive preliminary results,was proposed to produce corneal epithelial cells from human-iPSCs (hiPSCs)ESCs obtained from hair follicles or dermal fibroblasts[64].Results were also then confirmed in successive studies[65,66].The evolution of this approach led to the creation of corneal organoids from iPSCs[23],initially in two-dimensional,and then in 3D culture systems,with promising results as alternative for the treatment of bilateral LSCD[67].
MSCs have also been considered for ocular surface therapy[3,55].Preliminary results in animal experiments are encouraging,and the potential is great.Studies have shown how bone marrow-MSCs (BM-MSCs) are safe and can restore corneal epithelia from LSCD with the same results of cultivated limbal epithelial transplantation[68].BM-MSC associated with amniotic membranes have successfully been evaluated in LSD[69].Guet al[70] demonstrated how BM-MSCs could differentiate into cornel epithelial like cellsin vivoin rat damaged corneas[70].MSCs have been proposed also to promote graft survival[71,72].This can be explained by the potent immunomodulatory properties of SC,suppressing maturation and activation of atrial premature complexes and dendritic cells and cytotoxicity of natural killer cells.Several studies have attempted to exploit these features of MSCs and have shown interesting results by using topical therapies with eye drops containing MSCs (mainly BM-MSC and ASCs)[45,46].Topical application of MSCs can lead to an increase in aqueous tear volume and improvement in ocular surface evaluation tests.Moreover,it is a safe and easy applicable method.
Cells derived from epithelial UC-MSCs are capable of forming a stratified epithelial layer when seeded on artificial matrices.Rezaet al[73] first described the properties of UC-MSCs,finding how they have similar characteristics of LSCs[73].Garzónet al[74]utilized an epidermal growth factor enriched medium to induce UC-MSCs to transdifferentiate into corneal epithelial cells on a 3D human artificial anterior cornea model[74].The aim of the use of UC-MSCs derived epithelial cells is to circumvent the problem of the limited amount of limbal tissue available for transplantation,especially in cases of bilateral LSCD[29].
With regards to MSCs,human immature dental pulp and oral mucosa stem cells have been reported to be a promising cell source to correct LSCD,as described by Hassan and AbdelAziz[75] and Nishidaet al[76].Studies about ocular surface diseases and their treatment are reported in Table 1.
Research is currently searching for new ways of applications of stem cells therapy.As described above,MSCs derived exosomes is one of these[28].The other way recently described to facilitate and carry cells to the ocular surface is the use of contact lenses[77].
Corneal stroma and endothelium:The corneal stroma is the thickest corneal layer(approximately 90% of corneal thickness) and is composed of specialized extracellular matrix components and collagen fibrils.Corneal stromal keratinocytes (CSKs) are the major cell type in corneal stroma and are located between the collagenous lamellae,which are generally quiescent[29].Corneal insults cause death of CSKs at the injured site.Surviving CSKs near the injured site are activated to proliferate,while some fibroblasts are induced to transform in highly contractile myofibroblasts.When fibroblasts action prevails,scaring and consequent opacity of cornea can occur.The presence of dense opacities and persisting scars can interfere with the passage of light and cause an important loss of visual acuity if the opacity is large and localized in the center of the visual axis of the cornea,thus have an important burden on quality of life of patients[78].
Keratoplasty is a primary treatment option and current gold standard to treat numerous corneal conditions,including corneal injury,corneal dystrophy,keratoconus and corneal infractions.The ever-increasing number of patients needing keratoplasty has led to the shortfall of viable donor corneas.Moreover,it is important to note that post-surgical complications include important and sight threatening conditions,in addition to the risks of transplant rejections and elevated permanently induced astigmatism[23].Sepsakoset al[79] demonstrated that patients who underwent an ocular surface SCs transplantation (e.g.,with LSCs) before keratoplasty have better results than patients that did not do that[79].
MSCs express high levels of hepatocyte growth factor (HGF) in an inflamed environment,as is the case in an injured cornea.HGF inhibits the generation of opacity-inducing myofibroblasts and,alone,can restore corneal transparency in anin vivomodel of eye injury[83].This is the rationale that has been proposed for the use of these cells.All MSCs seem to have similar behaviorin vivo,being able to achieve keratocyte differentiation and modulate the corneal stroma.ASCs have been demonstrated to be an ideal source of autologous stem cells,while BM-MSCs have the same profile,but the extraction by bone marrow puncture is more complicated,not free of risks and painful.A pilot study by del Barrioet al[84] demonstrated the apparent safety of corneal stromal transplantation of autologous ASCs in humans,showing cell survivalin vivoand the ability of these cells to produce a low amount of new collagen in patients with advanced keratoconus.Several studies on animals have suggested that UC-MSC transplantation may be a feasible alternative to keratoplasty in treating congenital disorders of the cornea secondary to keratocyte dysfunction such as mucopolysaccharidoses,giving rise to potential future possibilities[85,86].UCMSCs could represent an attractive alternative,but the autologous use of these cells tends to be expensive and currently almost impossible.
An alternative stem cell population currently reported in clinical trials for the therapy of corneal stroma disorders include corneal stromal stem cells (CSSCs).These cells show similar properties of MSCs and can be obtained by specific cultures of LSCs.Experimental studies have shown that CSSCs can differentiate in CSKs after injection in the stroma[87].
Periodontal ligament stem cells and DPSCs could differentiate in CSKs and prove to be of potential clinical use[28,88].
The corneal endothelium is the posterior corneal surface.It is formed by a monolayer of endothelial cells (CECs) that regulate the stromal hydration,preventing edema and maintaining corneal deturgescence,which are all fundamental for normal vision.These cells are non-mitotic and have limited regenerative capacity.Diseases that affect the corneal endothelium cause stromal edema,central opacity and vision loss.Full thickness or partial corneal transplantation has been the gold standard for endothelial diseases to date;however,preliminary studies have shown that stem cells therapies could be of potential clinical use[28,29].MSCs could serve as a potential source to generate corneal endothelial cells for the treatment of corneal endothelial diseases,like Fuchs’ endothelial dystrophy and aphakic/pseudophakic bullous keratopathy.Joyceet al[89] demonstrated the potential of UC-MSCs to differentiate in CECs when treated in particular cultures[89].At this moment,however,there are no concrete data in current literature on the possible application of SCs in human endothelial disorders,which remains to be the primary cause of corneal transplantation[29].
Trabecular meshwork:Glaucoma is a degenerative optic neuropathy affecting approximately 70 million people worldwide.The most frequent cause of glaucoma is the increase of intraocular pressure (IOP) due to an alteration of aqueous outflow.Humor aqueous is continuously produced,and its outflow is controlled by the trabecular meshwork (TM),an avascular tissue located within the uvea and posterior to the corneal margin[90].TM is populated by specialized cells that decline in number with age,and these cells are particularly low in individuals with primary open glaucoma.It is probable that TM cells become dysfunctional prior to death and increasingly fail to carry out the physiological roles,leading to enhanced aqueous humor outflow resistance and the development of pathologically elevated IOP[91].Despite the importance of controlling IOP,the ultimate cause of glaucoma-associated vision loss includes axonal damage and the progressive loss of RGCs,the axons of retinal neurons that make up the optic nerve and transmit visual information from the eye to the brain[91].The presence of MSCs in TM have been suspected for years and then confirmed and propagatedin vitro[92].
TM cells are difficult to obtain from a living eye.Studies have faced this problem by using iPSCs generatedviagenetic reprogramming of adult somatic cells[19].To date,the use of TM-like cells derived from iPSCs from a patient’s own dermal fibroblasts may offer the best solution to the challenge of TM cell replacement therapy[92].Abu-Hassanet al[93] evaluated the results of iPSCs derived TM cell transplantation.The study showed that iPSCs differentiate to resemble TM cells in several keyways and have the potential to restore the primary TM cell function,thus maintaining IOP homeostasis[93].
Recently,other stem cells have been proposed to find the possibility of obtaining TM cells (Table 1).MSCs have been used in attempt to repair TM tissue predominantly in animals[94].Kumaret al[31] described the differentiation of ASCs in TM cells[31],and Manuguerra-Gagnéet al[95] studied the regeneration of TM from BM-MSCs with encouraging results[95].Data in stem cell models are of utmost importance and the basis when designing future studies with potential clinical benefits.
In her novelization of the tale, titled Deerskin, Robin83 McKinley gives Donkeyskin a talent with animal husbandry, specifically dogs, which she cares for on the prince s estate.Return to place in story.
Lens:Cataracts are the leading cause of blindness in the world.The current standard of care in congenital cataracts involves surgical removal of the cataractous lens and implantation of an artificial intraocular lens.Surgery is not free of complications,especially in patients with complicated cataracts and with other underlying ophthalmic conditions.
Human lens regeneration has not been demonstrated yet,however,lens epithelial stemprogenitor cells (LECs) have been isolated[96].The most significant functions of LECs are sustained self-renewal and protective capacities against external injuries[97,98].Liet al[99] have recently reported clinical trials on animals and humans (Table 1).They showed that with a new minimally invasive surgical procedure,with preservation of LECs,lens regeneration can be achieved with increased visual axis transparency and decreased rate of complications.The same conclusion has also been reported by Liet al[99].A further possibility of obtaining lenses through cell differentiation has been proposed by Murphyet al[98],who showed the possibility of creating micro-lenses starting from iPSC differentiation.
Optic nerve
RGCs are a population of CNS neurons located in the innermost layer of the retina and convey visual signals from the retina along their axons to the brain.Axonal injury leads to the functional loss of RGCs and subsequently induces death of neurons.Axon growth is essential for the restoration of neuronal connectivity and reestablishment of a functional visual system after optic nerve injury[100].Some optic neuropathies are very common,like glaucoma that is the first cause of irreversible blindness;while other conditions tend to be rare.Leber’s hereditary optic neuropathy is just one example of this heterogeneous category of ophthalmic pathologies.There is currently no treatment available for inherited optic neuropathies such as Leber’s hereditary optic neuropathy or dominant optic atrophy.With regards to glaucoma,current treatments aim at lowering the IOP.It is well known how the regenerative potential of the mammalian CNS is very limited.Research regarding the regeneration of elements of the nervous system is thus fascinating and usually limited to animal models.
There are two main objectives of cell therapy,including the delivery of a trophic and neuroprotective support[22,27] and (more ambitious) replacement of lost cells and functional restoration[101].The first step in developing cell replacement-based strategies for optic nerve regeneration requires a reliable,high-volume source of healthy RGCs[102].
iPSCsESCs represent the first source studied for the optical nerve regeneration.Takahashiet al[19] in 2007 reported a remarkable break-through technology whereby adult fibroblasts could be reprogrammed into iPSCs.These transdifferentiated cells exhibit similar characteristics to hESCs,including the ability to propagate indefinitely and the ability to differentiate into many different cell types,including RGCs.As explained by Gokoffskiet al[100],there are two different methods for generating RGSs from pluripotent stem cells:Organoid differentiation and planar differentiation.Organoids are self-organized 3D miniature organs developedin vitrofrom PSCs.Kuwaharaet al[102] first developed 3D retinal organoids from hESCs[102].Planarderived RGCs are transdifferentiated from traditional two-dimensional cell culture techniques[103,104].One of the limitations of this technique is the ability of iPSCsESCs to integrate into the ganglion cell layer.Preliminary results by Mesentier-Louroet al[105] reported that between 1% to 7% of iPSCs-derived RGCs integrated into the ganglion cell layer after intravitreal injection and about 20% after combined injection of RGCs and iPSCs[105] (Table 1).
The other limitation regarding the use of iPSCs currently being studied involves the possibility of formation of synaptic connections.Axonogenesis of derived RGCs is another crucial point:Neurotrophic factors,which theoretically could promote it,keep RGCs alive longer.The ideal local cellular environment is essential to permit and enhance RGCs in creating new axons;however,neuronal survival does not equate to new axon formation[106].An additional unresolved question involves the possibility of new axons in establishing functional synapse.
To overcome the difficulties in integrating RGCs,gene therapy has developed possible solutions,which are currently under investigation.Zhanget al[107]demonstrated the utility of pigment epithelium-derived factor[107].It is constitutively expressed by various ocular tissues,but its exogenous delivery is therapeutically advantageous to promote RGCs survival and axon regeneration.Zhanget al[107] used NSCs as cellular vectors to deliver continuously pigment epithelium-derived factor to adult retinas after optic nerve injury.These studies showed the utility of genetically modified NSCs to protect RGCs and promote axonal regeneration.
The effects of MSCs on RGCs have also been studied (mainly in animals).MSCs provide neuroprotection against RGC death;however,studies showed that these SCs did not tend to integrate[14].Preliminary studies by Zhanget al[107] have described the possible usefulness of UC-MSCs,which have shown a partial recovery of injured optic nerve.
Retina:Visual signalling starts in photoreceptor cells.In the human retina,rods and cones are responsible for dim light vision and daylight vision,respectively.Rods are located mainly in the peripheral retina,while cones are concentrated in a small portion of the retina,called macula.Bipolar cells receive visual signals from photoreceptors and transmit it to RGCs.RPE cells form a monolayer underneath the outer segment of the photoreceptors,constituting the outer blood-retinal barrier.In the visual system,RPE cells play diverse roles,which include absorption of scattered light,regulation of nutrients,ions and solutes,secretion of growth factors,regulation of the retinal cycle and phagocytosis of photoreceptor outer segment.
Retinal degenerative diseases are characterized by retinal cell loss,such as RPE and/or photoreceptor cell loss in AMD and retinitis pigmentosa,and RGC death in glaucoma,as seen previously.Studies that report retinal diseases and treatment are summarized in Table 2.
Binderet al[108] and van Meurset al[109] described the therapeutic potential of autologous RPE cells taken from the peripheral region of the retina in AMD.These promising results have prompted research towards stem cell therapies to extend it to degenerative retinal diseases,where cell replacement is needed[37].Retinal degenerative disorders requiring cell replacement include AMD,retinitis pigmentosa,Stargardt’s disease and glaucoma,which represent current challenges in cell therapy.RPE cell source is restricted for applications in RPE cell replacement,thus stem cells have been studied to solve this limitation.
The greatest potential for cell replacement has been seen with ESC/iPSCs[19],which can be successfully pre-differentiated prior to transplantation in the eye,with the greatest success shown in RPE/photoreceptor replacement for AMD[22].Studies involving cell reprogramming by Tuckeret al[110] and Lambaet al[111] have shown the possibility of creating ESC/iPSC-derived retinal progenitors capable of maturing in RPE or photoreceptors and capable of integrating in the retina[112].The same results,but with hESCs and hiPSCs,were confirmed by Nakanoet al[48] and Gonzalez-Corderoet al[113].The first clinical trials in humans have been done by Schwartzet al[40,114] in 2012 and 2015[40,114]:They successfully used hESCs-derived RPE cells in Stargardt’s disease without collateral effects (also noted by Mandaiet al[17]).Cell reprogramming allows the use of autologous PSCs,which guarantee no immune reactions with respect to allogenic cells.One of the observed collateral effects is the long-term hyperpigmentation of the macula[114].Potential future alternative approaches include the implant of stem cell derived RPE growing on a bioengineered scaffold,which can improve stability and maintain cell polarization[14].
Considering the functions of RPE on the overlying retina,it is fundamental to know that subretinal RPE transplantation aims at replacing these functions.Transplantation of non-RPE cells has been pursued with the rationale that they may counter disease through trophic factor secretion.For this reason,clinical trials with subretinal UCMSCs,NSCs and retinal progenitor cells are based on trophic support mechanism of action.These procedures,however,need further studies considering the potential increase of complications[115].
The future of retinal therapies could involve bioengineering.Sternet al[14] have reported that incorporating bioengineering approaches may better preserve retinal layering and integrationin vivomodels.The possibilities of this approach have been successfully seen in animal clinical trials[116].Nakanoet al[48] has described the formation of optic cup and stratified retina from hPSCs.Several successive studies have confirmed the possibility of creating retinal organoids and layers of differentiated photoreceptors,which can develop outer segment structures[117].Organoids may prove valuable in producing specific retinal cell types or 3D retinal structures for transplantation.This is particularly important for conditions such as geographic atrophy[14].
An alternative approach to retinal degenerative pathologies with SCs is the possibility of exploiting the paracrine capacities of some type of cells.This stem cell therapy is not disease specific and can have broad clinical applications,as seen previously.This is the potential of BM-MSCs and places them as a candidate cellular therapy to combat ocular neurodegeneration,even if these cells are less ideal for replacement cell therapy[22,27].Transplantation of BM-MSCs in experimentally induced glaucoma and optic nerve transection show no evidence of the differentiation into mature retinal cells (RPE or photoreceptors),despite some integration into the retina;but,improvement of retinal function by preserving photoreceptors and RPE cell viability have described[118].
ASCs have also been studied for this clinical use,which could prove to be beneficial in eye therapies for the paracrine effects,as seen for disorders of the SNC.Several studies suggest that ASCs have therapeutic potential for neurodegenerative conditions through neurotrophic factor production,with several of the active factors being different from those produced by BM-MSCs and DPSCs[27,119].ASCs and BM-MSCs have recently been evaluated with promising results in diabetic retinopathy.ASCs have shown the potential to be therapeutically effective in early-stage of diabetic retinopathy through paracrine factors and physical contact with endothelial cells[120].
Limits and why not
Stem cell therapy in ocular diseases certainly represents the future in the treatment of numerous serious eye diseases,especially if organ transplantation can be avoided.One of the main problems of these therapies,mainly the immunological tolerance to transplanted cells,has been solved thanks to the use of autologous cells[17].Graftvshost disease has been limited in this way.Despite all the advantages and promising results,what we know about stem cells therapies is still incomplete,especially concerning the possible side effects.The tumorigenicity of transplanted stem cells,to date,remains to be the most concerning issue for cell transplantation.Advances in genetic research and reprogramming have made it possible to reduce the incidence of this event[20].Other side effects may be caused by tissue sampling,particularly if performed in the contralateral,healthy eye.This can be avoided using SCs derived from other tissues,like ASCs[32].
Stem cell transplantation has the huge limit of the survival and engraftment of cells.To improve outcomes,methods considered include pre-treating the host tissue or the SCs culture with growth factors or cytokines,embedding SCs in biomaterials or expanding SCs in culture.Obtaining an adequate number of SCs is fundamental.For example,the expansion of ASCs in culture has been considered,but extensive cultures may change the properties of SCsin vivo,rendering them unfit for restoring injured or diseased tissues.Genetic research is thus of fundamental importance in this field.A further problem related to SCs treatment is the cost of this type of therapy,which is still very high today due to limited cost efficient technology to render this therapy available on a large scale[37].Conserving samples of one’s own tissue (i.e.adipose stem cells,which are the most represented) taken during other operations,similar to the methods used during lipofilling for aesthetic or reconstructive aims,could be a possible solution.Moreover,the ethical problem of the use and collection of certain cell types,such as ESCs,UC-MSCs and NSCs,remains considerable,which further limits SCs use in clinical trials.
Future prospectives
The vast literature and resources used to date underline the importance of stem cell therapies,especially for the future.To date,the results are still primordial,but the conditions seem leading to an important turning point.Further studies are surely needed to provide additional knowledge behind the mysterious physiological and potentially curative properties of stem cells,which can pave the way to clinical applications in ophthalmic care.Numerous current trials have been proposing different perspectives for the future of cell therapy in ophthalmic diseases.The therapeutic effects of MSCs in regenerative medicine,for example,can also be attributed to exosomes,which are secreted soluble factors[45].Exosomes are extracellular vesicles that are produced in the endosomal compartment of most eukaryotic cells and contain proteins that regulate tissues biogenesis.Several studies have considered the injection of MSCs exosomes in ocular tissues,with interesting results[28].Research should not only be limited at stem cell studies alone but also need to include a whole series of innovations brought on by bioengineering and nanotechnology[14,48,118].Modern studies seem to be aimed at the 3D development of fundamental parts of the eye,the use of specific vectors that can guarantee the replacing of tissues,and organoids[67].Novel studies are geared at the possibility of reproducing eye tissues in a normal 3D configuration.Transplantation of sophisticated multicellular 3D tissues can be an exciting opportunity.Nakanoet al[48] have shown that hPSCs can generate differentiated 3D structures similar to the embryonic eye cup,containing RPE and neural retina.Garzónet al[74] described the generation of the anterior cornea by tissue engineering.In addition,bioengineers have proposed to build ocular structures by incorporating biocompatible materials with stem cells products,for example to create TM or RPE monolayer[47].Bioengineering is fundamental for the development of optimal cell culture biomaterials for the expansion of SCs and the differentiation into ocular cells[16].Modified cultures (e.g.,using growth factors,biomaterials,injectable hydrogels,scaffolds or membranes containing cells) allow the maturation of cells and dedifferentiation,recreating the micro-environment of the host tissue.Consequently,there is an easier integration and a better survival of these SCs in the recipient site[42].The advantage of these approaches is to guarantee a reduction of the culture time to obtain a viable ocular tissue (such as a cornea[75]),thus avoiding massive tissue harvesting (preserving LSCs,for example[75]).
CONCLUSION
The potential therapeutic goals include being able to restore the sense of sight in patients who have lost it,by replacing or recreating the damaged eye structures,including restoration of physiological and functional connections with the CNS.Stem cell therapies seem to be interesting and promising,even concerning the more external structures,including the ocular surface and cornea.Finally,the possibility of using these cells topically,without having to subject patients to surgery,organ transplantation or hospitalization,is an element of great importance and hope.
 World Journal of Stem Cells2021年5期
World Journal of Stem Cells2021年5期
- World Journal of Stem Cells的其它文章
- Role of induced pluripotent stem cells in diagnostic cardiology
- Multidifferentiation potential of dental-derived stem cells
- Programmed cell death in stem cell-based therapy:Mechanisms and clinical applications
- Low complexity domains,condensates,and stem cell pluripotency
- Different kinds of stem cells in the development of SARS-CoV-2 treatments
- Disease modifying treatment of spinal cord injury with directly reprogrammed neural precursor cells in non-human primates
