Preharvest application of melatonin induces anthocyanin accumulation and related gene upregulation in red pear (Pyrus ussuriensis)
SUN Hui-li,WANG Xin-yue,SHANG Ye,WANG Xiao-qian,,DU Guo-dong,,LÜ De-guo,
1 College of Horticulture,Shenyang Agricultural University,Shenyang 110866,P.R.China
2 Key Laboratory of Fruit Quality Development and Regulation of Liaoning Province,Shenyang Agricultural University,Shenyang 110866,P.R.China
Abstract Anthocyanins are important components in the peel of red pears and contribute to the appearance of the fruit. Melatonin application is known to affect anthocyanin biosynthesis,but the effect of preharvest melatonin application on fruit coloration remains largely unknown. The objective of this study was to determine the effects of preharvest melatonin application on pigmentation,phenolic compounds,and the expression of related genes in Nanhong pear (Pyrus ussuriensis). The applications were performed during the pre-color-change period by spraying 50 or 200 μmol L-1 of melatonin on fruits. We found that treatment with melatonin had a significant effect on color development. The concentrations of anthocyanins and flavonols were enhanced by melatonin treatment,whereas hydroxycinnamate and flavanol concentrations were reduced. Quantitative real-time PCR analyses indicated that the transcription levels for most anthocyanin biosynthetic genes and anthocyanin-related transcription factors were induced by melatonin. Melatonin application also stimulated the expression of melatonin biosynthesis-related genes and consequently caused an increase in endogenous melatonin concentration. These results provide insights into melatonin-induced fruit coloration and will facilitate the application of exogenous melatonin in agriculture.
Keywords:melatonin,anthocyanin,pear,phenolic,peel pigmentation
1.lntroduction
Pear is an economically important fruit worldwide. In recent years,the demand for well-colored red pear cultivars has increased (Zhanget al.2013). Nanhong pear (Pyrus ussuriensis) is a red-skinned mutant of Nanguo pear. As the red-colored peel met with consumer approval,Nanhong pears rapidly became widely distributed throughout northern China. Unfortunately,the red color of the peel of Nanhong pear fruit proved unstable across regions and variable depending on culture conditions (Huanget al.2009).
Anthocyanins are widely synthesized in plants to provide various colors and protection for the fruits,flowers,and vegetables. These pigments are members of the phenolic class of secondary metabolites produced in plant tissues;they are recognized as health-promoting substances because of their potentially beneficial antioxidant,antiinflammatory,and anticarcinogenic properties (Sunet al.2019),and many factors influence their accumulation.
Anthocyanins are synthesizedviathe phenylpropanoid pathway,which has been well documented in various plant species,including petunia,maize,grape,apple,and pear (Qianet al.2014). The enzymes of this pathway include phenylalanine ammonia lyase (PAL),chalcone synthase (CHS),chalcone isomerase (CHI),flavanone 3-hydroxylase (F3H),dihydroflavonol 4-reductase (DFR),anthocyanidin synthase (ANS),and UDP-glucose:flavonoid 3-O-glucosyl transferase (UFGT) (Wanget al.2015). The three branches in this pathway include flavonol synthase (FLS),leucoanthocyanidin reductase (LAR),and anthocyanidin reductase (ANR),which synthesize flavonols and flavanols.The expression of these structural genes,which have been found to be positively correlated with anthocyanin biosynthesis,is transcriptionally regulated by the MBW (MYB-bHLH-WD40) protein complex (Niet al.2019). MYB transcription factors (TFs) have been identified as important TFs for the activation of anthocyanin biosynthesis-related genes in many fruit trees,including apple,grape,and pear trees (Baiet al.2017). In pears,the expression level ofPyMYB10in the peel was positively correlated with structural genes of the anthocyanin biosynthesis pathway and with anthocyanin accumulation (Fenget al.2010). Recently,PyMYB114 was reported to interact with PybHLH3 to coregulate anthocyanin biosynthesis in pear fruit (Yaoet al.2017).
Many environmental factors modulate anthocyanin biosynthesis in plants. For example,drought and light induce anthocyanin synthesis,thus enhancing fruit color (Chenet al.2016;Liuet al.2019). Further,plant regulators were shown to be involved in anthocyanin regulation,such as melatonin (Arnao and Hernández-Ruiz 2018). Melatonin (N-acetyl-5-methoxytryptamine) is produced in all plant species and has been identified as a free radical scavenger (Sharifet al.2018;Moustafa-Faraget al.2020). Further,recent studies have shown that plant melatonin is involved in multiple developmental processes and stress responses (Arnao and Hernández-Ruiz 2019a,b,c,2020a;Debnathet al.2019;Yanet al.2020). In addition,melatonin is involved in secondary metabolism,where it induces anthocyanin and flavonoid biosynthesis (Arnao and Hernández-Ruiz 2020b;Wang S Yet al.2020). Postharvest application of melatonin has been shown to improve anthocyanin accumulation in tomato,in conjunction with transcript levels of anthocyanin biosynthesis-related genes which were upregulated (Sunet al.2016). However,little is known about the effect of preharvest melatonin treatment on fruit coloring.
Previous research on pear fruit indicated that exogenous melatonin delayed fruit senescence during postharvest by limiting ethylene production and preventing the reactive oxygen burst (Zhaiet al.2018). However,the effect of melatonin on fruit color development and phenolic composition in pear fruit remains unclear. Here,we investigated the effect of melatonin on color development in the red-skinned Nanhong pear,an Ussurian pear cultivar of northeastern China. We investigated color development and phenolic composition of the fruit by spraying an exogenous melatonin solution before the fruit color changed naturally. In addition,the expression of both structural genes and TFs in the pathway of anthocyanin biosynthesis were analyzed by quantitative PCR. This study was conducted to provide sound experimental evidence for improving anthocyanin pigmentation by applying melatonin to red-skinned pear fruit.
2.Materials and methods
2.1.Plant materials and melatonin treatments
Seven-year-old Nanhong pear trees (Pyrus ussuriensis) were used as the experimental plant materials. The pear trees were cultured in an orchard at the Horticultural Experimental Station of Shenyang Agricultural University,Shenyang,Liaoning Province,China,where they received standard horticultural management. The pear fruit were subjected to the first melatonin application approximately 100 days after blooming,when the fruit had begun to change color. Two different concentrations of exogenous melatonin (50 and 200 μmol L-1) were sprayed on the fruit until they were dripping. Tween-20 detergent was used as a surfactant at a final concentration of 0.1% (v/v). Three days later,a second melatonin application was performed using the same method. At each application time,control plants were sprayed with 0.1% (v/v) Tween-20. The experiment was conducted in a completely randomized design with three treatments and three replications. The fruit were sampled randomly at 15 days after the first application.Nine fruit per replicate (three fruit per tree) were collected for measurements of color,and the peel of the fruit was frozen in liquid nitrogen immediately and stored at -80°C until use.
2.2.Fruit color measurement
Fruit peel color was measured on the most intensely colored part of fruit using a colorimeter (CR-400,Minolta,Japan),which provided L*,a*,and b* values. L* represents the relative lightness of color,ranging from 0 to 100. Both a* and b* scales extend from -60 to 60. Negative a* values indicate greenness while positive a* values indicate redness;in turn,negative b* values indicate blueness and positive b* values indicate yellowness (Zhuet al.2018).
2.3.Fruit quality measurement
Total soluble solids (TSS) (°Brix) were determined using a pocket refractometer (PAL-1,Atago,Japan). Total acidity (TA) was determined with 0.1 N NaOH using an automatic titrator (East Plus Titration,Mettler Toledo) and expressed as (%) malic acid equivalent fresh weight.
2.4.Phenolic extraction and analysis
Peel tissues were first ground in liquid nitrogen and thoroughly mixed. Frozen samples (0.2 g) were then extracted in 2.0 mL of 70% methanol containing 2% formic acid and centrifuged at 10 000×g for 10 min at 4°C. The supernatant was filtered through a 0.22 μm syringe filter prior to UPLC-MS analysis. Aliquots of 10 μL were subjected to UPLC-MS analysis using an ACQUITY UPLC H-Class System (Waters,USA),coupled to a Xevo triple quadrupole tandem mass spectrometer (Waters,USA).Chromatographic separation was performed on a Waters Acquity BEH C18 column (2.1 mm×50 mm,1.7 mm) by gradient elution,with the mobile phase consisting of 0.1% formic acid (A) and acetonitrile (B). The flow rate was 0.35 mL min-1. The parameters and conditions were set according to the methods described by Wang Xet al.(2020).
2.5.Melatonin extraction and analysis
Melatonin was extracted as described by Pothinuch and Tongchitpakdee (2011). Briefly,peel samples were extracted with methanol and ultra-sonicated (80 Hz) for 40 min at 4°C.After centrifugation at 10 000×g for 15 min,the supernatants were collected and dried under nitrogen gas. Analyses were performed in triplicate using an ACQUITY UPLC H-Class System (Waters,USA),coupled to a Xevo triple quadrupole tandem mass spectrometer (Waters). The flow rate was 0.30 mL min-1. Mobile phase A (0.1% formic acid) and mobile phase B (methanol) were used for a gradient elution as follows:25% B (0 min),40% B (8 min),95% B (10 min). The parameters and conditions were set according to the methods described by Zhaoet al.(2012).
2.6.Gene expression analysis
Total RNA was extracted from peel samples using the cetyltrimethyl ammonium bromide method. First-strand cDNA was synthesized using a reverse transcription system (TaKaRa,Japan) according to the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was evaluated with SYBR Premix Ex Taq II Kit (TaKaRa,Japan) on a 7500 Real-Time PCR System (Applied Biosystems,USA). A total of 18 genes involved in the anthocyanin and melatonin biosynthetic pathways were selected from the National Center of Biotechnology Information database and the pear genome database (https://www.rosaceae.org/species/pyrus/all). The primers are listed in Appendix A.
2.7.Statistical analysis
The data were analyzed with SPSS 17.0 using Duncan’s multiple range tests to determine significant differences between the treatments.
3.Results and discussion
3.1.Peel coloration and fruit quality
We analyzed the color parameters in pear fruit treated with melatonin and untreated controls. Fig.1-A shows that melatonin-treated pear fruit exhibited a deeper red color compared with the control fruit. Consistent with the changes in the visual appearance of the fruit,the L*,a*,and b* values all changed significantly following the application of the melatonin spray treatments. The effects of melatonin treatments on fruit peel color are summarized in Table 1. The a* value increased after melatonin application,whereas the L* and b* values decreased. The a* value for control fruit was -15.86;while the a* values for melatonin-treated fruit at 50 and 200 μmol L-1were 1.46 and -1.32,respectively,both much higher than the control fruit and significantly different from one another. Since positive a* values indicate redness,it can be inferred that fruit treated with 50 μmol L-1melatonin were the most red. In contrast,melatonintreated fruit had lower L* and b* scores than the control fruit,suggesting that the color of melatonin-treated fruit was not as light or yellow as the control fruit. However,this slight effect was not perceived by the unaided eye. Both melatonin treatments appeared to be effective in increasing a* values and in decreasing L* and b* values,which was consistent with the gradual change in fruit color from light yellow to red.
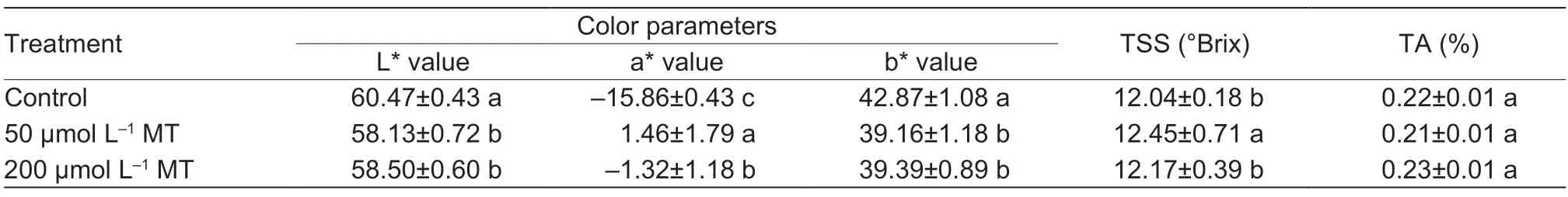
Table 1 Color (L*,a* and b* parameters),total soluble solids (TSS),and total acidity (TA) of pear fruits as affected by melatonin (MT) preharvest treatments.
According to the color parameters,control fruit showed a green to yellow color. In contrast,melatonin-treated fruit developed a remarkably deeper red color. This is consistent with the visual appearance observed in Fig.1-A.The red,green,and yellow colors depend on anthocyanin,chlorophyll,flavonoid,and carotenoid contents of the pear peel (Liuet al.2013). In the present study,L* and b* values decreased while a* increased in melatonin-treated fruit. The treated fruit developed a slightly deeper red color than non-treated fruit. Similarly,melatonin treatment has been reported to influence color intensity in cabbage sprouts (Zhanget al.2016). In general,melatonin treatment had a significant effect on color development,accelerating the pigmentation of pear fruit. Due to the significant increase in Values are mean±standard deviation (n=3),and mean values within a column followed by different letters are significantly different atP<0.05 according to Duncan’s test.the a* value,we hypothesized that the enhanced red blush on the fruit surface might be attributed to the accumulation of anthocyanin promoted by the melatonin treatments. This was confirmed by the anthocyanin and melatonin concentrations in pear peel (Fig.1-B and C).

Fig.1 Morphological and physiological changes in pear fruit 15 days after application of melatonin (MT) at 50 μmol L-1 (MT 50) and 200 μmol L-1 (MT 200). A,photographs showing changes in fruit appearance. B,changes in total anthocyanin content in pear peels. C,changes in melatonin content in pear peels. Error bars indicate the standard deviation of three replications. Different letters in each graph indicate significant differences at P<0.05 according to Duncan’s test.
We also analyzed the effect of melatonin treatment on fruit quality parameters for pear fruit collected 15 days after treatment. As indicated in Table 1,the concentration of TSS was slightly higher in fruit treated with 50 μmol L-1of melatonin than that in the control fruit,but it did not significantly differ between fruit treated with 200 μmol L-1melatonin and control fruit. Moreover,melatonin treatments had no significant effect on TA. These results agree with previous reports on the effect of melatonin application on TSS and TA in fruit (Xuet al.2017;Debnathet al.2018;Menget al.2019). In all these cases,the application of melatonin had little or no effect on these quality parameters.Similarly,Liuet al.(2018) did not observe differences in soluble solid accumulation or acid degradation in strawberries after melatonin treatment. Therefore,it can be inferred that soluble solids and titratable acidity in fruit do not respond to melatonin treatment.
3.2.Analysis of phenolic composition
To investigate the changes in the concentrations of phenolic compounds in melatonin-treated pear fruit relative to control fruit,23 phenolic compounds that can be classified into six groups were determined by LC-MS analysis (Table 2):hydroxycinnamic acids,hydroquinones,flavanols,flavonols,flavones,and anthocyanins. These phenolic compounds have been described previously in pear fruit.
Four different hydroxycinnamic acids were identified and quantified in pear peel at 15 days after application. Among them,chlorogenic acid was the most abundant,followed by caffeic acid,ferulic acid,and p-coumaric acid. As can be seen in Table 2,only the chlorogenic acid concentration decreased significantly after treatment with 200 μmol L-1melatonin,compared to the control,whereas no differences were observed in other hydroxycinnamic acids. Given the quantitative importance of chlorogenic acid,the total hydroxycinnamic acid concentration was also lower in pears treated with 200 μmol L-1melatonin than in control fruit. Conversely,a previous study on melatonin application in grapes showed that caffeic acid and chlorogenic acid were significantly enhanced by melatonin treatment (Xuet al.2017). Chlorogenic acid has a wide range of biological effects and it is a major phenolic compound in pear fruit that declines with fruit development (Heet al.2017). As mentioned above,this reduction might be explained by the promotion of fruit maturation induced by the 200 μmol L-1melatonin treatment,which likely resulted in the decrease in the concentration of chlorogenic acid in treated fruit.

Table 2 Phenolic profile of pear peels as affected by melatonin (MT) preharvest treatments1)
Arbutin (hydroquinone 1-β-D-glucoside),another important non-flavonoid compound present in a wide array ofPyrusspecies,accumulates in young organs such as leaf buds and young fruits (Cuiet al.2005). Our analysis revealed statistical differences in the arbutin concentrations among the different treatments. As shown in Table 2,arbutin concentration increased after application of 50 μmol L-1melatonin compared with that of control treatment. In contrast,application of 200 μmol L-1melatonin reduced the concentration of arbutin with respect to the control. To our knowledge,no research has previously reported the influence of preharvest treatment with growth regulators on arbutin compounds. Generally,our results indicated that the effects of melatonin treatment on arbutin concentration in pear peel were dependent on melatonin concentration.
Flavanols are products of the flavonoid pathway,which also leads to the production of anthocyanins and flavonols (Henry-Kirket al.2012). Epicatechin and its dimer,procyanidins B2,were the major flavanol compounds in pear fruit,followed by catechin and procyanidins B1. As seen in Table 2,individual flavonols in the peel significantly decreased with melatonin treatment relative to control fruit. Hence,the total amount of flavanols was also lower in melatonin-treated pears than in control fruit. Our results show that the effect of melatonin application on flavonols differed from its effect on anthocyanins. A possible explanation is that melatonin may preferentially activate the enzymes responsible for anthocyanin synthesis rather than those responsible for flavanol synthesis. This idea has also been suggested previously (Ruiz-Garcíaet al.2013).
Flavonols are another important flavonoid compound group that contributes to the color of fruit together with anthocyanins (Menget al.2019). Four types of quercetin and three types of isorhamnetin glycosides were identified here,the levels of which showed significant differences between melatonin-treated fruit and control fruit. As shown in Table 2,unlike flavanols,although peel flavonols responded to melatonin treatment by increasing remarkably relative to the control,these compounds increased to a larger extent at 50 μmol L-1melatonin. In addition,the treatment with melatonin also increased the total amount of flavonols in pear peel,especially at 50 μmol L-1. In agreement with these findings,Menget al.(2019) observed that melatonin promoted flavonol accumulation in grape skins. Flavonols are closely related to anthocyanins,as they share a considerable portion of their biosynthetic pathway (Portuet al.2015). Our results indicate that the influence of melatonin application on flavonol compounds is the same as that on anthocyanins;whereas the flavone concentration was independent of melatonin treatment,as only slight differences between treated and control fruit were detected for most of the flavones identified.
Anthocyanins are primarily responsible for fruit color,which is of great importance to pear quality and consumer perception (Portuet al.2015). The glycosides of cyanidin and peonidin were identified. Cyanidin-3-galactoside was the most abundant anthocyanin and accounted for more than 80% of the total anthocyanins detected after melatonin application. As can be seen in Table 2,both melatonin treatments had a significant impact on the concentrations of anthocyanins in pear peel. In particular,the cyanidin-3-galactoside concentration increased significantly in pear peel,from 0.86 mg kg-1in control fruit to 4.43 mg kg-1in fruit treated with 50 μmol L-1melatonin. Relative to control fruit,the total anthocyanin concentration increased by 4-and 3-fold after application of 50 and 200 μmol L-1melatonin,respectively. The enhancement of anthocyanin was an expected outcome from melatonin application,as it has been previously reported. Indeed,Xuet al.(2017) observed that the application of 100 μmol L-1melatonin to grape berries led to a 76.9% increase in total anthocyanin content at 63 days after veraison. Similar results were reported by Sunet al.(2016),who treated tomato with 50 μmol L-1melatonin and found that the total amount of anthocyanin increased by 50% compared with that in control fruit at 13 days after melatonin treatment. The enhancement of anthocyanin synthesis induced by melatonin is probably because of the accumulation of enzymes in the phenylpropanoid pathway (Flores and Ruiz del Castillo 2016). Our gene expression data confirmed this.
As presented in Table 2,compared with the control,both melatonin treatments significantly decreased the concentration of total phenolics. Similarly,application of melatonin to grape berries led to lower total phenolic levels compared with control plants (Menget al.2019). Chenet al.(2019) recently reported that melatonin treatments can significantly enhance the accumulation of anthocyanins and flavonols in crabapple leaves. We found similar results here,as melatonin treatment,particularly at 50 μmol L-1,was clearly associated with the accumulation of anthocyanins and flavonols in the peel of red-skinned Nanhong pears. Conversely,the concentrations of hydroxycinnamates and flavanols in pear peel decreased after the application of melatonin. Our data strongly suggest that melatonin treatment has differential effects on the various phenolic compounds.
3.3.Transcript levels of anthocyanin-related genes
Gene expression analysis provided further insight into the regulation of anthocyanin biosynthesis-related genes in the peel of melatonin-treated and control fruit. To further validate the results of anthocyanin accumulation,we used qRTPCR to analyze the effect of melatonin treatments on the transcriptional levels of structural genes in the anthocyanin biosynthetic pathway (Fig.2). The results indicated that both melatonin treatments increased the relative expression levels of the seven structural genes. In both 50 and 200 μmol L-1melatonin treatments,transcript levels for most of these genes were much higher than in control fruit. However,expression ofDFRwas similar to or even lower in melatonin-treated fruit than in control fruit,suggesting that this gene was not sensitive to melatonin treatment. Zhanget al.(2016) have shown that the expression levels of anthocyanin synthesis genes in red cabbage were upregulated by exogenous melatonin. Similar results were found with our peel samples,in which expression levels of most anthocyanin biosynthetic genes were markedly upregulated in melatonin-treated fruit. In addition,this result is consistent with the increased levels of anthocyanins in the peel of melatonin-treated fruit.

Fig.2 Relative expression of anthocyanin biosynthesis genes in pear peels that were treated with exogenous melatonin (MT)at 50 μmol L-1 (MT 50) and 200 μmol L-1 (MT 200). Error bars indicate the standard deviation of three replications.Different letters in each graph indicate significant differences at P<0.05 according to Duncan’s test.
Furthermore,theFLS,LARandANRgenes are associated with the synthesis of flavonols and flavanols (Wanget al.2015). In particular,FLS catalyzes the production of dihydroflavonols,the direct substrates for flavonol synthesis (Liet al.2019). In our study,the transcript level ofFLSwere significantly enhanced in melatonintreated fruit compared with control fruit,especially at the lower concentration (Fig.3). This response was consistent with the differences observed in the concentration of flavonols. Similarly,Chenet al.(2019) reported that the transcript level ofFLSin crabapple leaves increased at lower concentration melatonin treatments,whereas it decreased in response to the higher melatonin concentration tested. Thus,consistent with our results,the application of melatonin promotes the expression ofFLSand the accumulation of flavonols. Moreover,the conversion of anthocyanidins to the corresponding flavanols is catalyzed by LAR and ANR. Accordingly,the transcript level ofLARincreased only following the application of 50 μmol L-1melatonin,whereas that ofANRwas similar to or even lower in melatonintreated fruit than in control fruit. This could partly explain the decrease in flavanol concentration after melatonin treatment. Flavonoids are divided into several subclasses,including anthocyanins,flavonols,and flavanols,and they compete for the same substrate (Liet al.2019). Our results showed that the biosynthesis of flavanols was inhibited in melatonin-treated fruit. Thus,the substrates in the flavonoid pathway might flow mostly into the anthocyanin and flavonol synthesis pathways,leading to the rapid accumulation of anthocyanins and flavonols.
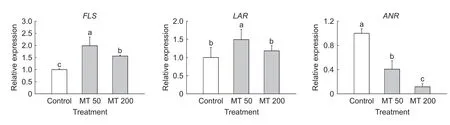
Fig.3 Relative expression of FLS,LAR and ANR in pear peels that were treated with exogenous melatonin (MT) at 50 μmol L-1 (MT 50) and 200 μmol L-1 (MT 200). Error bars indicate the standard deviation of three replications. Different letters in each graph indicate significant differences at P<0.05 according to Duncan’s test.
We also analyzed the expression of the TFs that regulate anthocyanin biosynthesis:MYB10,MYB114,bHLH,andWD40(Fig.4). After melatonin treatment,the expression of these four genes was upregulated to varying degrees compared to the control. Expression ofMYB10,MYB114,bHLH,andWD40increased 17-,2-,6-,and 4-fold,respectively. These results suggested thatMYB10was the most responsive TF to melatonin treatment in the peel of Nanhong pear. MYB TFs expressed in red pears have been shown to mediate anthocyanin biosynthesis by interacting with the bHLH and WD40 proteins (Schaartet al.2013).Numerous studies have shown that MYB10 has a key role in the regulation of the anthocyanin biosynthetic pathway. Qianet al.(2014) reported that methyl jasmonate treatment significantly increased mRNA levels ofMYB10in the peel of Mantianhong pears. In crabapple leaves,the expression levels of TFs (McMYB10,McbHLH3andMcbHLH33) were significantly induced by melatonin supplementation (Chenet al.2019). This is consistent with the results reported herein. We observed strong increases in the expression levels ofMYB10under melatonin treatment,which suggested that the upregulation ofMYB10is crucial to the regulation of melatonin-induced anthocyanin biosynthesis in pears.
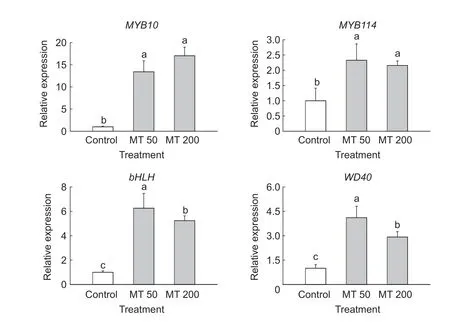
Fig.4 Relative expression of four transcription factors,MYB10, MYB114, bHLH and WD40,in pear peels that were treated with exogenous melatonin (MT) at 50 μmol L-1 (MT 50) and 200 μmol L-1 (MT 200). Error bars indicate the standard deviation of three replications. Different letters in each graph indicate significant differences at P<0.05 according to Duncan’s test.
As is well known,a major regulatory mechanism occurs in the expression of the anthocyanin pathway structural genes. These are known to be regulated by the MBW protein complex responsible for activating the transcription of the anthocyanin pathway structural genes by binding to their promoters (Xieet al.2019). In our study,the expression levels of almost all genes that participate in anthocyanin biosynthesis,such asPAL,CHS,CHI,F3H,ANS,andUFGT,were upregulated in response to melatonin application. In addition,changes in the transcript levels ofMYB10,MYB114,bHLH,andWD40were positively associated with the accumulation of anthocyanins. We propose that preharvest application of melatonin induced the expression of structural anthocyanin biosynthesisrelated genes by activating TFs,as has been proposed in the case of cabbage under the same treatment (Zhanget al.2016).
Recently,a number of models have been proposed for evaluating the effect of melatonin on the biosynthesis of anthocyanins (Xuet al.2019;Arnao and Hernández-Ruiz 2020b;Wang S Yet al.2020). Melatonin has been reported to enhance antioxidative defense mechanisms in ripening fruit,which was associated with increased biosynthesis of anthocyanins and flavonoids (Mukherjee 2019). Chenet al.(2019) recently reported that melatonin induced anthocyanin biosynthesis and promoted the accumulation of proanthocyanins and flavonols in crabapple leaves,and these changes were independent of light conditions. In contrast,Ai and Zhu (2018) found that melatonin could inhibit jasmonate-stimulated anthocyanin biosynthesis inArabidopsis thaliana. These results suggest that the application of melatonin at different concentrations and developmental stages have differing effects.
3.4.Endogenous melatonin concentration and transcript levels of melatonin biosynthesis genes
To understand the role of melatonin in the coloration of pear fruit,we measured the concentrations of melatonin at 15 days after melatonin treatment. Fig.1-C shows the melatonin levels in melatonin-treated and control fruit.Significant differences were observed between control and melatonin-treated fruit. Melatonin concentrations were 2.38 and 8.60 ng g-1FW after application of 50 and 200 μmol L-1melatonin,i.e.,there were 2-and 9-fold increases relative to control fruit,respectively. Indeed,fruit treated with 200 μmol L-1melatonin showed the highest concentration of melatonin. Therefore,application of melatonin caused a significant increase in endogenous melatonin concentration,suggesting absorption of the exogenous melatonin and thus successful donation of the compound.
Because melatonin in plants is synthesized from tryptophan sequentially,the expression levels ofTDC,T5H,SNAT,andASMTgenes were determined (Fig.5). The expression of melatonin biosynthetic genes was significantly upregulated in melatonin-treated fruit compared with that in control fruit,except forT5Hin the 50 μmol L-1melatonintreated fruits. Liuet al.(2018) explained that the delaying of strawberry fruit senescence caused by melatonin treatment (100 μmol L-1) may be attributed to the promotion of endogenous melatonin accumulation arising from melatonininduced expression of melatonin biosynthesis-related genes,includingFaTDC,FaT5H,FaSNAT,andFaASMT.Similarly,the expression of melatonin biosynthetic genes in crabapple tree leaves was induced by exogenous melatonin (Chenet al.2019). In accordance with previous studies,exogenously applied melatonin significantly enhanced the endogenous melatonin concentration in pear peel,and it was associated with increased expression levels of genes in the melatonin biosynthesis pathway. This implies that both endogenous melatonin and application of exogenous melatonin regulate anthocyanin accumulation in pear fruit.

Fig.5 Relative expression of melatonin biosynthesis genes in pear peels that were treated with exogenous melatonin (MT) at 50 μmol L-1 (MT 50) and 200 μmol L-1 (MT 200). Error bars indicate the standard deviation of three replications. Different letters in each graph indicate significant differences at P<0.05 according to Duncan’s test.
4.Conclusion
We examined the effect of melatonin treatment on the concentrations of phenolic compounds in pear peel.Additionally,we analyzed the expression of anthocyanin biosynthetic and regulatory genes. We found that melatonin treatments had a significant influence on phenolic composition. The anthocyanin concentration was increased by both the 50 and 200 μmol L-1melatonin treatments. Similarly,flavonol synthesis was enhanced by melatonin application. Conversely,the concentrations of hydroxycinnamates and flavanols in pear peel decreased after the application of melatonin. The expression levels of the anthocyanin biosynthetic genesPAL,CHS,CHI,F3H,ANS,andUFGT,were upregulated in melatonin-treated fruit. Such upregulated expression of structural genes occurred concomitantly with the increases in the transcript levels of TFs and the accumulation of anthocyanins. Moreover,melatonin treatment promoted the expression of melatonin synthesis genes and consequently increased the concentration of endogenous melatonin. Overall,this study provides evidence to position preharvest melatonin application as an innovative approach for the improvement of fruit color development in the red-skinned Nanhong pear.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31701865),the earmarked fund for the China Agriculture Research System (CARS-28),and the Liaoning Provincial Natural Science Foundation of China (2019-MS-276).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2021年8期
Journal of Integrative Agriculture2021年8期
- Journal of Integrative Agriculture的其它文章
- Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density
- Adoption of small-scale irrigation technologies and its impact on land productivity:Evidence from Rwanda
- Comparison of grain yield and quality of different types of japonica rice cultivars in the northern Jiangsu plain,China
- Natural nematicidal active compounds:Recent research progress and outlook
- lmproving grain appearance of erect-panicle japonica rice cultivars by introgression of the null gs9 allele
- Comparative transcriptome analysis of different nitrogen responses in low-nitrogen sensitive and tolerant maize genotypes
