A facile synthesis of 6-iodo-5H-[1,2,4]triazino[5,6-b]indole-3-thiols
WANG Hongxue, JI Ming, HUANG Wenyuan, CHANG Mingqin*
(1. College of Chemistry and Materials Engineering, Bohai University, Jinzhou 121000, Liaoning, China; 2. Liaoyang Market Supervision Service Center, Liaoyang 111099, Liaoning, China; 3. Fuxin Institute for Drug Control; Fuxin 123000, Liaoning, China)
Abstract: In this work, a facile synthesis of a series of structurally novel and intriguing iodo-functionalized [1,2,4]triazino[5,6-b]indole-3-thiols has been achieved in good yields through the condensation reaction of various iodo isatins with thiosemicarbazide using H2O-dioxane (VH2O∶Vdioxane, 5∶1) solvent system as reaction medium in the presence of K2CO3 as base. The molecular structures of these synthesized products were confirmed by spectral data and elemental analyses.
Keywords: iodo-substitution; [1,2,4]triazino[5,6-b]indole; condensation reaction; isatin; thiosemicarbazide
As well known, iodo-functionalized N-heterocyclic compounds have been an attractive synthetic targets because the iodine atom not only can play an important role in their bio-activity[1], but also is a very useful functional group for the development of chemical probes in pharmacological studies[2], or further chemical transformations to construct important drug-like small molecules[3], for example, through cross-coupling reactions and nucleophilic aromatic substitution (SNAr) reactions[4-5], which have been used extensively with a high level of success. For this reason, the construction of structurally novel and intriguing iodo-substituted aromatic heterocycles has been highly useful in the field of synthetic organic chemistry[6-7]. On the other hand, among nitrogen-containing heterocycles, [1,2,4]triazino[5,6-b]indole is a particularly fascinating structural motif present as pivotal skeleton in numerous significant natural products and pharmacologically relevant compounds[8-9], displaying significant biological properties such as antimalarial[10], antileishmanial[11]and anticancer activities[12]. Due to their striking biological activities and in order to have structurally diversified molecules for bio-screening, considerable synthetic efforts have been devoted surrounding the [1,2,4] triazino[5,6-b] indole ring for further modification and functionalization by both organic and medicinal chemists with the aim of enhancing the potency of this class of compounds[13-15].
Considering the aforementioned observations, we felt that it would be a worthwhile endeavor to the synthesis of iodo-substituted [1,2,4]triazino[5,6-b]indole derivatives, which might provide ample opportunity for further synthetic manipulation, and thus would make the targeted products particularly appealing. Thus, in the context of our continuing interest in the construction of interesting types of heterocyclic compounds[16-17], we would like to report herein a simple and efficient synthesis of iodo-functionalized [1,2,4]triazino[5,6-b]indole-3-thiols, which might be much more attractive and valuable for current medicinal chemistry needs. To the best of our knowledge, there are very few reports pertaining to the synthesis of such iodo-substituted compounds in the literature.
1 Experimental section
1.1 Apparatus and chemicals
The chemicals used in this work were obtained from Energy Chemical and were used without purification. The melting points were determined by using a WRS-1B melting point apparatus and were uncorrected. The IR spectra of the compounds in KBr pellets were obtained in the range of 400-4 000 cm-1on a Shimadzu FTIR-8400S spectrophotometer.1H (400 MHz) and13C (100 MHz) NMR spectra were recorded on a Brucker AVANCE NMR spectrometer using DMSO-d6as solvent. The reported chemical shifts (δvalues) are given in parts per million downfield from tetramethylsilane (TMS) as the internal standard. Elemental analyses were performed for C and H using an Elementar Vario EL-III element analyzer. The progress of reactions was monitored by thin layer chromatography (TLC) on silica gel GF254 using ethyl acetate/petroleum ether (1∶1) as eluent.
1.2 General procedure for the preparation 6-iodo-5-alkyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3a-g)
A mixture of the appropriate 5-iodo-1-alkylindoline-2,3-dione1(1 mmol), thiosemicarbazide2(0.1 g, 1.1 mmol), and K2CO3(0.21 g, 1.5 mmol) in H2O-dioxane (10 mL, v/v, 5/1) mixed solvent system was heated under refluxing temperature. The conversion was monitored by TLC. After the reaction was complete, the mixture was cooled to room temperature, quenched by addition of ice-water (8 mL), and acidified with acetic acid. The resulting crude product was washed with water, dried, and recrystallized from acetic acid to give the corresponding pure product3a-g. The yields, physical properties and spectral and analytical data are given below.
6-Iodo-5,8-dimethyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3a): Yellow solid, yield 74%,m.p. 280-281 ℃; IR (KBr)ν: 3 451, 3 000, 2 965, 2 365, 1 605, 1 580, 1 480, 1 340, 1 320, 1 175, 1 140, 1 040, 855 cm-1;1H NMR (DMSO-d6, 400 MHz):δ2.72 (s, 3H, 8-Me), 3.87 (s, 3H, 5-Me), 7.66 (s, 1H, Ar-H), 8.02 (s, 1H, Ar-H), 14.66 (s br, 1H, SH);13C NMR (DMSO-d6, 100 MHz): 20.66, 26.19, 109.76, 113.16, 117.35, 125.54, 133.63, 138.78, 149.23, 158.29, 179.33. MS (ESI,m/z): 357.0 [M + H]+. Anal. Calcd for C11H9IN4S: C, 37.09; H, 2.55; N, 15.73. Found: C, 37.31; H, 2.64; N, 15.56.
5-Ethyl-6-iodo-8-methyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3b): Yellow solid, yield 71%,m.p. 273-275 ℃; IR (KBr)ν: 3 441, 2 959, 2 838, 1 606, 1 566, 1 318, 1 148, 821 cm-1;1H NMR (DMSO-d6, 400 MHz):δ1.30 (t,J= 7.2 Hz, 3H, 5-CH2CH3), 2.68 (s, 3H, 8-Me), 4.35 (q,J= 7.6 Hz, 2H, 5-CH2CH3), 7.67 (s, 1H, Ar-H), 8.03 (s, 1H, Ar-H), 14.69 (s br, 1H, SH);13C NMR (DMSO-d6, 100 MHz): 15.37, 20.36, 37.79, 103.84, 121.55, 121.79, 135.11, 135.29, 137.57, 138.34, 149.26, 179.81. MS (ESI,m/z): 371.3 [M + H]+. Anal. Calcd for C12H11IN4S: C, 38.93; H, 2.99; N, 15.13. Found: C, 38.78; H, 2.81; N, 15.27.
5-Butyl-6-iodo-8-methyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3c): Yellow solid, yield 78%,m.p. 299-301 ℃; IR (KBr)ν: 3 431, 2 960, 2 838, 1 605, 1 566, 1 318, 1 148, 1 057, 810 cm-1;1H NMR (DMSO-d6, 400 MHz):δ0.94 (t,J= 7.6 Hz, 3H,n-Bu), 1.36 (sext,J= 7.6 Hz, 2H,n-Bu), 1.65 (quint,J= 7.6 Hz, 2H,n-Bu), 2.62 (s, 3H, 8-Me), 4.02 (t,J= 7.2 Hz, 2H,n-Bu), 7.63 (s, 1H, Ar-H), 8.18 (s, 1H, Ar-H), 12.32 (s br, 1H, SH);13C NMR (DMSO-d6, 100 MHz): 14.15, 19.73, 20.36, 31.95, 42.34, 103.91, 121.52, 121.78, 134.94, 135.33, 137.66, 138.38, 149.43, 179.81. MS (ESI,m/z): 399.1 [M + H]+. Anal. Calcd for C14H15IN4S: C, 42.22; H, 3.80; N, 14.07. Found: C, 41.97; N, 4.10; N, 13.85.
5-Benzyl-6-iodo-8-methyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3d):Yellow solid, yield 76%,m.p. 260-262 ℃; IR (KBr)ν: 3 436, 2 968, 2 850, 2 350, 1 605, 1 566, 1 464, 1 318, 1 148, 1 117, 1 008, 818 cm-1;1H NMR (DMSO-d6, 400 MHz):δ2.34 (s, 3H, 8-Me), 5.61 (s, 2H, N-CH2), 7.14 (d,J= 7.2 Hz, 2H, Ben-H), 7.27-7.32 (m, 3H, Ben-H), 7.57 (s, 1H, Ar-H), 8.09 (s, 1H, Ar-H), 14.78 (s br, 1H, SH);13C NMR (DMSO-d6, 100 MHz): 19.83, 43.93, 103.14, 121.89, 124.97, 128.75, 128.78, 131.97, 135.42, 136.72, 142.84, 144.92, 151.75, 159.94, 182.40. MS (ESI,m/z): 433.0 [M + H]+. Anal. Calcd for C17H13IN4S: C, 47.23; H, 3.03; N, 12.96. Found: C, 47.42; H, 2.95; N, 13.14.
8-(tert-Butyl)-6-iodo-5-methyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3e): Yellow solid, yield 82%,m.p. 279-281 ℃; IR (KBr)ν: 3 438, 2 965, 2 850, 1 605, 1 565, 1 464, 1 318, 1 148, 815 cm-1;1H NMR (DMSO-d6, 400 MHz):δ1.29 (s, 9H,tert-butyl), 3.51 (s, 3H, 5-Me), 7.73 (s, 1H, Ar-H), 7.90 (s, 1H, Ar-H), 12.27 (s br, 1H, SH).13C NMR (DMSO-d6, 100 MHz): 21.02, 26.08, 35.68, 107.68, 117.19, 124.46, 125.11, 127.94, 131.73, 142.73, 151.28, 179.18. MS (ESI,m/z): 399.2 [M + H]+. Anal. Calcd for C14H15IN4S: C, 42.22; H, 3.80; N, 14.07. Found: C, 42.50; H, 3.65; N, 14.29.
8-Ethyl-6-iodo-5-methyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3f): Yellow solid, yield 80%,m.p. 285-286 ℃;IR (KBr)ν: 3 435, 2 965, 2 849, 1 606, 1 567, 1 462, 1 319, 1 147, 816 cm-1;1H NMR (DMSO-d6, 400 MHz):δ1.26 (t,J= 7.6 Hz, 3H, CH2CH3), 3.07 (q,J= 7.6 Hz, 2H, CH2CH3), 3.85 (s, 3H, 5-Me), 7.64 (s, 1H, Ar-H), 8.03 (s, 1H, Ar-H), 14.66 (s br, 1H, SH);13C NMR (DMSO-d6, 100 MHz): 14.63, 27.50, 36.35, 103.62, 108.54, 120.78, 124.17, 141.54, 143.00, 145.56, 158.63, 179.18. MS (ESI,m/z): 371.1 [M + H]+. Anal. Calcd for C12H11IN4S: C, 38.93; H, 2.99; N, 15.13. Found: C, 38.71; H, 3.13; N, 15.31.
5-Benzyl-8-ethyl-6-iodo-5H-[1,2,4]triazino[5,6-b]indole-3-thiol(3g): Yellow solid, yield 77%,m.p. 246-247 ℃; IR (KBr)ν: 3 459, 2 969, 2 354, 1 487, 1 456, 1 382, 1 343, 1 117, 1 051, 820 cm-1;1H NMR (DMSO-d6, 400 MHz):δ0.98 (t,J= 7.2 Hz, 3H, 8-CH2CH3), 2.67 (q,J= 7.6 Hz, 2H, 8-CH2CH3), 5.57 (s, 2H, benzyl), 7.12 (d,J= 7.2 Hz, 2H, Ben-H), 7.22-7.32 (m, 4H, Ar-H and Ben-H), 7.90 (s, 1H, Ar-H), 14.66 (s br, 1H, SH);13C NMR (DMSO-d6, 100 MHz): 15.97, 27.59, 45.16, 104.04, 120.78, 121.78, 128.03, 128.59, 129.30, 132.25, 135.31, 136.25, 138.52, 141.99, 150.21, 180.02. MS (ESI,m/z): 447.2 [M + H]+. Anal. Calcd for C18H15IN4S: C, 48.44; H, 3.39; N, 12.55. Found: C, 48.68; H, 3.65; N, 12.27.
2 Results and discussion
We first carried out the reaction of 5-chloro-1-methyl indoline-2,3-dione (1a) and 1.1 equiv of thiosemicarbazide (2) by using H2O-dioxane (v/v, 5/1) solvent system as reaction medium with the presence of K2CO3as base at refluxing temperature as outlined in Scheme 1.
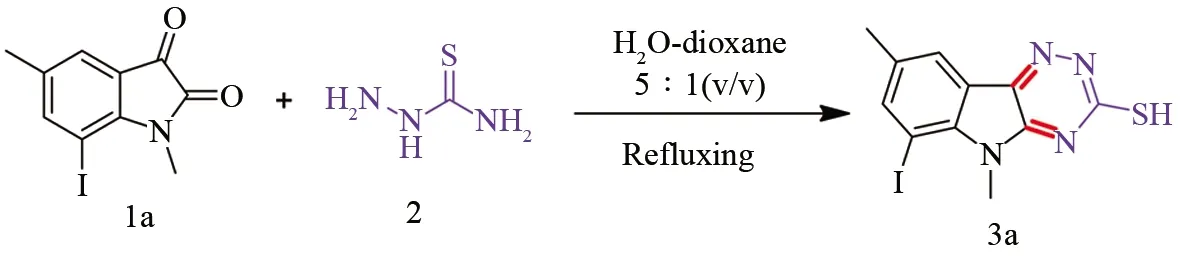
Scheme 1 Synthesis of 6-iodo-5,8-dimethyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol (3a)
In fact, our initial investigation towards the reaction was conducted following a published method by using H2O as solvent with the presence of K2CO3as base at refluxing temperature[11]. Although the procedure was very easy, eco-friendly, and impressive, the desired product 6-iodo-5,8-dimethyl-5H-[1,2,4]triazino[5,6-b]indole-3-thiol (3a) was obtained only in a low yield of 37% even after refluxing 12 h. In an effort, we investigated the aqueous reaction by employingβ-cyclodextrin as the catalyst, which was reported to yield better results in this type of reaction[18]. Although this impressive method resulted in an increase in the product yield to 46%, this was still not satisfactory as we expected. Moreover, increasing the reaction temperature to reflux or using microwave irradiation resulted in no further improvement in the product yield as well. Further, attempts to use other organic solvents such as dioxane, MeOH, EtOH, MeCN or DMF, no further improvement in the product yield was observed.
After these unsuccessful trials, we were delighted to find that the mixed solvent system of H2O/dioxane could be used as a reliable approach for this reaction, resulting in a much increase in the product yield, and after a simple optimal experiment the volume ratio 5∶1 was found to be optimal to complete the reaction as shown in Scheme 1, providing the most striking yield of 80% within 8 h as monitored by TLC. Subsequently, we further extended the reaction to other iodo-substituted isatins (1b-g) in a similar fashion. Satisfactorily, these substances were equally amenable to the reaction process without any experimental difficulties, successfully furnishing corresponding3b-gin satisfactory yields 71-82%. The results of this series of experiments were listed in Table 1.

Table 1 Yields and physical properties of the targeted compounds 3a-g a
aIsolated yield.
To the best of our knowledge, all these newly synthesized compounds have never been reported, and their structures were easily confirmed by spectral data and elemental analyses with the results being in good agreement with the expected compounds. For example, the1H NMR spectrum of3aexhibited the presence of one distinct singlet signal at 14.66 ppm, readily recognizable as arising from ─SH proton, together with four singlets at 2.72, 3.87, 7.66 and 8.03 attributable to tow methyl protons and two aromatic protons, respectively, which were in good agreement with the assigned structure of3a. Further, the structure was confirmed by its mass spectrum through the appearance of a quasi-molecular ion peak atm/z371.1 ([M+H]+). Finally, the structure assigned for3awas fully supported by its elemental analyses, which established the molecular formula C12H11IN4S in accordance with the suggested molecular structure. The other compounds exhibited similar spectral characteristics except the substituents, which exhibited characteristic signals with appropriate chemical shifts.
3 Conclusion
In summary, we have demonstrated an easy access to the synthesis of structurally intriguing iodo-functionalized [1,2,4]triazino[5,6-b]indole-3-thiols through the condensation reaction of iodinated isatins with thiosemicarbazide. The reaction protocol described here could be attractive as it is simple, easy to handle, and does not involve the use of expensive reagents or catalysts. These newly synthesized compounds contain a derivatizable iodo functional group, which makes them particularly appealing, since the functional group provides ample opportunity for further synthetic manipulation, for example, by metal-catalyzed coupling reactions or aromatic substitution (SNAr) reactions to obtain more complex and significant chemical diversity for use in medicinal chemistry. Currently, work is ongoing, mainly focusing on the further elaboration and application of these compounds, which represent an intriguing goal that we are contemplating, and these results will be a part of future reports.

