Colorimetric quantification of aqueous hydrogen peroxide in the DC plasma-liquid system
Renze YU (俞仁泽), Zhaoyuan LIU (刘钊源), Jiao LIN (林娇),
Xinyi HE (何心怡)1, Linsheng LIU (刘林生)3, Qing XIONG (熊青)4,Qiang CHEN (陈强)1 and Kostya (Ken) OSTRIKOV (欧思聪)5
1 Shenzhen Research Institute of Xiamen University, Institute of Electromagnetics and Acoustics, Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance,Key Laboratory of Electromagnetic Wave Science and Detection Technology, Xiamen University, Xiamen 361005, People’s Republic of China
2 State Key Laboratory of Electrical Insulation and Power Equipment, Centre for Plasma Biomedicine,Xi’an Jiaotong University, Xi’an 710049, People’s Republic of China
3 College of electronic engineering, Guangxi Normal University, Guilin 541004, People’s Republic of China
4 State Key Laboratory of Power Transmission Equipment & System Security and New Technology,Chongqing University, Chongqing 400044, People’s Republic of China
5 School of Chemistry and Physics,Queensland University of Technology,Brisbane,QLD 4000,Australia
Abstract The quantification of hydrogen peroxide(H2O2)generated in the plasma-liquid interactions is of great importance,since the H2O2 species is vital for the applications of the plasma-liquid system.Herein, we report on in situ quantification of the aqueous H2O2 (H2O2aq) using a colorimetric method for the DC plasma-liquid systems with liquid as either a cathode or an anode.The results show that the H2O2aq yield is 8–12 times larger when the liquid acts as a cathode than when the liquid acts as an anode.The conversion rate of the gaseous OH radicals to H2O2aq is 4–6 times greater in the former case.However,the concentrations of dissolved OH radicals for both liquid as cathode and anode are of the same order of tens of nM.
Keywords: plasma-liquid interactions, hydrogen peroxide, OH radical, atmospheric-pressure plasma
1.Introduction
In 1785, Henry Cavendish performed his famous work of‘Experiments on air’ in which he proposed that nitric acid is likely formed by contacting air discharge with water[1].This investigation might be the first documented study of the discharge plasma-liquid interactions.Since then, applications of the plasma-liquid systems have been extensively explored[2], such as in wastewater treatment [3–12], nanomaterial synthesis [13–29], plasma medicine [30, 31], plasma agriculture [32, 33], and analytical chemistry [34–42].These applications are mainly based on the physical and chemical processes induced by the interplay between the plasma and the liquid.The reactive species generated from the plasmaliquid interactions are the key for all the applications.Therefore, characterization of the formation and evolution of the reactive species in the plasma-liquid system is of great importance.
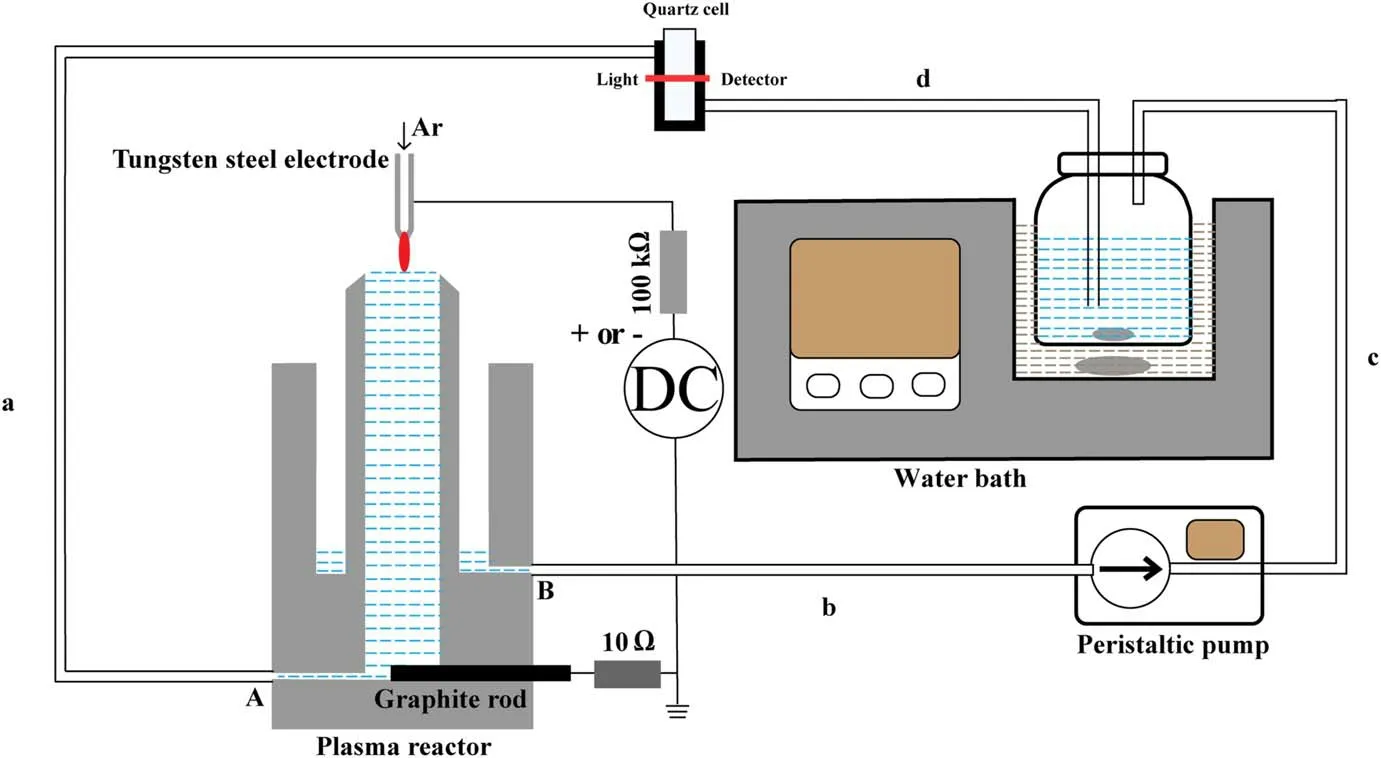
Figure 1.Schematic diagram of the experimental setup.Lengths of silicone tubes a,b,c,d are 25 cm,20 cm,20 cm and 20 cm,respectively.
When the discharge plasma is operated in ambient air,the participation of oxygen and nitrogen gases in the discharge leads to the formation of reactive oxygen and nitrogen species(RONS), such as hydroxyl (OH) radicals, atomic oxygen,hydrogen peroxide(H2O2),nitrite and nitrate[43–47].Among these RONS,OH radicals present very strong oxidizing ability which leads to the short depth of the OH penetration into the liquid(of the order of ~102nm[48]).As a relatively long-lived reactive species, aqueous H2O2(H2O2aq) can participate the bulk solution chemistry.In other words, the dissolved OH(OHaq)radicals,as a short-lived species,can affect only a very thin layer of the solution surface, while the H2O2aqcan affect the bulk solution chemistry.Quantification of gaseous OH radicals in plasmas has been extensively performed by several diagnostic techniques, including broadband UV absorption[49,50],laser induced fluorescence spectroscopy[51–53],and chemical ionization mass spectrometry [54].The OH radicals in liquid phase can also be quantified by laser flash photolysis[55], electron paramagnetic resonance spectroscopy [56],electron spin resonance spectroscopy [57], molecular probe[58] and some other methods.
In an AC plasma jet-liquid system,the H2O2aqis formed by the dissolution of gaseous H2O2generated at the plasma tube in a low frequency plasma where aqueous solution is not an electrode of the discharge [59].However, the case is different when the liquid acts as an electrode of the discharge.In the DC plasma-liquid systems with the liquid as a discharge electrode,the H2O2aqhas been found to be formed predominantly from the recombination of OHaqas shown in scheme 1 [60, 61].
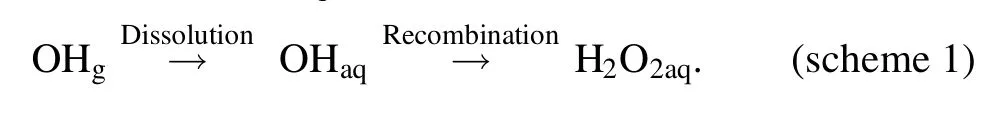
We emphasize that the H2O2aqis formed by the recombination of dissolved gaseous OH radicals which are generated by the plasma-liquid interactions at the plasma-liquid interface or are delivered from the gaseous plasma discharge into the surface layer of the liquid.Therefore, the quantification of H2O2aqin the DC plasma-liquid systems is expected to give us some insight of the dissolution behavior of the OH radicals from the gaseous plasma.
In this paper, we use a colorimetric method to quantify the H2O2aqin the DC plasma-liquid system with the liquid acting either as a cathode or an anode.The results show that there are different behaviors of the gaseous OH radical dissolution and the H2O2aqyield between the systems that use the liquid as a cathode or an anode.
2.Experimental setup
Figure 1 presents the schematic illustration of the discharge plasma device,while the details of the device can be found in our previous papers [60, 62].Argon gas (20 sccm) was fed through the top hollow tungsten steel electrode (slightly tapered at the nozzle, 1.02 mm in inner and 6.35 mm in outside diameters).A DC voltage (BOHER HV, LAS-20 kV–50 mA, negative or positive polarity) was applied to the hollow electrode to ignite the discharge between the electrode and the flowing liquid surface with the discharge gap of 3 mm.The liquid was maintained and circulated in a polytetrafluoroethylene cylinder-like plasma reactor by a peristaltic pump with a flow rate of 100 ml min−1.The inner and outside diameters of the cycling tube are 3 and 5 mm,respectively.Inside the pump,there is a 10 cm silicone tube(4 and 6 mm in inner and outside diameters) for connecting tubes b and c.Using the peristaltic pump,the liquid is pushed to enter the centric cylinder from port A and then flows out from the top of the centric cylinder where the liquid is treated by the plasma.The plasma treated liquid flows down to the outside cylinder and then flows out from port B.As the negative or positive voltage was applied to the top electrode,the liquid acted as either the cathode or the anode.A ballast resistor (100 kΩ) was connected in series in the circuit to avoid the discharge conversion from glow to arc mode.The discharge current was obtained by dividing the voltage across a 10 Ω resistor connected in series with a graphite electrode.
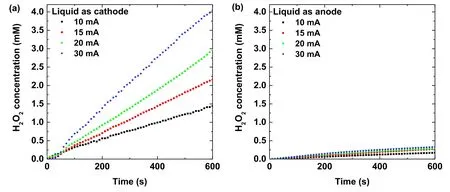
Figure 2.Time evolutions of the H2O2 concentration in the plasma treated Ti(SO4)2 solution at several discharge currents as the solution acts as (a) cathode, and (b) anode.
The aqueous H2O2concentration in the plasma-liquid system was measured by a colorimetric method.The method is based on the reaction between H2O2and titanium sulfate[Ti(SO4)2] in a strongly acidic environment (Ti4++H2O2+2H2O→H2TiO4+4H+).The yellow-colored complex,H2TiO4, has an absorption band at around 407 nm, and this absorbance is proportional to the concentration of H2O2.We used an aqueous solution of titanium sulfate and sulphuric acid as the treated liquid, by which we can record the H2O2aqconcentration during the plasma operation.7.5 ml of titanium sulfate(120 g l−1)was added to 250 ml sulphuric acid(H2SO4,1.5 M) to prepare the treated solution.The mixed solution(100 ml)was circulated by the peristaltic pump with a flow rate of 100 ml min−1.After the plasma treatment, the solution is pumped into a blending bottle which is placed in a water bath to keep the solution temperature at 25°C.Next to the blending bottle,the solution absorbance is recorded at a flow quartz cell each ten seconds by Ocean Optics USB 2000+coupled with a light source (DH-2000, 200–2500 nm).The Cell (made of JGS1 quartz)is 10 mm in optical length and 0.48 ml in volume with a suitable wavelength range of 200–2500 nm.
3.Results and discussion
In our previous work [63, 64], the proportionality of the absorption intensity at 407 nm of the acidic Ti(SO4)2solution and H2O2concentration was 0.27.Using this proportionality,the time evolution of the H2O2concentration in the plasma treated Ti(SO4)2solution at several discharge currents is presented in figure 2.The results indicate that the H2O2concentration increases linearly with the discharge time for the solutions acting as the cathode.When the solution is used as the anode, the H2O2concentration increases much more slowly with the discharge time.
When gaseous OH radicals delivered into the solution are solvated (OHaq), some OHaqspecies will recombine to form H2O2aq, while unreacted OHaqspecies will be consumed by the OH scavengers in the solution.The formed H2O2aqthen reacts with Ti4+in the strong acid solution to form the yellow-colored complex H2TiO4(equations (1)–(3)),
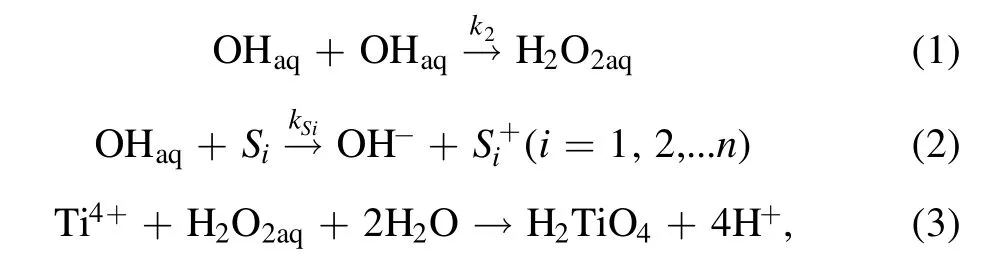
whereSiis theith OH scavenger in the solution,k2andkSiare the rate constants of reactions (1) and (2), respectively.
From the above equations, we obtain
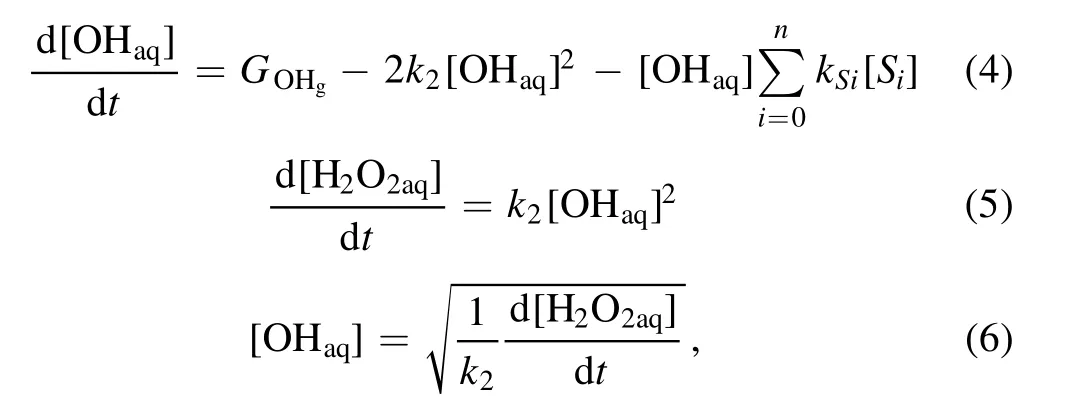
whereGOHgis the dissolution rate of gaseous OH radicals.
By differentiating the curves in figure 2, we obtain the time dependence of the rate for the H2O2aqyield as presented in figure 3.The results show that the rate for the H2O2aqyield for the liquid acting as the cathode is around ten times larger than when the liquid is used as the anode.
From equation (6) and usingk2=5.5×109M−1s−1[65], the concentration of OHaqcan be calculated (figure 4).The OHaqconcentration for the liquid as the cathode case is 2 to 3 times larger compared to the liquid as the anode case.In both cases,the OHaqconcentrations(~tens of nM)are almost constant for a fixed discharge current during the plasma treatment except for at the beginning of the discharge.
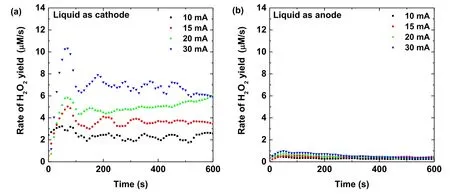
Figure 3.Time evolutions of the rate for the H2O2aq yield in the plasma treated Ti(SO4)2 solution at several discharge currents as the solution acts as (a) cathode, and (b) anode.
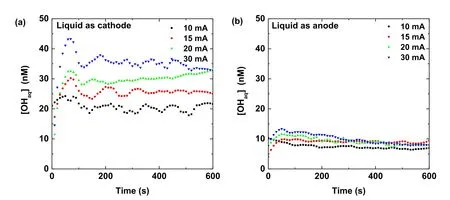
Figure 4.Time evolutions of the OHaq concentration in the plasma treated Ti(SO4)2 solution at several discharge currents as the solution acts as (a) cathode, and (b) anode.
If a molecular probe is used, most of the dissolved OH species will be captured by the molecular probe.Therefore,the measured OHaqconcentration increases with the plasma treatment time, as observed by Chen Zet al[61] and Kovačević V Vet al[66].When the molecular probe is present,the estimated OHaqconcentration should correspond to the concentration of the OHaqspecies after the scavenging.However,the OHaqconcentration estimated in our case is in fact a steady-state concentration,and it is almost a constant and very small for a fixed discharge current.
The following analysis is carried out to obtain some insight into the observed phenomena.In a quasi-steady state(d[OHaq]/dt= 0), equation (4) turns out to be
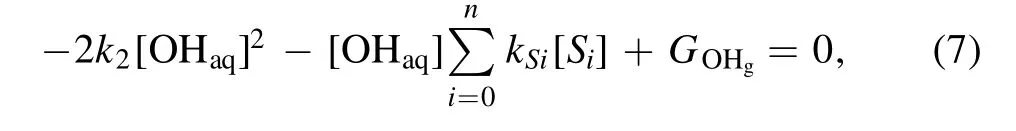
then we obtain
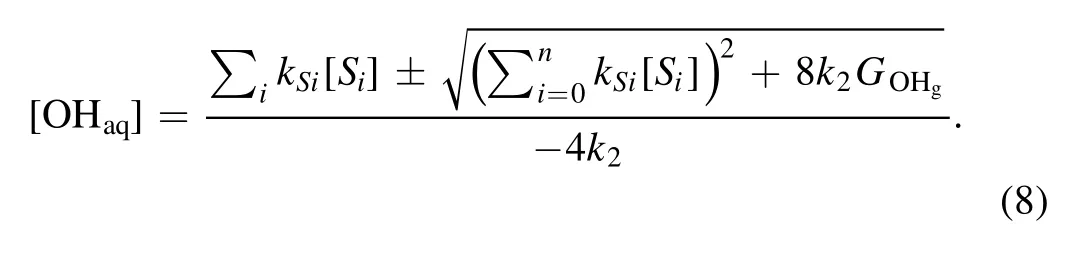
Because the OHaqconcentration should only have the positive values, we have
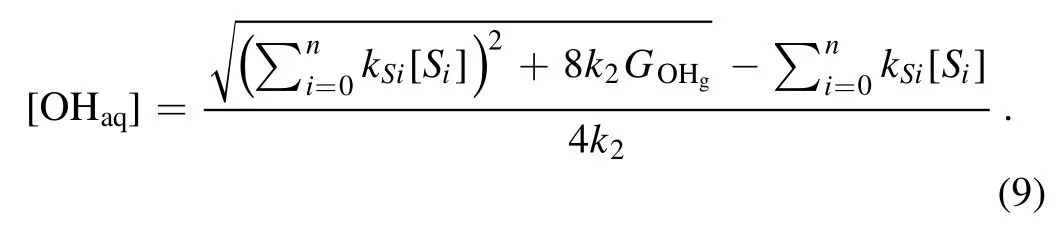
We assume thatGOHgis a constant for a fixed discharge current, the OHaqconcentration is controlled by the terms ofin equation (9).Therefore, the OHaqconcentration is affected by the OH scavengers in the solution.It has been reported that the number densities of the gaseous OH radicals generated in the both cases when the liquid is used either as the cathode or anode are of the same order[50,67],and consequently theGOHgshould be also of the same order for cases with a liquid cathode and a liquid anode at a fixed discharge current.Therefore,the large difference in the rate of the H2O2aqyield is likely caused by the different scavengers in each specific case (see equations (5) and (9)).In the same solution, the common OH scavengers should be the same for both the cathode and anode cases.It has been reported that the positive ion irradiation is the dominant physical process in theliquid as the cathode case, while the electron shower on the solution surface is the key process in the other case [68].The positive ion irradiation will not lead to much variation of the scavengers in the solution,while the electron shower will induce the formation of hydrated electrons (eaq) in the solution.Therefore, the hydrated electron (eaq+OHaq→OH−) and hydrated electron-induced species such as OH−and atomic hydrogen(eaq+HO−2→2OH−+OHaq,eaq+eaq→H2+2OH−,eaq+H+→H, OH−+OHaq→O−+H2O&H +OHaq→H2O)can play as the additional OH scavengers when the liquid acts as the anode [63].This explains the low generation rate of H2O2aqand the low OHaqconcentration in the latter case.
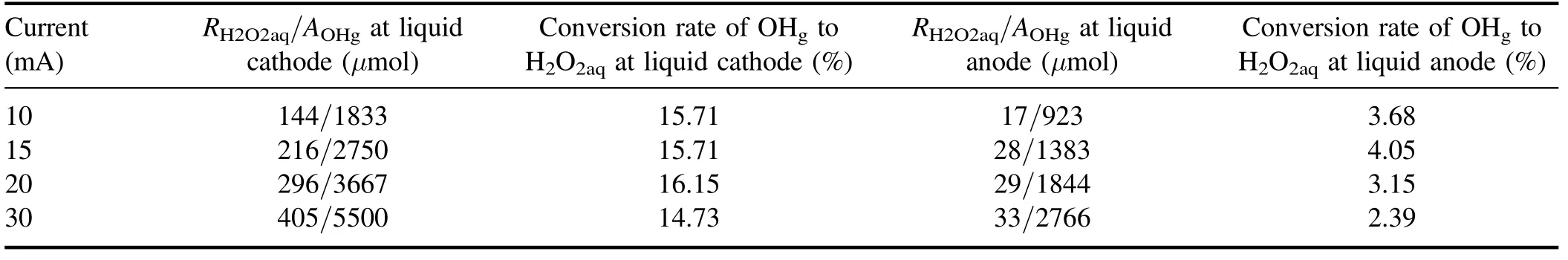
Table 1.Total H2O2aq yield(RH2O2aq),amount of OHg impinging on the solution surface(AOHg),and the conversion rate of OHg to H2O2aq for both liquid as cathode and anode.
From the kinetic theory of gases,we can estimate the rate of gaseous OH radicals (OHg) impinging on the solution surface (ROHg) by equation (10) [69],
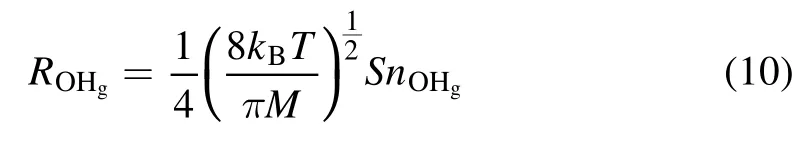
wherekBis the Boltzmann constant(1.38×10−23J K−1),Mis the molecular mass of the OH radical(2.82×10−26kg),Tis the temperature of the OHg(assumed to be ~2000 K[60]),nOHgis the OHgnumber density, andSis the contact area of the plasma and the solution surface.
IfnOHgis expressed in the unit of m−3, equation (10)will be
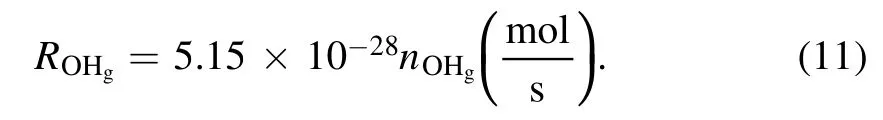
The number densities of gaseous OH radicals for the plasma-liquid systems have been quantified by several different techniques,the OHgnumber density spans a range from 1018to 1023m−3depending on the discharge type and plasma parameters [50, 67, 70–73].In an experimental setup similar to this work and using laser-induced fluorescence spectroscopy, Nikiforov Aet al[73] observed that the OHgnumber density increases almost linearly with the discharge current for both liquid cathode and liquid anode.The estimated OHgnumbers are 8.95×1021m−3and 1.78×1022m−3for the liquid cathode and anode cases, respectively, at a discharge current of 28.5 mA [73].If we take the values of OHgconcentration from [73], we can achieve the amount of OHgimpinging on the surface during the plasma treatment(10 min).The results are summarized in table 1 with the total H2O2aqyield and the conversion rate of OHgto H2O2aq.Obviously, the conversion rate of OHgto H2O2aqfor the liquid as the cathode case is 4–6 times larger than for the anode case.According to equations (1) and (2), the way to enhance the conversion rate of OHgto H2O2aqin the latter case is either to increase the OHaqconcentration [74] or to decrease the amount of OH scavengers in the solution [63].
4.Conclusions
The quantification of H2O2aqconcentration is performed during the DC discharge plasma treatment of a solution using a colorimetric method.The results show that the H2O2aqconcentration increases with the plasma exposure time.The H2O2aqyield is much larger for the liquid as the cathode case than when the same liquid is used as the anode.After analyzing the processes in the solution, we conclude that the additional OH scavengers,hydrated electrons and their derivatives such as OH−and atomic hydrogen, introduced into the solution by the plasma contribute the low yield of H2O2aqand the low conversion rate of gaseous OH radicals to H2O2aqwhen the liquid acts as the anode.
Acknowledgments
Q Chen thanks National Natural Science Foundation of China (No.52077185) and the Basic Research Program of Science and Technology of Shenzhen, China (No.JCYJ20190809162617137) for partial financial support.L Liu thanks for the financial supports from the Basic Ability Promotion Project for Young and Middle-Aged Teachers in Universities of Guangxi (No.2018KY0083) and Doctoral Scientific Research Fund of Guangxi Normal University(No.2017BQ019).Q Xiong thanks for the financial supports from National Natural Science Foundation of China (No.11975061), the Technology Innovation and Application Development Project of Chongqing (No.cstc2019jscxmsxmX0041), the Construction Committee Project of Chongqing (No.2018-1-3-6), and the Fundamental Research Funds for the Central Universities(No.2019CDQYDQ034).K Ostrikov thanks the Australian Research Council (ARC) for partial support.
 Plasma Science and Technology2021年5期
Plasma Science and Technology2021年5期
- Plasma Science and Technology的其它文章
- UV and soft x-ray emission from gaseous and solid targets employing SiC detectors
- The acceleration mechanism of shock wave induced by millisecond-nanosecond combined-pulse laser on silicon
- Investigation of non-thermal atmospheric plasma for the degradation of avermectin solution
- Laser-induced breakdown spectroscopy for the classification of wood materials using machine learning methods combined with feature selection
- Plasma-assisted Co/Zr-metal organic framework catalysis of CO2 hydrogenation:influence of Co precursors
- Quantitative analysis of uranium in electrorecovery salt of pyroprocessing using laserinduced breakdown spectroscopy
