Predictors of hospitalization plus airway support among infants with recurrent wheezing in the emergency department
Jefferson Antonio Buendía, Ranniery Acuña‐Cordero, Carlos E Rodriguez‐Martinez
(1.Department of Pharmacology and Toxicology, School of Medicine, Research Group in Pharmacology and Toxicology (ⅠNFARTO), Universidad de Antioquia, Medellín, Colombia;2.Departamento de Neumología Pediátrica, Hospital Militar Central, Departamento de Pediatría,Facultad de Medicina, Universidad Militar Nueva Granada, Bogotá, Colombia;3.Department of Pediatrics, School of Medicine, Universidad Nacional de Colombia, Bogota, Colombia;4.Department of Pediatric Pulmonology and Pediatric Critical Care Medicine,School of Medicine, Universidad El Bosque, Bogota, Colombia)
Abstract: Objective Most patients with recurrent wheezing are infants under 2 years of age.Clinical prediction models of the risk of receiving airway support during the hospital stay in this population have been poorly studied in tropical countries.This study aimed to evaluate the clinical predictors of hospitalization plus airway support among infants with recurrent wheezing evaluated in the emergency department in Colombia.Methods A retrospective cohort study was performed.This study included all infants with two or more wheezing episodes who were younger than two years old in two tertiary centers in Rionegro, Colombia, between January 2019 and December 2019.The primary outcome measure was hospitalization plus any airway support.A multivariable logistic regression model was used to identify factors independently associated with hospitalization plus any airway support.Results A total of 85 infants were hospitalized plus any airway support, of whom 34(40%) were treated with high flow nasal canula, 2(2%) received non‐invasive ventilation, 6(7%) were mechanically ventilated, and 43(51%) received conventional oxygen therapy.The multivariable logistic regression model showed that predictors of hospitalization plus airway support included prematurity (OR=1.79, 95%CI: 1.04‐3.10), poor feeding (OR=2.22,95%CI: 1.25‐3.94), nasal flaring and/or grunting (OR=4.27, 95%CI: 2.41‐7.56), and previous wheezing episodes requiring hospitalization (OR=3.36, 95%CI: 1.86‐7.08).The model has a high specificity (99.6%) with acceptable discrimination and an area under the curve of 0.70(95%CI: 0.60‐0.74).Conclusions The present study shows that prematurity, poor feeding, nasal flaring and/or grunting, and more than one previous episode of wheezing requiring hospitalization are independent predictors of hospitalization plus airway support in a population of infants with recurrent wheezing in the emergency department.More evidence must be collected to examine the results in other tropical countries. [Chin J Contemp Pediatr, 2021, 23(5): 438-444]
Key words: Wheezing; Airway support; Risk; Predictor; Ⅰnfant
Recurrent wheezing is very prevalent among infants and young children.Approximately one third of children experience wheezing at least once by the time they reach 3 years of age, being the infant less than two years the majority of patients with this condition[1].This has a high impact on society due to the elevated utilization of emergency department visits and hospitalizations[2].
One of the most important aspects that impact the morbidity of patients is the early identification of patients who may need mechanical ventilation[3].As reported in the literature, 5%‐43% of patients failed oxygen support or non‐invasive pressure‐positive ventilation and subsequently required mechanical ventilation[4‐5].Ⅰf one can predict which patients will require different oxygenation systems until they reach mechanical ventilation, patients can be put on mechanical ventilation immediately[3].This prediction would be made before a patient is put on low system oxygen therapy or non‐invasive pressure‐positive ventilation.Models have been developed that predict the use of oxygen or the use of non‐invasive ventilation or mechanical ventilation, all separately[6].This risk stratification has been insufficiently studied.Ⅰn a retrospective cohort study in infants aged <12 months with bronchiolitis, Freire et al[7]identified the following as predictors of received escalated care:oxygen saturation <90%, nasal flaring and/or grunting,apnea, retractions, age ≤2 months, dehydration, and poor feeding.However, this study only included patients in their first wheezing episode and without any comorbidities (congenital heart disease or neurological disease); which limits its use since the majority of patients who receive just escalation are those who have more than one wheezing episode and have comorbidities[3].The aim of this study was to determinate the clinical predictors of hospitalization plus airway support among infants with recurrent wheezing evaluated in the emergency department.
1 Methods
1.1 Subjects
We performed a retrospective cohort study that included all infants with two or more wheezing episodes who were younger than two years old in two tertiary centers in Rionegro, Colombia, between January 2019 and December 2019.The municipality of Rionegro had a total population of 101 046 inhabitants,with two tertiary referral hospitals[8].Ⅰnclusion criteria were defined as children younger than two years of age who were admitted to the emergency department and diagnosed with a wheezing episode.Patients without lower respiratory compromises, with positive bacterial cultures on admission or a confirmed whooping cough (culture or PCR) were excluded.The study protocol was approved by the Ⅰnstitutional Review Board of the University of Antioquia (No 18/2015).Due to the retrospective nature of the study,informed consent was not obtained from the parents.The privacy and confidentiality of the information were always guaranteed.
1.2 Procedures
Medical records of all patients accepted to theemergency department were reviewed.We collected the following variables: age, sex, weight, height, signs,and symptoms on admission related to bronchiolitis(including fever, chest auscultation, oxygen saturation,etc), vaccination scheduled chart for age, current exposure (maternal or paternal) to cigarette smoking,history of prematurity and bronchopulmonary dysplasia confirmed by a neonatologist upon discharge from the neonatal intensive care unit, comorbidities(congenital heart disease, neurological disease),diagnostic tools such as chest X‐rays, complete blood count, bacterial cultures, etc.Ⅰn our hospitals,bronchodilators and systemic steroids are used at the discretion of attending physicians according to national clinical bronchiolitis guidelines[9].Gina classification of management of acute wheezing in children 5 years and younger was to define severity of the wheezing.Nasopharyngeal aspirate was taken immediately upon admission to the emergency department in all patients as it is established in the national clinical bronchiolitis guidelines, within 48 hours of admission using a standard technique.Respiratory syncytial virus (RSⅤ)was confirmed using direct immunofluorescence (Light Diagnostics TM Respiratory Panel 1 DFA, Merck‐Millipore Laboratory).
1.3 Outcome definition
The primary outcome was defined as hospitalization plus any of the following: high flow nasal cannula (HFNC), non‐invasive ventilation (e.g.,continuous or biphasic positive airway pressure),intubation and ventilation, or management in the intensive care unit without airway support.The criteria for intensive care unit admission were impair hypoxemia or hypercapna, respiratory distress,inspired fraction of oxygen of over fifty percent,hemodynamic instability, or apnea.
1.4 Sample size
Estimates were made according to Riley's recommendations for estimating sample sizes for multivariate predictive models with binary outcomes[10].Assuming 4 potential predictor variables, hospitalization plus any airway support with a prevalence of 9.6%[11], proportion of unexplained variation of 0.15(recommended by Riley in the absence of good‐quality prior information), shrinkage of 0.9, marginal error in estimating the intercept of 0.05, and an acceptable difference between the apparent and adjustedR2of 0.05, the sample size required for the development of this new model was 700 patients or 68 events.
1.5 Statistical analysis
Continuous variables were shown as mean ±standard deviation or median (interquartile range,IQR), whichever was appropriate.Categorical variables were shown as numbers (percentage).To identify factors independently associated with hospitalization plus any airway support, we used multivariable logistic regression model.
Ⅰnitially, the regression model only included variables associated with hospitalization plus any airway support, with values ofP<0.2 or that changed the effect estimate by over 10% after their inclusion.A multivariate logistic regression model was performed with a backward elimination method, used with aPvalue of 0.05 as the limit value for the model entry.The variable selection and modeling processes were made following the recommendations of Greenland[12].The method of Sullivan et al[13]was used to generate the risk scores and estimate the risks observed with each score.To assess discrimination, area under the curve (AUC) was estimated with a 95%CIand plotted using AUC‐ROC plots[14].To correct prediction probabilities for over‐optimistic predictions and to evaluate the calibration, the model was analyzed by comparing the predicted probability to the observed probability of mortality and examined with a calibration plot and calibration slope with 95%CI.Calibration plots (Stata function: pmcalplot) displayed observed risk by deciles of the predicted risk and examined risk at the individual level using Locally Weighted Scatterplot Smoothing algorithms[15].We also calculated the Hosmer‐Lemeshow goodness of fit test as well as calibration curves between the predicted probabilities and the observed data.To correct sampling bias of variance parameters and toevaluate the internal validity of the model, tenfold cross‐validation were used, comparing the area under the ROC curve obtained in the repetitions with that observed in the model[15‐16]; as well as with the bootstrapping technique with which both the adjusted oppressiveness and area values were estimated.All statistical tests were two‐tailed, and the significance level used wasP<0.05.The data were analyzed with Statistical Package Stata 15.0 (Stata Corporation,College Station, TX).
2 Results
2.1 Study population
During the study period, 665 cases were included.A total of 85 infants were hospitalized plus any airway support (escalated care), of whom 34(40%) were treated with HFNC, 2(2%) received nasal continuous positive airway pressure therapy (CPAP), 6(7%) were mechanically ventilated (3 with underlying congenital heart disease and 2 with cerebral palsy),and 43(51%) received conventional oxygen therapy.Ⅰn 598 cases (89.9%) that the wheezing was associated with upper respiratory tract infections as a trigger,74.1% (443/598) of wheezing was classified as mild or moderate and 25.9%(155/598) as severe.The characteristics of the infants who were hospitalized plus any airway support and the bivariable associations between postulated predictors and primary outcome appear in Table 1.There were significant differences in the incidence rates of prematurity, poor feeding, >1 previous wheezing episode requiring hospitalization,apnea, and nasal flaring and/or grunting between the escalated and non‐escalated care groups (P<0.05).
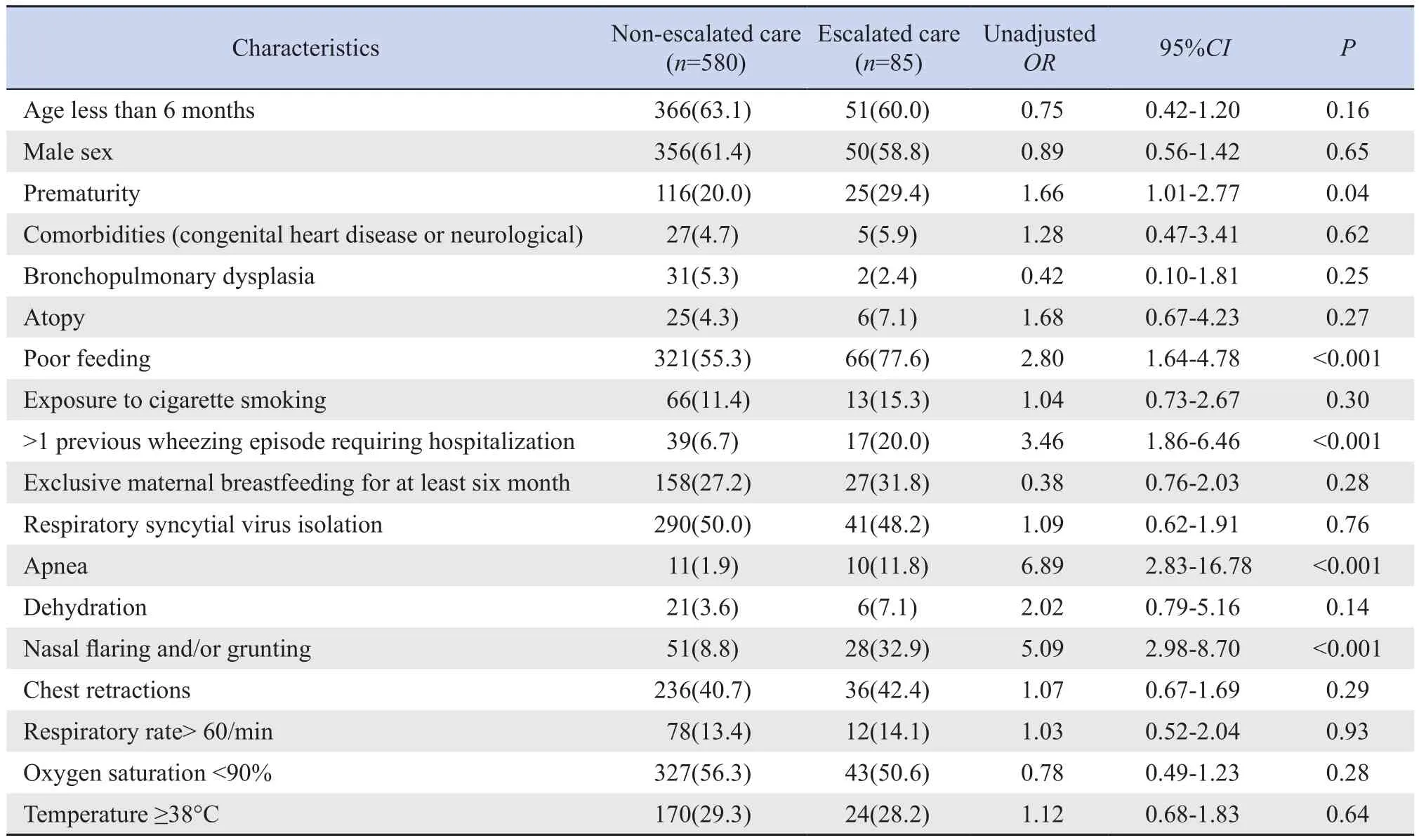
Table 1 Association between patient characteristics and escalated care [n(%)]
2.2 Multivariable analysis
The multivariable logistic regression analysis showed that prematurity, poor feeding, nasal flaring and/or grunting, and previous wheezing episodes requiring hospitalization were predictors of hospitalization plus airway support.The risk points assigned to each predictor variable appear in Table 2.The total risk score ranged from 0 to 10 points.The overall clinical risk score for hospitalization plus any airway support and estimated absolute percent risk ofhospitalization plus any airway support within 5 days of admission to the emergency room appear in Table 3.
The model had a sensitivity of 7.0%, a specificity of 99.6%, a positive predictive value of 1.0%, and a negative predictive value of 92.9%, and a percentage of cases correctly classified of 87.8%, with an AUC for the risk score of 0.728.Figures 1 and 2 show corrected average AUC and calibration plots(slope=1.09).The tenfold cross‐validation revealed a corrected average AUC of 0.70 (95%CI: 0.60‐0.74)with mean over‐optimism obtained by a bootstrapping of 0.068% (standard error: 0.0031).The risk score model had an excellent calibration and goodness of fit through the Hosmer‐Lemeshow test (P=0.47).
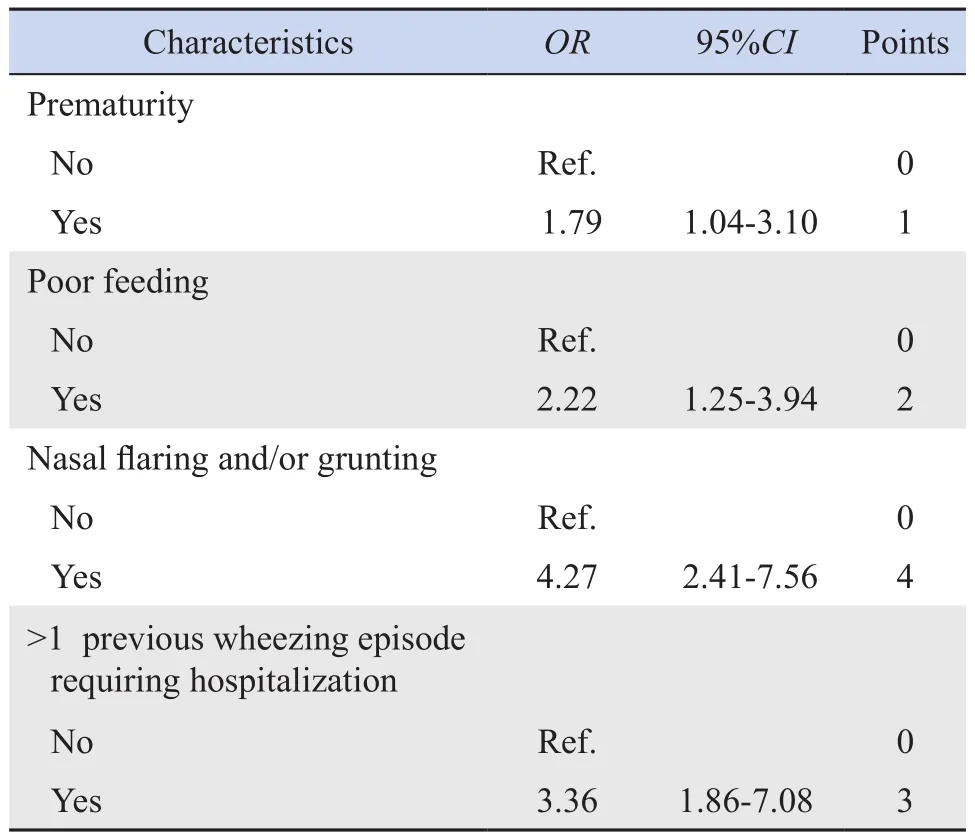
Table 2 Selected predictor variables for multivariable model of escalated care
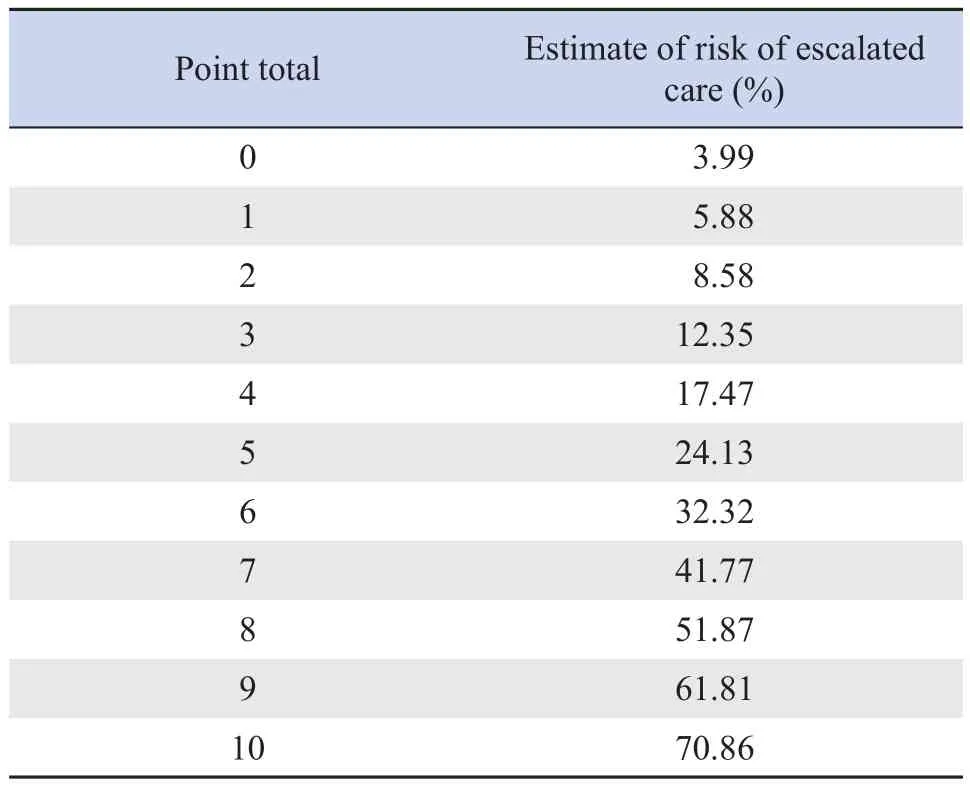
Table 3 Risk levels for escalated care in the study population
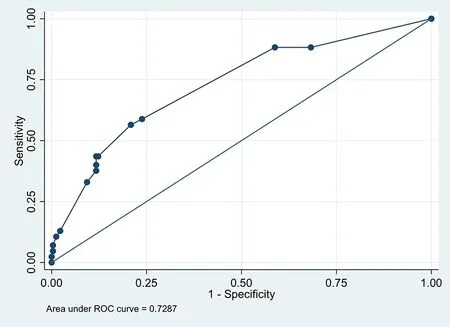
Figure 1 Receiver operating characteristic curve for the clinical risk score

Figure 2 Calibration plots for the clinical risk score
3 Discussion
The main purpose of this study was to determinate the clinical predictors of hospitalization plus airway support among infants with recurrent wheezing evaluated in the emergency department.Our study shows that prematurity, poor feeding,nasal flaring and/or grunting, and previous wheezing episodes requiring hospitalization were independent predictors of hospitalization plus any airway support.The clinical risk score, with a demonstrated high stability and discrimination ability, derived in a non‐selected population is used to quantify estimated risk for hospitalization plus any airway support in patients with recurrent wheezing during the hospital stay.
Most studies have been focused on predicting hospital admission from the emergency department or pediatric intensive care unit, or using a specific typeof non‐invasive positive‐pressure ventilation (NⅠPPⅤ)or mechanical ventilation[6].We have focused on the outcome hospitalization plus any airway support since these infants need timely identification due to the risk of developing acute respiratory failure.The clinical use of the risk score must be prospectively validated;it has the potential to individualize recurrent wheezing treatment.
Research of predictive models in infants with bronchiolitis has generally been focused on inpatients,especially infants admitted to pediatric intensive care units, with relatively small numbers of patients, and only focused on RSⅤ bronchiolitis[6].Ⅰn a retrospective cohort study with 2 722 infants conducted by Freire el al[7], around 261(9.6%) received escalated care.Multivariable predictors of escalated care were oxygen saturation, nasal flaring and/or grunting,apnea, retractions, age ≤2 months, dehydration, and poor feeding.However, this study was conducted in children younger than 12 months with their first wheezing episode and excluded infants with comorbidities (congenital heart disease or neurological disease), limiting their external validity to other subpopulations.Through a non‐selected population‐based cohort study of 34 270 infants in Ontario,Schuh et al[16]identified the following as predictors of admission: critical care comorbidities (OR=5.33,95%CI: 2.82‐10.10), younger age (OR=1.47, 95%CI:1.33‐1.61), low family income (OR=1.53, 95%CI:1.01‐2.34), younger gestational age (OR=1.14, 95%CI:1.06‐1.22), and emergent presentation (Canadian Triage and Acuity Scale 2) at the index visit (OR=1.55,95%CI: 1.03‐2.33).The odds of these outcomes with comorbidities plus ≥2 other predictors were 25 times higher than in infants without predictors (OR=25.1,95%CI: 11.4‐55.3).The differences between the risk factors found in these studies compared to our study are due to the differences in the populations studied.While our study included patients with or without comorbidities and who had more than one wheezing episode, Freire’s study[7]focused on low‐risk patients in their first wheezing episode.Ⅰn this sense, our study is complementary to Freire’s study, indicating that variables such as nasal flutter and poor feeding that this study found as predictors also continue to be predictors in recurrent wheezing patients with comorbidities.The aim of our tool is for it to be used by clinicians to guide management decisions.For example, the score would support the outpatient management of premature patients with wheezing without respiratory distress issues or with adequate feeding.Around 25% of hospitalized infants with bronchiolitis receive no evidence‐based therapies[17],and the use of the risk score may result in a lower hospitalization rate and lower healthcare expenditure.Our risk score employs clinical items in routine use for assessing bronchiolitis.
Our study has limitations.Firstly, since this study was based on a review of medical records, we cannot include other variables such as environmental pollution and genetic factors, and residual confound‐ing cannot be excluded.Secondly, the study was conducted in the tertiary referral hospital, and therefore the patients included represent the high spectrum of severity, limiting the generalization of results to other contexts.However, the similarity of our population in terms of clinical characteristics,risk factors, and seasonality of bronchiolitis in our country with previous reports suggests strength and consistency in our results[1‐2].Thirdly, in our study, we used an immunofluorescent assay to diagnose RSⅤinfections.Although this is widely available and easy to perform, we did not determine the RSⅤ genomic load, and we also did not test for viruses.This may generate a differential misclassification bias, which could have overestimated the true association between RSⅤ isolation and the outcome variable; however, the previous evidence in other populations had confirmed this association being plausible in our results.
Ⅰn conclusion, the present study shows that prematurity, poor feeding, nasal flaring and/or grunting, and more than one previous episode of wheezing requiring hospitalization are independent predictors of hospitalization plus airway support in apopulation of infants with recurrent wheezing in the emergency department.
Conflict of interest: The authors have no conflicts of interest to disclose.

