Effect of proteolytic starter culture isolated from Chinese Dong fermented pork(Nanx Wudl)on microbiological,biochemical and organoleptic attributes in dry fermented sausages
Xi Chen,Ruifang Mi,Biao Qi,Suyue Xiong,Jiapeng Li,Chao Qu,Xiaoling Qiao,Wenhua Chen,Shouwei Wang,∗a
bBeijing Key Laboratory of Meat Processing Technology,Beijing 100068,China
Keywords:
Staphylococcus xylosus
proteolytic activity
Lactobacillus plantarum
Nanx
Wudl
fermented sausage
ABSTRACT
The effect of a proteolytic starter culture isolated from Nanx Wudl,on microbiological,biochemical and organoleptic attributes of dry fermented sausages was investigated during processing.Based on preliminary screening,the combination of Staphylococcus xylosus SX16 and Lactobacillus plantarum CMRC6,showing excellent proteolytic activity in vitro,was selected as the multi-strain starter(starter LS).For comparison,the single-strain starter culture of L.plantarum CMRC6(starter LB)and non-inoculated control were also tested.During fermentation,lactic acid bacteria and staphylococci dominated the microbiota and suppressed the Enterobacteriaceae growth in LS-inoculated sausages.The addition of LS starter accelerated acidification and proteolysis during ripening,improving the contents of total free amino acids and several essential free amino acids(Phe,Ile and Leu).Volatile compounds analysis revealed that LS-fermented sausage showed the highest abundance of 3-methyl-1-butanol,probably due to the inoculated S.xylosus.The inoculation of LS starter improved the sensory properties of sausages,especially the flavor attribute.Therefore,S.xylosus SX16 and L.plantarum CMRC6 are promising candidates for inclusion as multi-strain starters in the manufacture of gourmet fermented dry sausage.
1.Introduction
Fermented dry sausages have been produced in many countries.Nowadays,consumers are becoming increasingly aware of these products for their unique sensory properties and specific health benefits[1–4].Fermented sausages usually consist of pork/beef meat,fat,salt and spices.Under certain temperature and relative humidity conditions,complex reactions take place leading to specific color,texture and flavor development of fermented sausages[5].However,traditional fermented sausages generally undergo spontaneous fermentation by empirical methods,thus the quality of the products cannot be guaranteed.
For product safety and quality consistency,starter cultures are now commonly used in fermented sausage production[6].Meat starter preparations consist mainly of microorganisms with desired metabolic activity[7].In fermented sausage,lactic acid bacteria(LAB)are considered as the most important contributor in the suppression of pathogens through their antibacterial metabolites,including organic acids,bacteriocins and hydrogen peroxide[8].Coagulase-negative staphylococci(CNS)contribute to the limitation of lipid oxidation and formation of nitrosomyoglobin,favoring the development of typical red color with their nitrate-reducing ability[9].SeveralStaphylococcusspecies,such asStaphylococcus xylosus,also participate in aroma development through their proteolytic and lipolytic activities.During ripening,proteolysis is responsible for the sensory and nutritional properties of fermented meats[10].Many studies over the last decade suggested that protein hydrolysis is not only attributed to the activity of endogenous meat enzymes,but also related to the activity of some bacterial groups,such asStaphylococcus[11–15].However,the suc-cess ofStaphylococcusin proteolysis during meat fermentation is affected by lactic acid bacteria[16,17].Therefore,a good interaction(no inhibition or mutualism)between LAB andStaphylococcusshould be considered in screening multi-strain starter cultures[18].According to Tremonte et al.[19],the mixture ofS.xylosuscombined withLactobacillus sakeishowed a stronger degradation effect on sarcoplasmic protein thanS.xylosuscultured alone,suggesting a relationship between the microbial interactions and the proteolytic activity of staphylococci.Yet,the microbial interactions in mixed culture fermentation and effects on proteolytic behavior of the staphylococci,and the subsequent influence on the quality of fermented slices have been ignored.
Microorganisms isolated from traditional meat products are promising candidates for meat starter culture because of their good adaptability to the ecological niche of fermented meats[20].Moreover,LAB and CNS isolated from spontaneous fermentation may offer distinct and attractive aromas based on their specific metabolic activities[21,22].Nanx Wudl is a Chinese fermented pork product popular in Dong,Tujia,and Miao nationalities[23].The natural fermentation favors indigenous microbiota growth,improving the microbiological safety and flavor attribute of the final product[24–26].Therefore,the use of bacteria isolated from Nanx Wudl could conceivably improve organoleptic properties and shelf-life of fermented meats,such as fermented sausage.
In order to obtain the proteolytic starter culture,S.xylosuswith protease activity were isolated from Nanx Wudl.LAB used in this study were isolated from Nanx Wudl as previously reported[27].Based on the proteolytic behavior of the mixed culturein vitro,a starter formulation was developed includingS.xylosusSX16 andLactobacillus plantarumCMRC6.Therefore,the present study aimed to obtain proteolytic meat starter that is suitable for producing fermented sausage and provide useful information for an appropriate choice of multi-strain starters.
2.Materials and methods
2.1.Nanx Wudl sampling
Ten different Nanx Wudl samples were collected from smallscale facilities in the Dong ethnic minority region of Guizhou province(China).These small-scale facilities used traditional manufacturing procedures,without the use of starter cultures.Samples were kept at 4◦C and analysed within 4 days.
2.2.Screening of proteolytic staphylococci
The strains of staphylococci were isolated according to Cachaldorai et al.[28].Brie fly,10g sample was aseptically taken from Nanx Wudl,transferred to a sterile bag containing 90mL physiological saline(0.85%NaCl)and homogenized at 500r/min for 2min in a SCIENTZ-11 stomacher(Scientz Biotechnology,China).The homogenate was spread-plated in duplicate on MSA and incubated at 37◦C.After 48h of incubation,up to 10 colonies were selected from each plate and purified by streaking on MSA plate.The cell morphology,gram-stain,catalase,proteolytic and nitrate reductase activities of strains were tested.The 16S rDNA sequencing was carried out for identification of the isolates as described by Casaburi et al.[29].
2.3.Proteolytic starter culture screening and preparation
Two strains of LAB isolated from Nanx Wudl[27]were used in this study.The proteolytic behavior of mixed starters was detected in the sarcoplasmic protein extract(SPE)[19],inoculated with different combinations of staphylococci and lactic acid bacteria.After propagating twice in fresh NB medium(staphylococci)or MRS broth(LAB),each strain was incubated(106CFU/mL)in 20mL SPE with 1% glucose.For comparison,uninoculated SPE was incubated under the same conditions.After 0,48 and 96h of incubation,samples(2mL)were taken for assays.Protein degradation was detected by SDS-PAGE method[30].The experiments were performed on two independent batches.Lyophilized starter culture powders were prepared as previously described[27].
2.4.Sausage manufacture and sampling
The sausage formulation included pork meat(76%),pork fat(18%),glucose(1%),sodium chloride(2%),red wine(2%),black pepper(0.5%),potassium nitrate(0.005%),sodium nitrite(0.005%)and fresh garlic(0.49%).Three different formulation groups of sausage(20kg each)were manufactured as follows:(1)CG:control group(non-inoculated),(2)LB:starter LB(L.plantarumCMRC6)inoculated group,and(3)LS:starter LS(L.plantarumCMRC6 combined withS.xylosusSX16)inoculated group.Lyophilized powder of each strain was used for the inoculation(106CFU/g batter).After mixing for 15min in a vacuum tumbler,the meat batter was stuffed into 38mm cellulose casings.The processing temperature and relative humidity(RH)were as follows:30◦C for 2 days,(90 ± 3)%RH;the following 19 days,15◦C and(80±3)%RH.For sampling,three sausages were obtained from each treatment after 0,1,2,6,9,14,and 21 days.For the sensory evaluation,volatile compounds and free amino acids analysis,the sausages were obtained at the end of processing(day 21).
2.5.Microbiological analysis of sausages
After removing the casing,sample(10g)was aseptically taken from the center of sausage.Each sample was placed in a sterile bag containing 90mL physiological saline(0.85%NaCl)and homogenized.The homogenate was poured in duplicate on different growth plate.LAB,staphylococci andEnterobacteriaceaecounts were determined on MRS,MSA and VRBA medium,respectively.The enumeration was performed at 37◦C after 48h of incubation except forEnterobacteriaceaeafter 24h.
2.6.Physical and chemical analysis of sausages
Water activity(aw)of sausages was determined at 25◦C using a LabMaster hygrometer(Novasina,Switzerland).For the pH measurement,a SevenGoTMpH electrode(Mettler Toledo,Switzerland)was inserted directly in the sample.For color measurement,samples were cut into pieces and the values ofL*,a*andb*were obtained by a CR-400 colorimeter(Konica-Minolta,Japan).
The extraction of proteins was performed according to the method described previously[31].After the determination of protein concentration by the Biuret method,the assays were carried out using SDS-PAGE[30].The determination of free amino acids was performed using a fully automated analyzer L-8900(Hitachi,Japan)according to Chen et al.[32].
2.7.Volatile compounds analysis of sausage
The detection of volatile compounds was performed according to Montanari et al.[33]with some modifications.Minced sausage(3g)was placed in 10mL extraction vial.After heating for 30min at 45◦C,a solid-phase microextraction(SPME)fibre was introduced and held in the headspace of the vial for 30min.Subsequently,adsorbed molecules were desorbed in GCMS 1310/TSQ8000(Thermo Fisher Scientific,USA)for 5min at 230◦C.Volatile molecules were separated in a DB-WAX capillary column(30m×0.25mm×0.25 um).The oven temperature was programmed as follows:40◦C for 3 min,raised to 50◦Cat10◦C/min,heated to 120◦C at 4◦C/min,finally raised to 230◦C at 12◦C/min,and kept for 8min.The C5–C20saturated alkanes(Sigma-Aldrich,USA)were used to determine retention index(RI).The identification of volatiles was conducted by comparing the RI with those in the NIST 11 mass spectral library.Semi-quantification of each volatile compound was performed using 2-octanol(Sigma-Aldrich,USA)as the internal standard.The heatmap was generated using a programming tool(HemI,version 1.0).
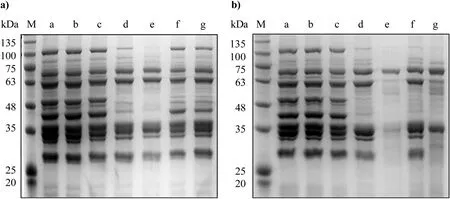
Fig.1.Electrophoretic profile of sarcoplasmic proteins inoculated with different strains after 48h(a)and 96h(b)of incubation.Lane M:prestained protein marker;lane a:non-inoculated sarcoplasmic protein at 0h;lane b:non-inoculated sarcoplasmic protein at 48 or 96h;lane c:L.plantarum CMRC6;lane d:S.xylosus SX16;lane e:L.plantarum CMRC6 combined with S.xylosus SX16;lane f:S.xylosus SX2;lane g:L.plantarum CMRC6 combined with S.xylosus SX2.
2.8.Sensory analysis
Twelve experienced panelists were chosen to evaluate the sensory properties.The evaluations were conducted in a sensory panel room at 25◦C.The sausage sample was cut into slices(3mm thick)and placed in a white plastic dish.The samples were blind-coded with 3-digit random numbers and evaluated three times.Unsalted crackers and water were used to cleanse the palate between samples.The differences in color,external appearance,firmness,and flavor attributes of sausage samples were evaluated using a continuous 7-point intensity scale as described previously[27].
2.9.Data analysis
Experiments were conducted in triplicate.Data analysis was performed using analysis of variance(one-way ANOVA)in SPSS Statistics 19.0 Core System(International Business Machines,USA).The results were expressed as the mean±standard deviation for each experimental group. Significant differences(P<0.05)between sample means were compared using Tukey’s test.
3.Results and discussion
3.1.Selection of proteolytic starter culture
A total of 58 g-positive staphylococci strains with catalase activity, were isolated from Nanx Wudl samples.The proteolytic activity was assessed by electrophoretic method,showing that 35 strains possessed the ability to hydrolyze sarcoplasmic proteins.Efficient nitrate-reducing staphylococci strains can be used to lower the amounts of curing salts,which is beneficial for human wellness[34].The nitrate-reducing ability of strains was measured at 15 and 30◦C using agar method,indicating that 35 proteolytic isolates exhibited nitrate reductase activity.Through genetic analysis based on 16S rDNA sequencing,20 strains were identified asS.xylosusspecies,whilst 15 strains identified as other staphylococci(S.equorum,S.sciuri,S.epidermidisandS.saprophyticus).
Besides coagulase-negative staphylococci,meat starter cultures frequently consist of lactic acid bacteria as acidifying components[7,35].Staphylococci fraction most often includeS.xylosus,whileL.sakeiandL.plantarumare generally representing the LAB group[7,36].In this study,L.plantarumCMRC6, which isolated from Nanx Wudl previously,was selected as the LAB fraction based on its technological characteristics[27].The proteolytic activity of mixed starters was assessed amongL.plantarumCMRC6 and 20 strains ofS.xylosus.After 96h of incubation,two strains ofS.xylosus(SX16 and SX2)in combination withL.plantarumCMRC6 showed a stronger degradation effect on sarcoplasmic protein thanS.xylosuscultured alone(Fig.1).L.plantarumCMRC6 could not hydrolyze sarcoplasmic protein,because its electrophoretic profile was identical to that of uninoculated protein.After 48h of incubation,S.xylosusSX16 hydrolyzed sarcoplasmic protein, showing a decreased intensity of protein fractions of about 100,63,48 and 40kDa.When this strain was co-inoculated withL.plantarumCMRC6,a complete disappearance of these protein bands was observed.On the other hand,S.xylosusSX2 showed some proteolytic activity,resulting in a decreased intensity of protein bands at 100,63,48 and 40kDa.WhenS.xylosusSX2 was co-cultured withL.plantarumCMRC6,the profile was not different from that of sarcoplasmic protein inoculated with SX2 alone until after 96h(Fig.1b).Therefore,a starter culture includingL.plantarumCMRC6 andS.xylosusSX16(starter LS), showing the highest proteolytic activityin vitro,was developed for the production of fermented sausages.For comparison,a singlestrain starter ofL.plantarumCMRC6(starter LB)and non-inoculated control were also tested.
3.2.Microbiological analysis of sausages
The microbial counts of LAB,staphylococci andEnterobacteriaceaeduring ripening was shown in Fig.2.Before fermentation(day 0),the LAB counts of the inoculated samples(>5.15 log(CFU/g))were significantly higher than that of control(4.35 log(CFU/g))because of the presence ofL.plantarumCMRC6.After 48h of fermentation,the CG,LB and LS sausages experienced a burst in LAB growth,and reached values of 7.51,9.13 and 9.05 log(CFU/g),respectively.During ripening,the LAB population in control sausage was significantly(P<0.05)lower than that of sausages fermented with starters. The results were similar to Chen et al.[27],confirming the fast growth rate ofL.plantarumCMRC6 and its good adaptability to the fermented sausage environment.
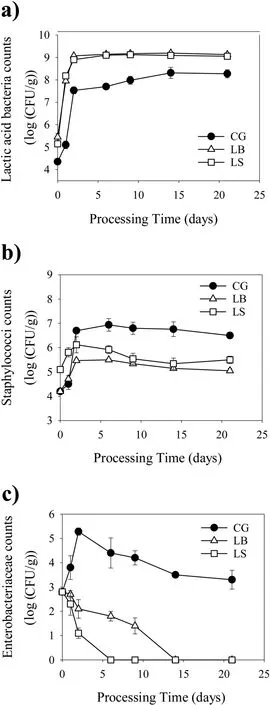
Fig.2.The viable counts of lactic acid bacteria(a),staphylococci(b),and Enterobacteriaceae(c)of sausages during ripening.CG:non-inoculated control sausage;LB:sausages inoculated with starter LB(L.plantarum CMRC6);LS:sausage inoculated with starter LS(L.plantarum CMRC6 combined with S.xylosus SX16).
Coagulase-negative staphylococci contributed to the development of color and flavor properties[13].As expected,the initial staphylococci count of LS-fermented sausages were higher(5.10log(CFU/g))than that in other sausages because of the inoculation ofS.xylosusSX16(Fig.2b).After 48h of fermentation,the staphylococci population was significantly higher in CG sausage than that of inoculated sausages probably due to the lower acidifying ability of the autochthonous microflora inoculated by chance.The results agreed with many reports that strongly acidification leads to the inhibition of pH-sensitiveS.xylosusin fermented sausages[16,17,37].
Enterobacteriaceaeare generally considered useful biomarkers of potential microbiological contamination during processing.As shown in Fig.2c,the initial level ofEnterobacteriaceaewas about 2.8 log(CFU/g).Within the first 48h,theEnterobacteriaceaepopulation in control sausage increased sharply(P<0.05),reaching 5.28 log(CFU/g),probably because of the lower acidifying ability of the autochthonous LAB.In contrast,a continuing population drop ofEnterobacteriaceaewas found in inoculated sausages during ripening.It is important to note that theEnterobacteriaceaein LS-inoculated sausage was not detected after 6 days of ripening,suggesting the high competitiveness of multistrain starters.Therefore,the selected multi-strain starter could dominate the microbial community during ripening,improving the safety of fermented sausages by suppressingEnterobacteriaceaegrowth.
3.3.Physicochemical properties of sausages
The pH change of sausages during ripening is displayed in Fig.3a.Initially,the pH level was about 5.90.After 48h of fermentation,the pH value of the LB and LS-fermented sausages declined sharply(P<0.05),whereas the CG sausage displayed a slight reduction.During ripening,the pH level of the control sample was significantly(P<0.05)higher than that in LB and LS-fermented samples.The accumulation of organic acids,mainly lactic acids produced byL.plantarumCMRC6,led to the pH decline of inoculated sausages.The result was similar to other research that inoculated sausages showed lower pH values because of the strong acidifying activity of selective LAB starters[39].However,the occurrence of autochthonous LAB with low acidifying ability gave higher pH values in non-inoculated control.It is believed that the growth of LAB at the beginning of processing favors a rapid pH drop of sausages,contributing to the suppression of pathogens and undesirable microorganism,such asEnterobacteriaceae[8,40].The increase of pH values during the latter stage(day 14)could be attributed to proteolytic degradation,which generated peptides,free amino acids and amines[41].
Fig.3b depictsawof all the sausages during ripening.The initialawvalues of all samples were 0.96.Over the entire ripening period,theawvalues of all sausages gradually declined because of water loss.Throughout ripening,no significant difference was found amongawvalue of control and inoculated samples.After 21 days of ripening,theawvalues of CG,LB and LS batches were 0.86,0.86 and 0.88,respectively,favoring the inhibition of pathogens and undesirable bacteria.Dalmıs¸et al.[42]reported that different processing parameters like relative humidity and ripening period could influence theawof sausages,rather than addition of starter cultures.
3.4.Color of sausages
Color plays an important role in consumers’acceptability to fermented sausages[43].Fig.4a shows theL*values of sausages throughout ripening.Within the first 48h,theL*values of all sausage exhibited a significant increase(P<0.05),then declined steadily probably due to water loss[44].After 21 days of ripening,no significant difference was found betweenL*values of control and inoculated samples.During ripening,thea*value of control sausage increased slightly,while that of inoculated sausages increased rapidly(P<0.05).After 21 days of ripening,thea*values of LB and LS-fermented sausages were significant(P<0.05)higher compared to non-inoculated CG.Staphylococci and lactic acid bacteria are involved in nitrite degradation to nitric oxide,favoring the formation of nitrosomyoglobin,thereby intensifying the red color of inoculated sausages[27,45,46].As shown in Fig.4c,theb*values of all sausages dropped steadily during ripening.The results agreed with other research that the oxygen content declined caused by consumption of microorganisms,leading to the reduction of oxymyoglobin and the decrease ofb*value[38].
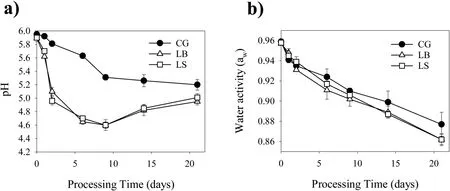
Fig.3.The pH(a)and water activity(b)of sausages during ripening.
3.5.Proteolysis of sausages
The sarcoplasmic and myofibrillar protein degradation was assayed by electrophoretic method.Fig.5 shows the profiles of the sarcoplasmic proteins.In inoculated sausages,a total disappearance of the protein fraction of about 100kDa was observed after 2 days of ripening,and sarcoplasmic profiles of these sausages displayed a complete degradation of the bands ranging from 35 and 55kDa after 6 days of ripening.It is worth noting that,the protein fraction of about 25kDa disappeared after 9 days in LS-inoculated sample compared to 14 days in samples inoculated with starter LB.This could be explained by the more pronounced proteolytic activity of starter LS,with effects on mainly the sarcoplasmic proteins degradation(Fig.1).During ripening,degradation of large proteins originated lighter protein fractions in the range of 15 and 25kDa in inoculated sausages after 2 days of ripening.In contrast,the electrophoretic pattern of the sausage in CG group did not show significant change except an increased intensity of the bands between 15 and 25kDa after 6 days of ripening.
Fig.6 depicts the profiles of myofibrillar proteins.Over the ripening period,the intensity of the myosin heavy chain(MHC)fraction at about 200kDa declined and a complete disappearance was observed in LS-inoculated sausage after 6 days of ripening,while the total disappearance of this band was found in LB-fermented sausages after 9 days.The actin band in the range of 35 and 55kDa was also affected by different starter cultures because it disappeared after 6 days in LS-cultured sample compared to 9 days in LB-cultured samples.In contrast,the intensity of MHC and actin fractions in control sausages was maintained and decreased slightly after 21 days of ripening.During ripening,the intensity of the protein fractions between 25 and 35kDa increased in all formulation treatments,probably because of the release of the peptides originating from MHC hydrolysis[15,42,47].
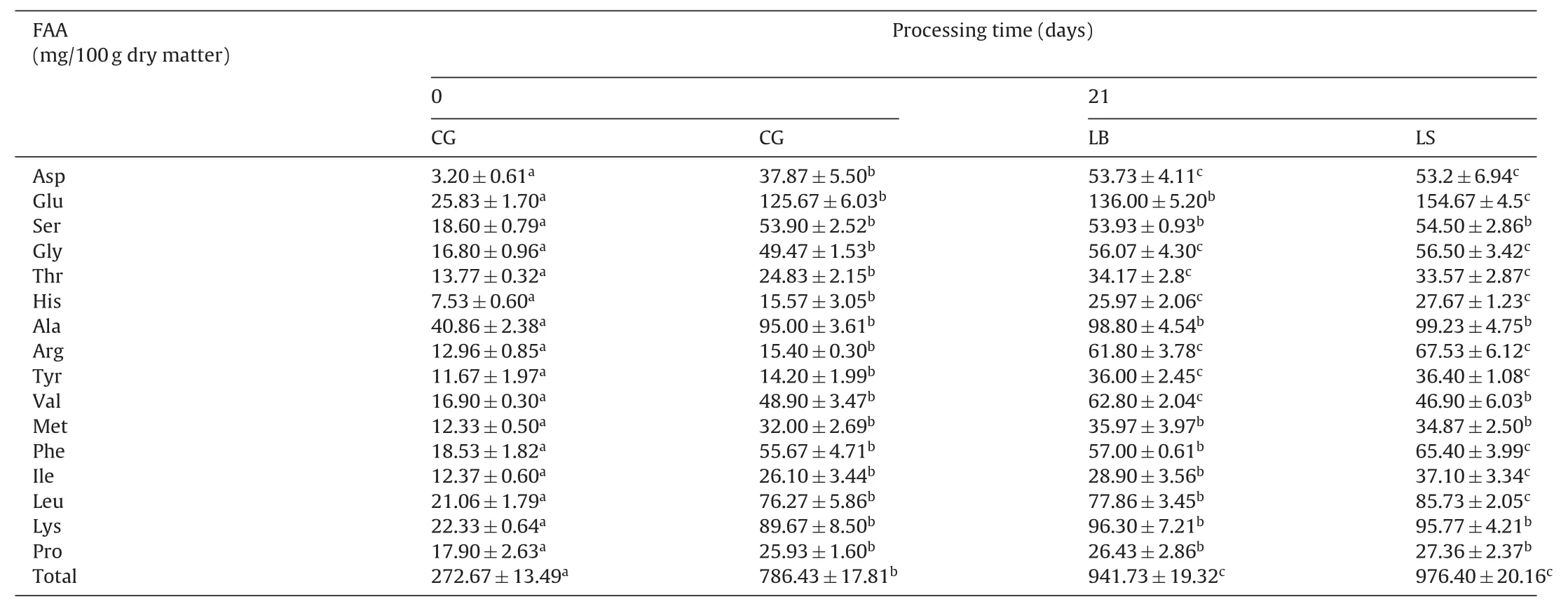
Table 1Free amino acids(FAA)composition of sausages after 21 days of ripening.

Fig.4.The lightness(a),redness(b),and yellowness(c)of sausages during ripening.

Fig.5.Electrophoretic profile of sarcoplasmic proteins during ripening.CG sausages(a),sausages inoculated with starter LB(b)and LS(c).Lane M:prestained protein marker;lanes 1-7:sarcoplasmic proteins at 0,1,2,6,9,14 and 21 days of ripening.
In this study,sausages cultured with LB and LS starters showed similar protein patterns of the sarcoplasmic and myofibrillar proteins during ripening,which could be attributed to the activation of endogenous proteases by the decline in pH.In fact,there was no significant difference between the pH levels of LB and LS-fermented sausages during ripening,leading to the activation of endogenous proteases and similar proteolysis profiles in the two types of sausages.Another explanation may be the short processing time(21 days)used in this study.It is reported that the action of the endogenous proteases contributes to the degradation of meat proteins at the beginning of fermentation,while bacterial proteases play an important role during latter stages of processing[48,49].
3.6.Free amino acids of sausages
Table 1 summarized the specific concentration of free amino acids after 21 days of ripening.The total free amino acids of CG,LB and LS samples increased significantly(P<0.05),reaching levels of 786.43,941.73 and 976.40mg/100g sausage,respectively.The increase in total free amino acids of fermented sausages during ripening has been reported by some studies[41,50].After 21 days of ripening,the LB and LS-fermented samples showed higher total free amino acids content levels(P<0.05)than non-inoculated CG,suggesting that the selected strains were involved in the production of free amino acids.Therefore,total free amino acids was affected by addition of starter cultures in fermented sausages.However,there was no significant difference between total free amino acids concentrations of LB and LS-inoculated sausages,which was similar to the studies of Wang et al.and Du et al.[50,51].Among the 16 free amino acids determined in control and inoculated sausages,those found in the highest abundance were glutamic acid,contributing to fresh taste,followed by alanine,associated with sweet flavor[49,52].The LS-fermented sausage had a greater increase in essential free amino acids(Phe,Ile and Leu)compared to other sausages.Therefore,the proteolytic activity ofS.xylosuscould contribute to the generation of the free amino acids. It is worth noting that,different processing parameters like relative humidity and temperature will affect biochemical reactions driven by the micro flora,which could further affect specific free amino acids,making it difficult to compare the results of different studies[53].

Fig.6.Electrophoretic profile of myofibrillar proteins during ripening.CG sausages(a),sausages inoculated with starter LB(b)and LS(c).Lane M:prestained protein marker;lanes 1-7:myofibrillar proteins after 0,1,2,6,9,14 and 21 days of ripening.
3.7.Volatile compound profile
After 21 days of ripening,the identification and quantification of volatile compounds were performed.A total of 50 volatiles were identified in control and inoculated sausages(Fig.7),including alcohols(9),aldehydes(16),ketones(3),esters(5),acids(7),terpenes(4),sulphur and nitrogen compounds(4)and others(2).Most volatiles identified in fermented sausages have been reported by many other studies[54–56].Table S1 summarized the specific contents of volatiles.In this study,volatiles were studied in terms of their most probable origin to understand the effect of starter cultures on aroma generation pathways.However,some volatiles may have more than one source or be the products of secondary reactions between substances derived from different metabolic pathways[36].Nearly 21%-52% of compounds originated from spices such as black pepper used in sausage preparation.The most abundant pepper terpenes identified in this study was 3-carene.Regarding the volatiles originating from lipid oxidation,the values of hexanal,nonanal,(Z)-2-heptenal,octanal,2,4-decadienal showed significant differences(P<0.05)between experimental treatments,suggesting the influence of starters on lipolysis and autoxidation rates.The volatiles originating from microbial esterification could exert important impact on the flavor attribute of fermented sausages.In this study,the most abundant was ethyl lactate,which exhibited significant differences(P<0.05)among treatments.It is important to note that 3-methyl-1-butanol was found only in LS-fermented sausages,probably due to the inoculation ofS.xylosusSX16.This compound contributes to the complexity of flavor and mainly originates from the leucine catabolism by CNS[56].In comparison withS.carnosus,a higher level of 3-methyl-1-butanol generated byS.xylosushas been reported previously[54],indicating the contribution ofS.xylosusSX16 to the flavor attribute of fermented sausages.
3.8.Sensory attributes of sausages
The sensory evaluation was conducted by the trained panel after 21 days of ripening(Table 2).The LB and LS-fermented sausages had significantly higher(P<0.05)scores compared to CG sausages in most sensory attributes(color,firmness and flavor).The higher color scores of inoculated sausages may be attributed to the nitrate and nitrite reductase activities of starters[57].Flavor attribute for sausages fermented with starters had higher(P<0.05)scores than control sausage,indicating that LB and LS-inoculated sausages would be more popular with consumers.It is important to note thatthe flavor score of LS-fermented sausage was higher than that of LB-inoculated sausage,which agreed with many other reports thatS.xylosusstrains participate in aroma generation and could give a“rounder” flavor to fermented sausages[34].Therefore,the strains ofS.xylosusand lactic acid bacteria isolated from Nanx Wudl are promising candidates for inclusion as multi-strain starters in the manufacture of gourmet fermented dry sausage.
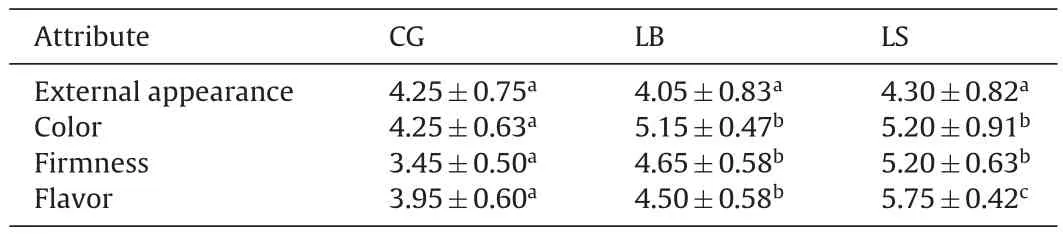
Table 2Sensory evaluation of sausages fermented by different starter cultures.
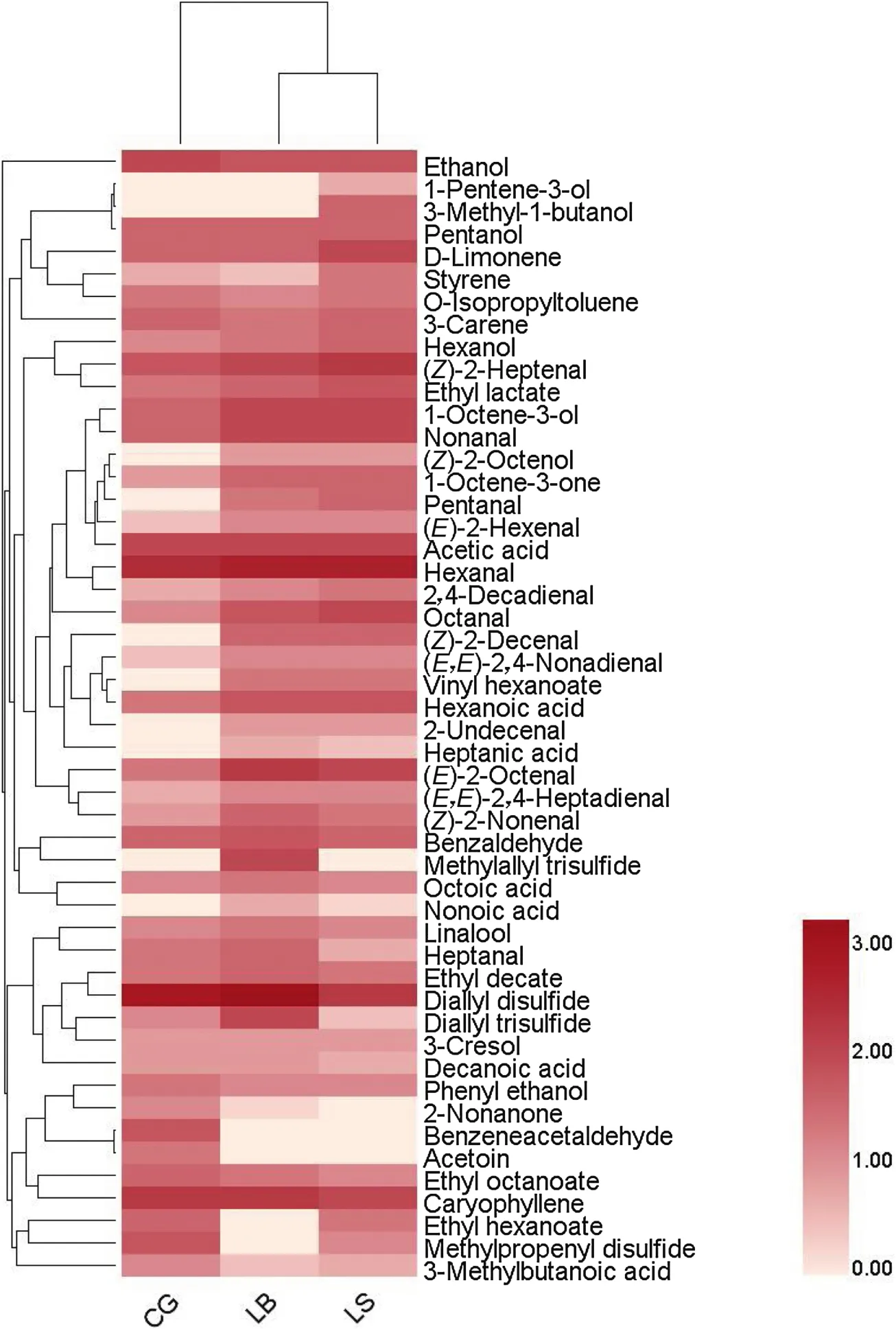
Fig.7.The content distribution of the volatile compounds in dry fermented sausages at the end of ripening.The clustering was performed with Pearson distance and complete method.The colors corresponded to normalized mean levels from low(white)to high(red).CG,LB and LS represented different starter formulation sausages.
4.Conclusions
To the best of our knowledge,for the first time,the present research investigated the effect of proteolytic microorganisms isolated from Nanx Wudl as promising candidates in the manufacture of fermented sausage. The use of themulti-strain starter includingS.xylosusSX16 andL.plantarumCMRC6 accelerated acidification and proteolysis during ripening,improving the microbiological safety and sensory attributes of fermented dry sausage.
Declaration of Competing Interest
The authors declare that no conflict of interest exits in the submission of this manuscript.
Acknowledgements
The authors acknowledge the financial support of the National Key R&D Program of China(grant no.2018YFD0400404).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.fshw.2020.05.012.
- 食品科学与人类健康(英文)的其它文章
- Effect of xanthan gum on the quality of low sodium salted beef and property of myofibril proteins
- Peptide fraction from sturgeon muscle by pepsin hydrolysis exerts anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via MAPK and NF-κB pathways
- Chronic consumption of thermally processed palm oil or canola oil modified gut micro flora of rats
- Hypoglycemic polysaccharides from Auricularia auricula and Auricularia polytricha inhibit oxidative stress,NF-κB signaling and proinflammatory cytokine production in streptozotocin-induced diabetic mice
- Simultaneous determination of 15 pesticide residues in Chinese cabbage and cucumber by liquid chromatography-tandem mass spectrometry utilizing online turbulent flow chromatography
- Characterization of spoilage bacterial communities in chilled duck meat treated by kojic acid

