Three Photochromic Co-crystals Based on Viologen Moiety
LIU Jin-Jian LIU Na LU Yi-Wei ZHAO Guo-Zheng( 04000)( 04004)
Abstract:The reactions of 1,2,4,5-benzenetetracarboxylic acid(H4BTEC)with the transition metal ions in the presence of neutral viologen moiety,1-carboxyethyl-4,4′-bipyridine(CEbpy),led to three isostructural co-crystals[M(H2O)5(BTEC)0.5]·(CEbpy)(M=Mn(1),Zn(2),Co(3))in an alkaline environment.Upon UV irradiation,these three compounds exhibited obvious photochromic behaviors owing to the formation of viologen radicals through photo-induced electron transfer.Meanwhile,compound 3 exhibited irreversible hydrochromic behavior upon heating,while compounds 1 and 2 did not.The influence of metal ions on their chromic behaviors has been discussed.CCDC:1953749,1;1954911,2;2032783,3.
Keywords:1-carboxyethyl-4,4′-bipyridine(CEbpy);co-crystals;photochromism;hydrochromism
Co-crystals,formed by two or more different molecular moieties through intermolecular non-covalent interactions,such as hydrogen bonding,van der Waals interactions and halogen bonding,have the advantages of easy processing,highly adjustable structures and performances[1].Drug co-crystallization has been widely studied as the strategy to improve the stability and physicochemical properties of drugs[2].In recent years,molecular co-crystal materials have attracted great interest because of their excellent optical functionality in many fields,such as fluorescent polarizer,chemical sensors,light-driven actuator and nonlinear optics[3].Therefore,the design and development of photofunctional co-crystals are of great significance for ad-justing and controlling the property.In this respect,the formation of co-crystals not only provides a platform for the synthesis of new photo-functional materials,but also provides an effective way to explore new chemical and physical phenomena[4].
In the past years,photochromic materials have received extensive attention not only due to their unique photophysical and photochemical properties,but also due to their potential technological applications ranging from chromic devices to clean energy[5].Among these photochromic materials,photoactive cocrystals have received more and more research interest in the construction of photo-functional system because of the vital role in changing the photo-related property of molecular solids[6].Viologens(4,4′-bipyridinium derivates)have excellent electron-accepting ability,and contain the charged bipyridinium moiety in the conjugation process,which have been widely used in the construction of chromic materials[7].Thus,as an effective component of the redox activity,viologens are ideal units to construct photochromic co-crystals due to the reversibility of electron transfer(ET)[8].Recently,we have reported some viologen coordination polymers(CPs)with different metal ions to adjust their photochromic behaviors,indicating that metal ions can regulate photo-induced intermolecular ET[9].However,the influence of metal ions on the viologen photochromic co-crystals has rarely been reported.Therefore,it is still a huge challenge to develop metal-controlled responsive co-crystals with adjustable performances.
Herein,we report three viologen photochromic materials with different metal ions based on the co-crystal formation.The neutral viologen derivate,1-carboxyethyl-4,4′-bipyridine(CEbpy)has been selected to act as the template.CEbpy has terminal nitrogen and oxygen atoms simultaneously serving as coordination sites,where one acetic acid group is introduced to 4,4′-bipyridine,which is usually used to construct viologen CPs and rare co-crystals[10].On the other hand,we used low cost,non-toxic and environmentally friendly metal ions,namely Mn,Znand Co,to coordinate with 1,2,4,5-benzenetetracarboxylic acid(H4BTEC)to form the[M(H2O)5(BTEC)0.5]component in an alkaline environment.The assembly of two molecular moieties guides the formation of co-crystals,[M(H2O)5(BTEC)0.5]·(CEbpy)(M=Mn(1),Zn(2),Co(3)),through the intermolecular non-covalent interactions.In addition to characterizing these three co-crystals,the photochromic behaviors of compounds 1~3 were also investigated.As expected,these three compounds exhibited photochromic behaviors,resulting from both photoinduced electron transfer(PET)and the formation of CEbpy radicals upon irradiation.In addition,compound 3 exhibited irreversible hydrochromic behavior because of dehydration,while compounds 1 and 2 did not.The influence of metal ions on their chromic behaviors has been discussed.
1 Experimental
All chemicals used in the synthesis were purchased from commercial sources without further purification.CEbpy was synthesized according to the literature[11].A Rigaku Ultima Ⅳ-185 diffractometer was used to collect powder X-ray diffraction(PXRD)patterns at 40 kV,40 mA with CuKαradiation(λ=0.154 06 nm)and a graphite monochromator scanning from 5°to 50°.A Vario EL ⅢCHNOS elemental analyzer was used to perform elemental analysis of C,H and N.FT-IR spectra(4 500~500 cm-1)was obtained by a Nicolet 5DX spectrometer by use of KBr pellets.A Varian Cary 5000 UV-Vis spectrophotometer was used to perform UV-Vis diffuse reflectance spectrum(DRS)at room temperature.A Bruker A300-10/12 spectrometer was used to record electron paramagnetic resonance(EPR)spectrum at room temperature.A ThermoFisher ESCALAB 250 X-ray photoelectron spectrometer(powered at 150 W)was used to perform X-ray photoelectron spectroscopy(XPS)by AlKαradiation(λ=0.835 7 nm;spot size,500 m).A HTG-3 equipment was used to perform thermogravimetric(TG)experiments in air between 30 and 800℃at a rate of 10℃·min-1.
1.1 Preparation of 1~3
The general procedures for preparations of compounds 1~3 were as follows:a mixture of Mn(CH3COO)2·4H2O for 1(24.5 mg,0.10 mmol)/Zn(CH3COO)2·2H2O for 2(22.0 mg,0.10 mmol)/Co(NO3)2·6H2O for 3(29.0 mg,0.10 mmol)and CEbpy(21.4 mg,0.10 mmol)was added into 10 mL water.Another mixture of NaOH(8.0 mg,0.2 mmol)and H4BTEC(12.7 mg,0.05 mmol)was heated to dissolve in 20 mL water,and filtrated.After mixing the above two mixtures and stirring for 10 min,the residue was filtered.The filtrate was allowed to stand for several days to give block crystals.Yield for yellow 1:42%,for colorless 2:35%,for orange 3:46%,based on CEbpy.IR(KBr pellet,cm-1):2 026,1 484,1 459,1 417,1 371,1 322,1 220,1 191,1 139,1 076,1 002,925,819,763,723,692,617,580,524.Anal.Calcd.for C17H21MnN2O11(compound 1,%):C 42.16,H 4.38,N 5.78;Found(%):C 42.37,H 4.31,N 4.50.Anal.Calcd.for C17H21ZnN2O11(compound 2,%):C 41.27,H 4.29,N 5.66;Found(%):C 41.47,H 4.32,N 5.45.Anal.Calcd.for C17H21CoN2O11(compound 3,%):C 41.81,H 4.34,N 5.74;Found(%):C 42.02,H 4.40,N 4.37.PXRD patterns were recorded to confirm the phase purity,and all main peaks were consistent with the simulated PXRD,indicating the high purity and homogeneity of these three compounds(Fig.S1,Supporting information).The thermal stabilities of 1~3 were investigated by TG analysis in a temperature range of 30~800℃ as shown in Fig.S2.
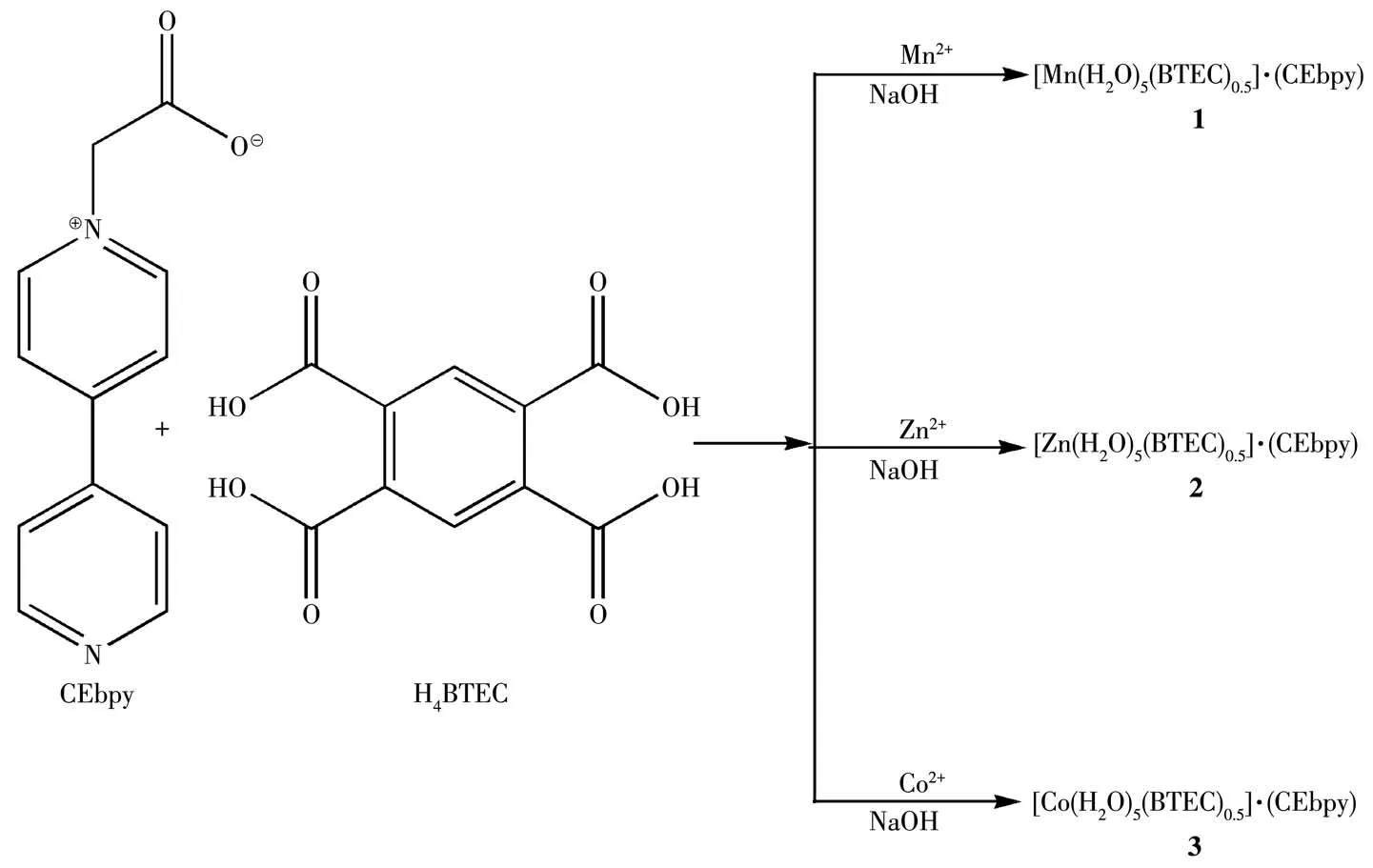
Scheme 1 Preparation of compounds 1~3
1.2 X-ray crystallorgraphy
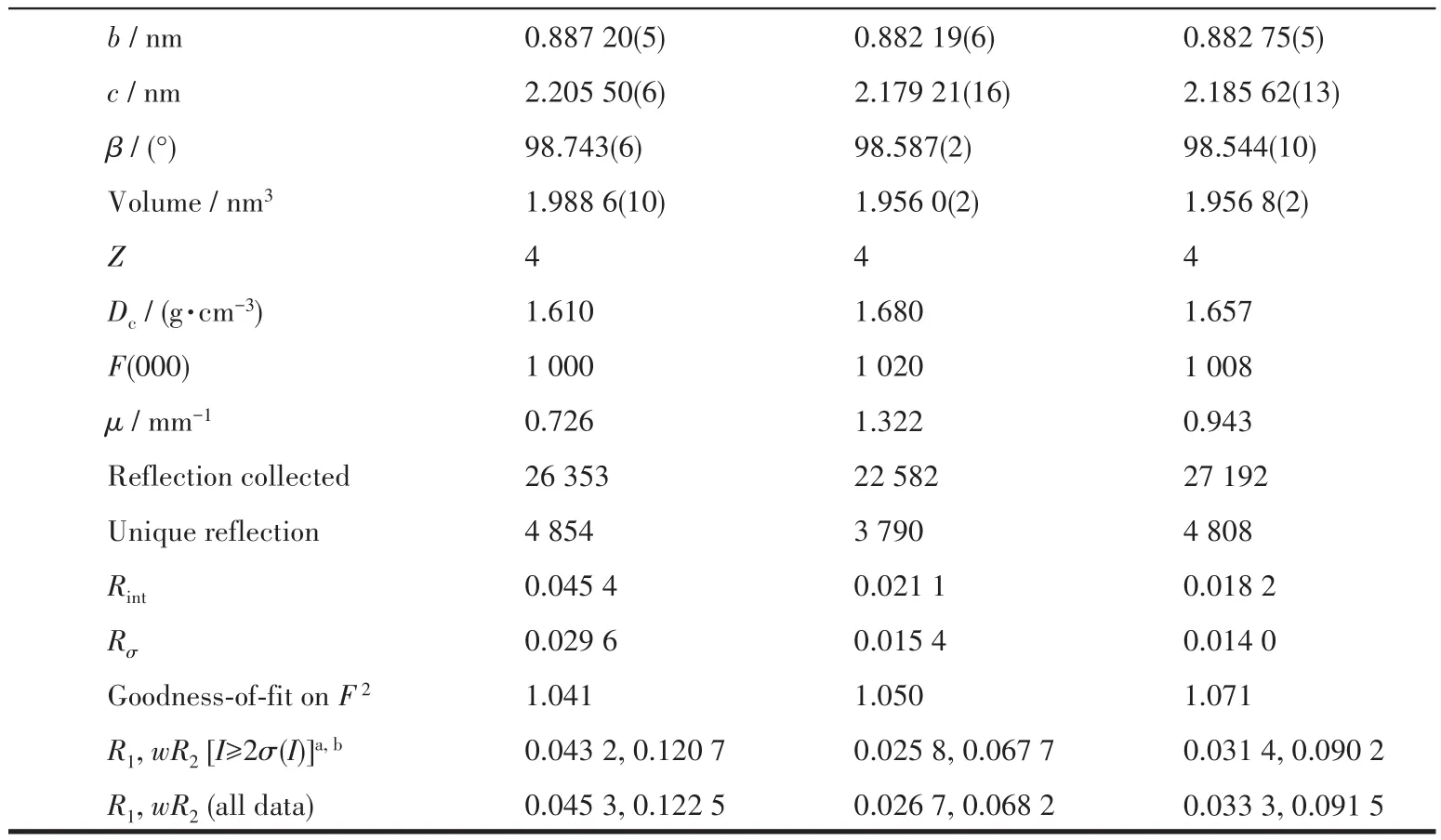
X-ray diffraction data of compounds 1~3 was collected on an Oxford Gemini diffractometer at 293 K using graphite monochrome MoKα(λ=0.071 073 nm).The SCALE3 ABSPACK scaling algorithm was used to perform empirical absorption correction of spherical harmonics[12].The SHELXTL-97 program was used to solve and refine the structure onF2by direct method and full matrix least squares technique[13].All nonhydrogen atoms were anisotropically refined.The crystallographic data of 1~3 are listed in Table 1,and the selected bond lengths and bond angles are listed in Table S1.

Table 1 Crystal data and structure refinement for 1~3
Continued Table 1
CCDC:1953749,1;1954911,2;2032783,3.
2 Results and discussion
2.1 Structure description
X-ray single crystal diffraction analyses reveal that the three compounds are isostructural and crystallize in the monoclinic system with the space groupP21/n.Because of the same structure for the three compounds,compound 1 was chosen as the representative of the discussion.The asymmetric unit of 1 contains one CEbpy molecule,one Mnion,half one coordinated BETC4-ligand and five coordinated water molecules.As shown in Fig.1,each Mnion is located in a distorted octahedral coordination sphere,which is surrounded by five water molecules and one oxygen atom from the BETC4-ligand to form the[Mn(H2O)5(BTEC)0.5]molecule.For the neutral CEbpy,the N and O atoms do not take part in the coordination process.In the three crystal structures,the CEbpy molecule binds to[M(H2O)5(BTEC)0.5]through O—H…N hydrogen bonds with H…N distances of 0.193 9,0.193 2 and 0.188 5 nm for 1,2 and 3,respectively(Fig.S3).The intermolecular O—H…N hydrogen bond interactions formed between CEbpy and coordinated H2O molecule immobilize the functionalized CEbpy moiety and enhance the stability of the structure.In addition,other intermolecular interactions including C—H…O and O—H…O hydrogen bonds promote the formation of the entire molecular aggregate.

Fig.1 X-ray crystal structure of 1 drawn with ellipsoids at 30% probability level
2.2 Photochromism
Compounds 1~3 containing the viologen moiety typically showed photochromic behavior owing to PET from the electron-rich group to the viologen moiety[14].As expected,all the three compounds are photosensitive.Compound 1 turned from yellow to blue(1P)within 10 min upon sunlight or UV irradiation(Hg lamp,365 nm,175 W)(Fig.2a),compound 2 changed from colorless to blue(2P)within 2 min upon sunlight or UV irradiation(Fig.2b),while compound 3 turned from orange to dark red(3P)within 30 min only upon UV irradiation(Fig.2c).The PXRD patterns of these three photoproducts matched the primary crystals,further proving that the photochromism is not photoisomerization or photolysis[15].When the photoproducts of them were bleached,their crystal structures also matched the original samples well(Fig.S1).We believe that this behavior should be attributed to the free radicals produced by PET.To confirm this supposition,the UV-Vis and EPR spectra were measured.The UV-Vis DRS spectra of three compounds all displayed two characteristic bands located at about 410 and 630 nm after irradiation(Fig.2a,2b and 2c),which are the typical characteristics of those photo-generated viologen radicals[16].The EPR spectra of these three compounds had strong signals(Fig.2d,2e and 2f):1 ofg=2.004,2 ofg=2.002,3 ofg=2.002.These signals were also consistent with those reported viologen radicals[17].All the characteristics indicate that CEbpy was reduced to CEbpy·-radicals upon irradiation,leading to the color changes.Prior to irradiation,characteristic bands of compound 2 were detected in the UV-Vis and EPR spectra(Fig.2b and 2e),which may be due to its strong photosensitivity.In synthetic 2,the production of viologen radicals may be caused by indoor photoactivation,which is indeed supported by the fact that atg=2.002.The narrow signal of CEbpy radicals was increased upon further UV irradiation.The photoproducts of three compounds can remain stable in the air,and the samples were left in the dark for about a week before the colors returned to their original states.In addition,the faded samples showed color changes after re-irradiation,indicating that the photo-induced coloration-decolorization processes of these three compounds are reversible.These reversible processes all can be repeated at least five times without significant color losses.

Fig.2 Photographs and UV-Vis DRS spectra before and after irradiation for 1(a),2(b)and 3(c);EPR spectra before and after irradiation for 1(d),2(e)and 3(f)
It is well known that for these viologen-based compounds,the distances and orientations between the donor and acceptor in the solid state play an important role in PET[18].In order to better understand the pathway of ET,the molecular stacking structures of these three compounds have been analyzed.Among the three photochromic compounds,electron-deficient pyridine groups are electron acceptors,and electron-rich carboxylate groups are widely reported as electron donors[19].The structural analysis shows that in compound 1,the distance of O1…N1 is 0.272 8 nm(Fig.S3a)and the angle of O1…N1…C2 is 61.07°;in compound 2,the distance of O5…N1 is 0.273 4 nm(Fig.S3b)and the angle of O5…N1…C7 is 60.84°;in compound 3,the distance of O1…N2 is 0.273 3 nm(Fig.S3c)and the angle of O1…N2…C2 is 61.08°.Such distances and orienta-tions are suitable for PET[20].It is reasonable to assume that CEbpy radicals originate from ET,from the O atom of the carboxylate group to the N atom of the pyridinium ring.So,when they are exposed to irradiation,the color changes happen.In addition,we have performed XPS test of 2 to identify the PET process.It showed that the core-level spectra of Zn2pand C1sdid not change before and after irradiation(Fig.3a and 3b),while the core-level spectra of O1sand N1swere significantly changed.The O1speak of 2 was located at 531.06 eV,and moved to 531.36 eV after irradiation.The binding energy was increased by 0.3 eV because of electron dissociation of the O atom(Fig.3c),showing that the O atoms of the carboxylate group are electron donors.For N1s,it transited to the lower binding energy from 400.08 to 399.88 eV after irradiation(Fig.3d),showing that N atoms of CEbpy are electron acceptors.The results show that the photochromic behavior of 2 is owing to the presence of PET from the O atom of the carboxylate group to the N atom of the pyridinium ring.
According to the structural analysis,these three compounds own O—H…N hydrogen bonds,and the H…N distances of 1,2 and 3 are 0.193 9,0.193 2 and 0.188 5 nm,respectively(Fig.S3).The O—H…N hydrogen bonds have been systematically demonstrated to be applicable to so-called through-space ET[21].Thus,if PET is supported by such O—H…N hydrogen bonds,then logically the photochromic behavior of 3 is greater than or close to 1 and 2.In fact,on the contrary,the photochromic behavior of 3 was the worst,apparently.Therefore,the O—H…N hydrogen bonds are not the key factor in photochromism.It is well known that metal ions play a crucial role in the structure and properties of viologen-based compounds[22].The metal ions with good electron-withdrawing ability have positive effects on photochromism.We know that the electronegativity value of Mnis 8.88,Znis 10.38 and Cois 9.10[23].Thus,the strongest electronwithdrawing ability of Znleads to the best photochromic behavior in 2.But compound 3 has the worst photochromic performance,not 1.The possible reason is that the color of 3 was much darker than 1,leading to the rate of color change in 3 slower than 1.

Fig.3 XPS core-level spectra of 2 before(black line)and after irradiation(red line)
2.3 Hydrochromism
Interesting,solid compound 3 exhibited distinguishable hydrochromic behavior,while compounds 1 and 2 did not.Compound 3 changed from orange to purple(3T)at room temperature after heating at 70℃within 3 min(Fig.4a).Unfortunately,the X-ray single crystal data of 3T cannot be obtained due to dehydration.PXRD studies indicate that the dehydrated phase was a little amorphous,different from 3(Fig.S1c).Therefore,it is speculated that 3T is the dehydrated phase,formed by losing water molecules after heating at 70℃,which was confirmed by the TG test(Fig.S2).The solid UV-Vis spectrum of 3 showed a wide absorption band in a range of 400~600 nm,which is attributed to thed-dtransition of Co[24].The UV-Vis spectrum of 3T shows another absorption at 450~700 nm,similar to another geometric red shift of the Cocoordination fieldd-dtransition,with the absorption band extending to a higher frequency[25].Thus,this chromic behavior should be attributed to the coordination environment change of Codue to dehydration.To further study the effect of water molecules on heating,compound 3 was heated in water at 70℃,and no color change was observed.In addition,the EPR signal of 3T was silent,consistent with that of 3,excluding heat-induced ET and free radical generation(Fig.4b).Thus,the main cause of discoloration during heating is dehydration.The heated sample cannot return to the original crystalline state even after exposed to moisture for almost one month,indicating that the color change due to dehydration is irreversible.The three compounds have the same structure,and the difference in their chromic behaviors may be owing to their different metal ions.The Coion,a classical substitutive active metal ion,is known for its chromic behavior caused by coordination geometry[26].The configuration ofd-orbital will be significantly affected by the rearrangement of the coordination sphere,and the color changes will be obvious after heating.
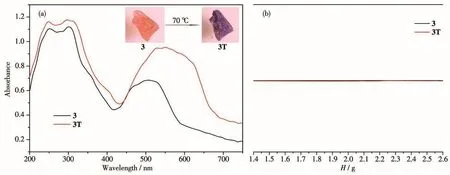
Fig.4 Photographs and UV-Vis spectra(a)and EPR spectra(b)for 3 before and after heating
3 Conclusions
In summary,we present the synthesis and crystal structures of three isostructural co-crystals containing different metal ions based on the neutral viologen derivative,1-carboxyethyl-4,4′-bipyridine.Interestingly,they all show obvious photochromic behaviors due to photo-induced electron transfer when exposed to irradiation.In addition,compound 3 exhibits irreversible hydrochromic behavior due to dehydration.This work illustrates the effect of metal ions on the chromism of viologen-based co-crystals and we hope that it will provide valuable insights into the design of new chromic materials.
Supporting information is available at http://www.wjhxxb.cn
- 无机化学学报的其它文章
- Synthesis and Characterization of Metal-Organic Framework Based on 2,6-Bis(4-carboxybenzylidene)cyclohexanone
- Two Metal-Organic Frameworks Built from 2,2′-Dimethyl-4,4′-biphenyldicarboxylic Acid
- Effect of Mass Ratio of Ni and Co in Initial Solution on Oxygen Evolution Reaction Performance of Ni-Co-S-O/NF Catalyst in Alkaline Water Electrolysis
- Structure and Fluorescence Properties of Three 1D/2D/3D Zn/Cocomplexes Based on Flexible Tetracarboxylic Acid
- Two Nitronyl Nitroxide Biradical-Bridged Lanthanide One-Dimensional Chains:Crystal Structure,Magnetic Properties and Luminescent Behavior
- Synthesis and Visible-Light-Driven Photocatalytic Properties of Floating BiFeO3/Expanding Perlite Photocatalysts

