Symmetrical Dirutheniumcomplex Based on 1-Isopropyl-4-methylbenzene and Dimethylbiguanide:Synthesis and Anticancer Activity in Vitro
YU Qun-Ying( 332000)
Abstract:A novel symmetrical dinuclear bridging complex(NH4)2[Ru(Cym)(L)]2Cl2·4H2O(1)(Cym=pcymene=1-isopropyl-4-methylbenzene,H2 L=1,1-dimethylbiguanide)was obtained by treatment of the precursor[Ru(Cym)Cl2]2 with metformin hydrochloride.In aqueous base solution,deprotonation of the proligand(1,1-dimethylbiguanide)occured and the corresponding neutral ruthenium complex 1 was obtained.The structure of complex 1 has been established by FT-IR and NMR spectroscopy and single-crystal X-ray diffraction analysis.The number of crystal water was obtained by thermogravimetric analysis.The inhibition of cell proliferation activity against four human cancer cell lines(HepG-2,A549,Hela,MCF-7)of complex 1 relative to cisplatin was measured by MTT method in vitro.Notably,the novel complex displayed comparable potency toward HepG-2(hepatocellular carcinoma,HCC)compared to cisplatin.CCDC:2058702.
Keywords:ruthenium complex;crystal structure;anticancer activity;cisplatin
0 Introduction
Today platinum-based complexes including cisplatin,carboplatin,and oxoplatin are potent antitumor agents,and they are precious class of antitumor metallotherapeutics commonly prescribed in the clinic.However,the platinum-based chemotherapeutics are far from ideal:they cause drug resistance and a range of side effects,by which their therapeutics value is curtailed.Serious adverse drug reactions in hospitalized patients rank as the 4th~6th leading cause of death,highlighting the desire need for safer and selective metallotherapeutics[1].
Ruthenium-based antineoplastic agents are among the most investigated non-platinum metallodrugs,and they are appealing candidates that have certain merits over platinum-based therapeutics.Ruthenium anticancer agents show high selectivity for tumor cell lines,and low cytotoxicity to normal cells.Furthermore,they have cytotoxicity against some cisplatin resistant cell lines.The reported mechanisms of action by which the ruthenium therapeutics work include acting as protein kinase inhibitors,DNA binding,protein binding,apoptosis,and so on[2].However,the oncotherapeutic value of ruthenium based anticancer agents can be influenced by the coordination mode of ligands.Typically,the N,N-,S,O-,S,N-,C,N-bidentate donating ligands generally yield potent antineoplastic metallodrugs[3].
In more recent times,research groups have endeavored to tether bioactive ligands to ruthenium center.The strategy may obtain a new molecular entity(NMEs)having pharmacokinetic and therapeutic profiles distinct to the free ligands themselves.The metal center and bioactive ligand may exert synergistic effect,endowing the new molecular with multitargeted and reduced toxicity properties of which many chemotherapeutics lack.For example,Wang et al.[4]demonstrated that when the 4-anilinoquinazolines(4-AQs)epidermal growth factor receptor(EGFR)inhibitor ligands(2~7)(Fig.1)were incorporated to the ruthenium center,their ability to induce early stage apoptosis was enhanced compared to the ligands alone,while also retaining the DNA binding capacity ascribed to the rutheniumcenter.
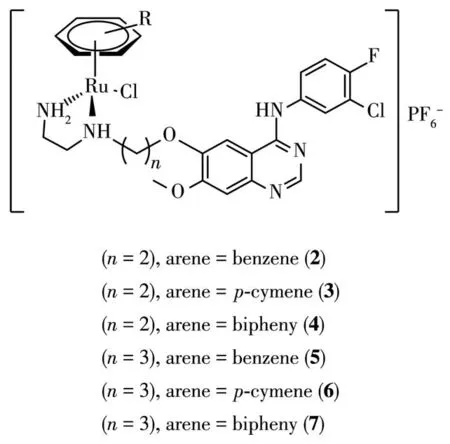
Fig.1 Chemical structures of organorutheniumcomplexes incorporating 4-AQs(2~7),analogues of the EGFR inhibitor gefitinib
Metformin(1,1-dimethylbiguanide),a derivative of biguanide,has been prescribed for the treatment of type 2 diabetes for over 30 years.It is a magic molecule that exhibits antimalarial biological properties[5],and recently,it has been assessed for therapeutic treatment of pain,anxiety,and memory disorders.Furthermore,there are concrete evidences that metformin has the ability to reduce the incidence of overall cancer,liver cancer,pancreatic cancer,colorectal cancer and breast cancer as well as the mortality of overall cancer,liver cancer and breast cancer.Possible modes of action could be ascribed to anti-inflammatory effects,antioxidant effects and killing of cancer stem cells,suppressing tyrosine kinase receptors such as HER1 and HER2,inhibiting cancer cells by initiating the pivotal LKB1/AMPK/m TOR axis which regulates energy metabolism and protein synthesis of the cell[6].
In light of above,we want to incorporate the pharmacophore of metformin into ruthenium center to get a new ruthenium complex and test its cytotoxicity against cancer cell linesin vitro.
1 Experimental
1.1 Materials and methods
NMR spectra were recorded on Bruker DRX-400 instrument with TMS as internal standard.Chemical shifts were reported asδvalues,relative to internal DMSO(δ2.50 for1H NMR and 39.50 for13C NMR).ESI-MS spectrum was determined on API QSTAR Pulsari spectrometer.X-ray diffraction was obtained by APEX DUO.Infrared spectra were recorded on a FT-IR spectrometer with KBr pellets.Yield referred to spectroscopically (1H NMR)homogeneous material.Unless otherwise noted,materials obtained from commercial suppliers were used without further purification.
1.2 Synthesis of the complex
Metformin hydrochloride(330 mg,2 mmol),dichloro(p-cymene)rutheniumdimer(613 mg,1 mmol),massive KOH(448 mg,8 mmol),NH4Cl(107 mg,2 mmol)and distilled H2O(40 m L)were placed into the reaction vessel,sealed and stirred for 30 min at room temperature,during which time the reation turned dark red.Then the magnetic stir bar was removed,and the mixture was crystallized in refrigerator(4℃)for ten days furnishing the desired complex 1,(NH4)2[Ru(Cym)(L)]2Cl2·4H2O(Cym=p-cymene=1-isopropyl-4-methylbenzene,H2L=1,1-dimethylbiguanide),as a dark red triclinic crystal.Yield: 483 mg, 65%. Anal. Calcd. for C28H62Cl2N12O4Ru2(%):C,37.21;H,6.91;N,18.59.Found(%):C,37.42;H,6.91;N,18.36.ESI-MS:m/z364.0,Calcd.for[1a+H]+:364.10(indicating the formation of 1a in light methanol solution:monomeric species of 1,Scheme 1).1H NMR(400 MHz,DMSO-d6):δ5.80(d,J=6.08 Hz,4H),5.68(d,J=6.08 Hz,4H),5.67(s,1H),5.45(s,4H),5.06(s,2H),2.94(s,12H),2.78~2.67(m,2H),2.15(s,6H),1.15(d,J=6.88 Hz,12H).13C NMR(101 MHz,DMSO-d6):δ159.56,158.27,109.15,104.58,87.57,86.49,37.85,29.76,22.00,17.53.IR(KBr,cm-1):3 340,3 278,3 200,3 155,2 961,2 925,2 869,1 617,1 584,1 493,1 431,1 402,1 303,1 215,1 110,1 025,865,803,775,706,671,520,470.
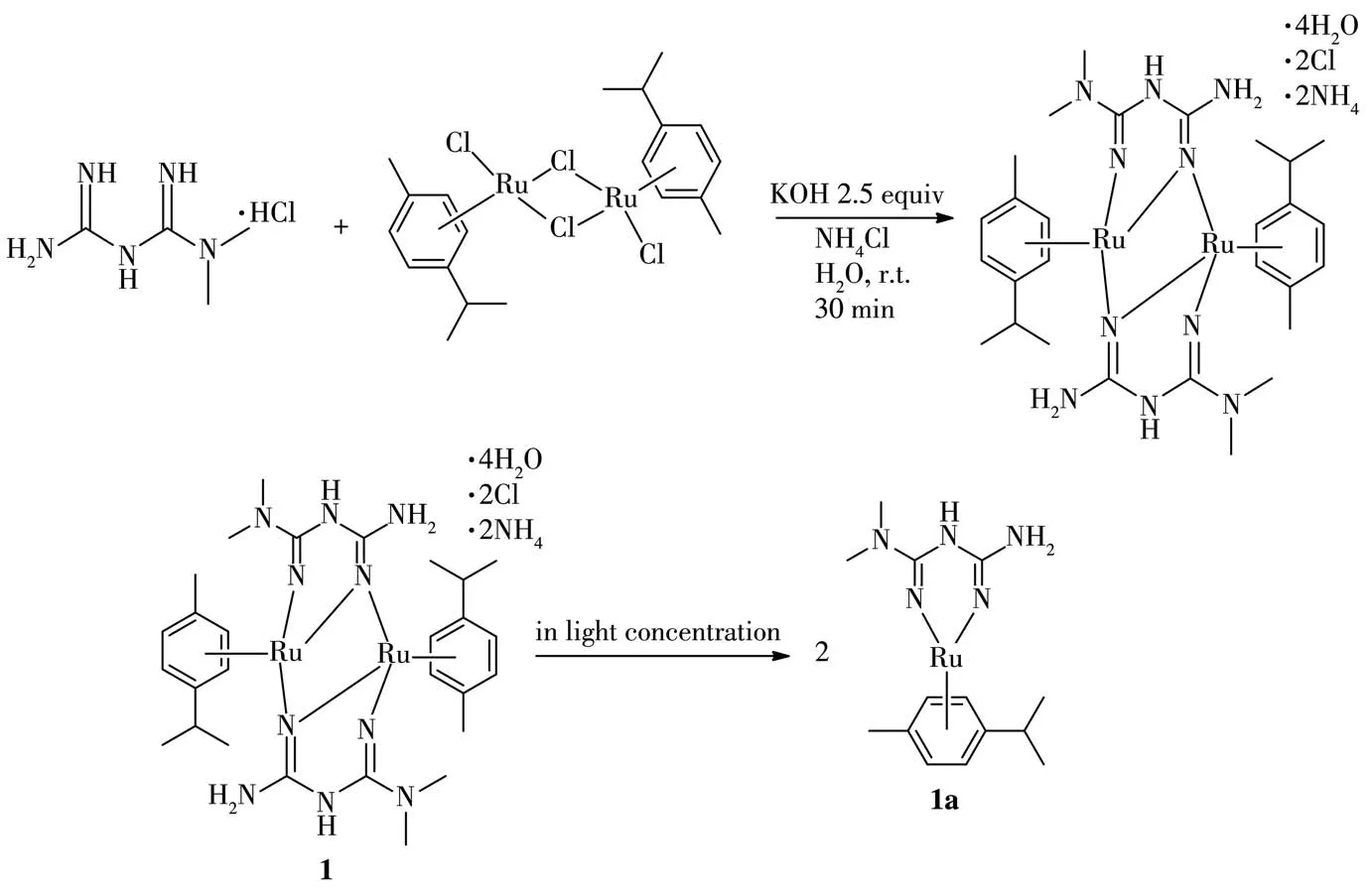
Scheme 1 Synthesis route of complex 1 and formation of 1a
1.3 Crystal structural determination
The crystal data for complex 1 was collected on APEX DUO using CuKαradiation.Absorption corrections were applied using multi-scan methods.This structure was solved by direct methods and refined by full-matrix least-squares using the SHELXL-2018/1[9].All non-hydrogen atoms were refined anisotropically.Hydrogen atoms were located geometrically and treated as a riding atom.The diffraction data and selected bond lengths and bond anglesare listed in Table 1 and 2,respectively.CCDC:2058702.

Table 1 Crystallographic data of complex 1
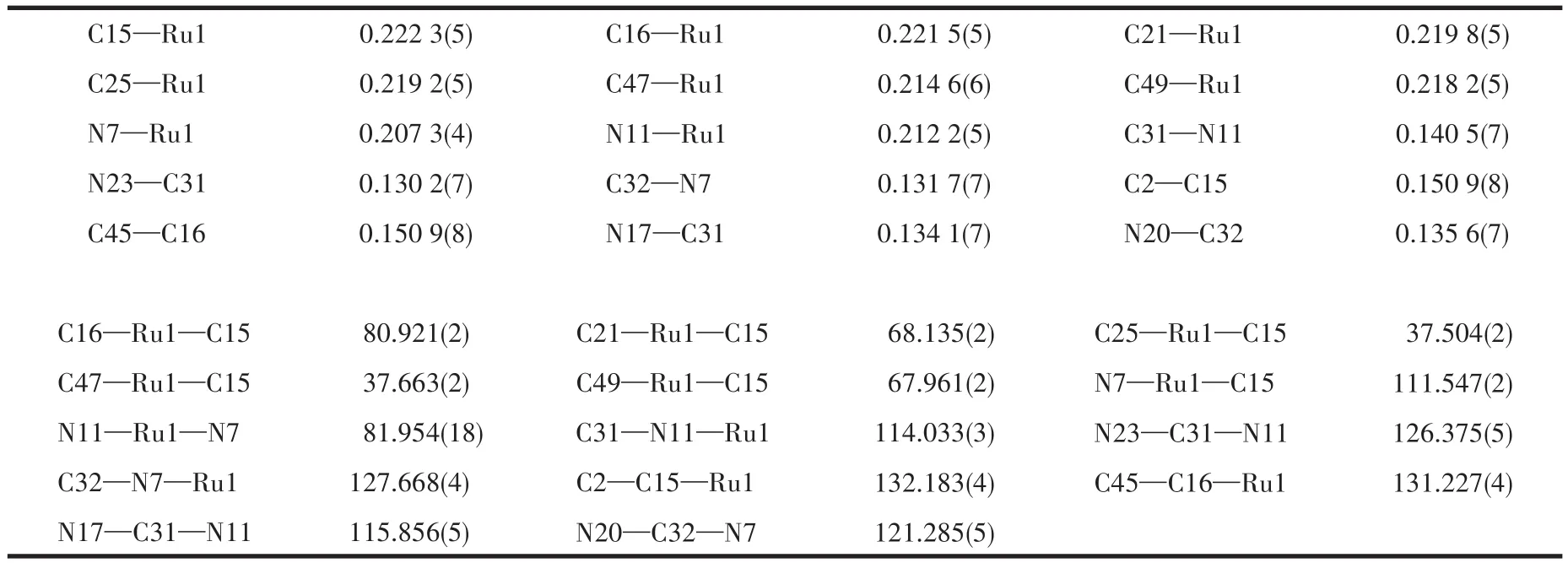
Table 2 Selected bond distances(nm)and angles(°)for complex1
1.4 Cancer cell grow th inhibition assay
1.4.1 Cell culture
Cancer cell lines(HepG-2,Hela,A549,MCF-7)were routinely grown in Dulbecco′s modified Eagle′s medium(DMEM/H)containing 10% heatinactivated fetal bovine serum(FBS)at 37℃in 5%(V/V)CO2.Cell suspensions were seeded in 96-well plates at a density of 8 000 cells per well for 12 h.Then,a fresh complete drug-containing medium with 5% FCS was added,and incubated for another 48 h.
1.4.2 Determination of IC50values
An MTT assay was used to evaluate cell viability.Following drug exposure(the required concentration varied from 0~100 μg·m L-1),MTT solution(final concentration 0.5 mg·m L-1)was added to each well,and staining for 4 h.The optical density,which was directly proportional to the number of surviving cells,was measured at 490 nm using microplate reader(Molecular Devices,Inc.).The percentages of surviving cells were calculated by using absorbance ratios of drug-treated cells versus untreated cells.The IC50values for the inhibition of cell growth were calculated by fitting the plot of the logarithmic percentage of surviving cells against the logarithm of drug concentration with a linear regression function.
2 Results and discussion
2.1 Synthesis
The dinuclear complex 1 has been prepared by reaction between the precursor complex[Ru(Cym)Cl2]2with the proligand metformin hydrochloride in aqueous base condition at room temperature.In alkaline solution,deprotonation of the proligand(1,1-dimethylbiguanide)occured and the corresponding neutral ruthenium complex 1 was obtained by exchange reaction.Good dark red triclinic crystal suitable for X-ray diffraction studies was grown from stock solution.Orgnometallic rutheniumcomplex encompassingN,N-bidentate ligand of 1,1-dimethylbiguanide is stable,because the vacantdorbitals of the metal in oxidated state may overlap with the filledπorbitals of the ligand which are considered as strongσ-andπ-donating system.Interestingly,in light concentration of methanol solution,compound 1 was converted into compound 1a,indicating the formation of monomeric species,that can be reflected in the ESI-MS spectrum.
2.2 Spectroscopy
The infrared spectrum of the complex exhibited an intense absorption band in a range of 3 100~3 500 cm-1assignable to the stretching vibration of the NH groups.It is probable that inter-or intramolecular hydrogen bonds overlap with NH vibrations and are responsible for this broad band.A set of strong bands observed in a range 1 400~1 700 cm-1may be attributed to C=N stretch and NH deformation.A new band appearing at 1 320~1 220 cm-1is assigned to ring vibration and supports the formation of a chelate ring.
The proton NMR spectra of the metal complex,recorded in DMSO-d6solution,showed a downfield shift of the aromatic protons resonances with respect to those of the dichloro(p-cymene)rutheniumdimer,while aliphatic protons did not undergo significant chem ical shifts.The same pattern was also observed in the carbon NMR spectra of the complex.This fact may be attributed toπelectron delocalisation on the chelate ring.A total of four singlets were observed,among themδ=5.45(s,4H),5.06(s,2H),2.94(s,12H)are attributable to 2(—NH2),2(—NH—),2(CH3)2N— protons,respectively,δ=2.15(s,6H)is assignable to 2(CH3—Ar).The carbon NMR spectra appearing atδ=159.56,158.27 are assignable to C=N carbons.
2.3 Crystal structure
An ORTEP representation of the coordination environment of complex 1 including the atom labeling scheme is shown in Fig.2.Single-crystal X-ray structure analysis reveals that complex 1 crystallizes in the triclinic system space groupP1.The asymmetric unit(Fig.3)of 1 contains a crystallographically unique Ruion,one L2-block,onepcymene moiety,a lattice chloride anion,an ammonium cation and two water molecules.View of the pack drawing of 1 is shown in Fig.4.The Ru1—N7 and Ru1—N11 bond length are 0.207 3(4),0.212 2(5)nm,respectively,significantly longer than the values reported in the dinuclear[(μabpy){Ru(acac)2}2],due to the effect of theπ-accepting ancillary ligand,Cym.The N7—Ru1—N11 angle is 81.954(18)°,close to the values found in some reported Rucompounds[7].The average Ru—C(Cym)bond distance of 0.219 3 nm is comparable to the values reported in other{Ru-Cym}complexes[8].
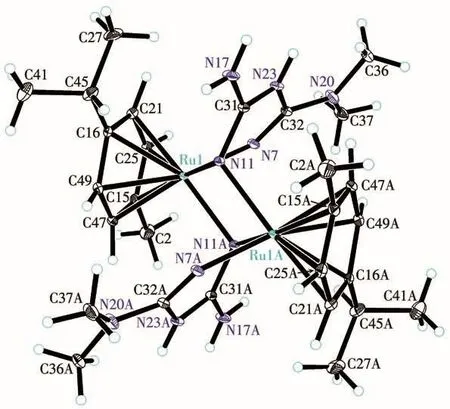
Fig.2 Drawing of complex 1 with the atom-labelling scheme with 30% probability displacement ellipsoids
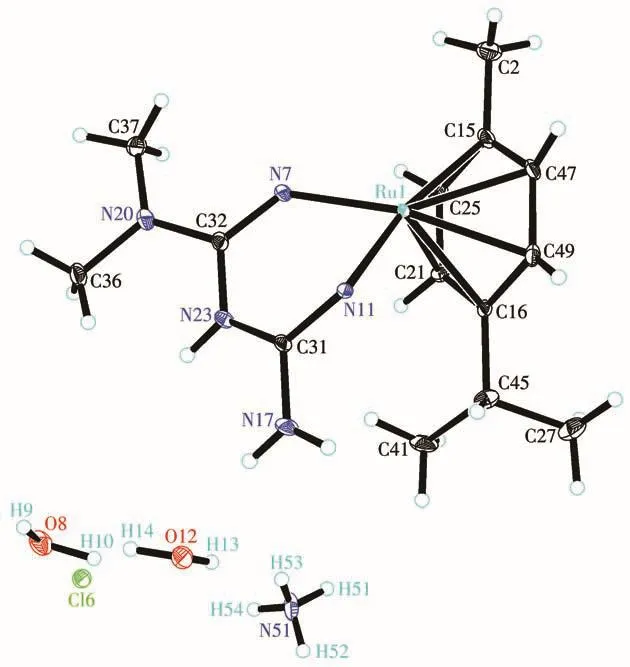
Fig.3 Drawing of asymmetric unit of complex 1 with 30% probability displacement ellipsoids
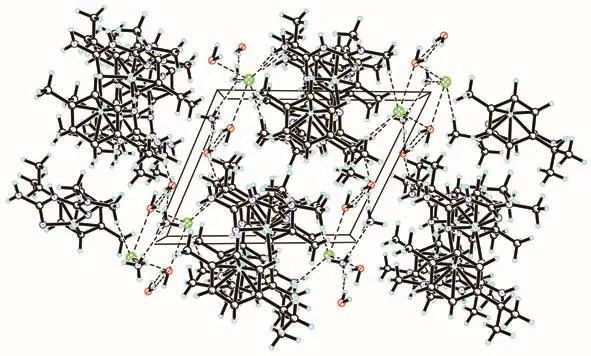
Fig.4 Pack drawing of complex 1 with hydrogen-bonds shown as dashed lines
2.4 In vitro proliferation assays
The anticancer efficacies of 1in vitrowere assessed by an MTT assay in four human cancer cell lines,including HepG-2(hepatocellular carcinoma,HCC),A549(lung cancer),Hela(cervical),MCF-7(breast).The clinically prescribed platinum based therapeutic cisplatin was tested as well for comparison.These cells were treated with various concentrations of complex 1 and cisplatin for 48 h,and the IC50values are given in Table 3.From the data,it is obvious that complex 1 displayed comparable cytotoxic potency toward HepG-2(hepatocellular carcinoma,HCC)compared to cisplatin.

Table 3 IC50 values of metal complexes cisplatin and 1 against HepG-2,A549,Hela and MCF-7 cell lines for 48 h
3 Conclusions
In summary,a novel symmetrical dinuclear bridging complex encompassing the framework of metformin has been synthesized,characterized and tested against four cancer cell lines(HepG-2,A549,Hela,MCF-7).It is worth noting that the complex shows similar cytotoxicity toward hepatocellular carcinoma cell line to cisplatin.In most countries,hepatocellular carcinoma(HCC)occupies 70%~85% of all cases of liver cancer.Drug companies are pushing hard for ideal drugs to cure advanced HCC,though blows kept coming.We report herein a novel ruthenium complex which would provide good handle for further development.
Supporting information is available at http://www.wjhxxb.cn
Acknowledgements:This work was financially supported by Scientific Research Fund of Jiangxi Education Department(Grant No.GJJ180907)and Jiangxi Health Department(Grant No.202131075).

