Multiple sclerosis: why we should focus on both sides of the (auto)antibody
Jeroen den Dunnen, Lynn Mes, Willianne Hoepel, Joost Smolders
Various clinical and experimental findings suggest a pathogenic role of antibodies in multiple sclerosis (MS). Yet, whether antibodies contribute to the pathogenesis or progression of MS is still a subject of intense debate. This controversy particularly results from unclarity regarding the target antigens of the antibodies that are found in the central nervous system (CNS) of MS patients. The identification of such target antigen(s) at disease onset remains an important topic of investigation, but these antigens may be heterogeneous and not the decisive factor for the initiation of MS development. In addition to antigen-specific binding of IgG, IgG may also promote pathology in MS patients by binding in an antigen nonspecific manner. Therefore, we propose that we should not only focus on the antigen-binding part of MS antibodies,but also should pay attention to the other side of the antibodies in the CNS of MS patients, i.e. the fragment crystallizable(Fc) tail (Figure 1A). The characteristics of the Fc tail, particularly the (combination of) IgG subclass, allotype, and glycosylation determine the pathogenicity of IgG, but these characteristics are still poorly defined in MS. Unraveling these characteristics may not only lead to better understanding of MS pathogenesis, but may also yield new strategies for therapy.The efficacy of CD20-targeted therapies indicates a role for B cells and their multiple effector functions in the disease process of MS, including their differentiation towards antibodysecreting plasma cells. While antibody concentrations are very low in healthy CNS,IgG antibodies are present in increased concentrations in the cerebrospinal fluid(CSF) in the majority of MS patients. Using immune electrophoresis, these IgGs show a CSF-unique oligoclonal pattern in more than 90% of MS patients. These oligoclonal bands are important for MS diagnosis, but are not specific for this disease. They also appear in CSF in infections and several other immune-mediated diseases, such as autoimmune encephalitis, and incidentally in demyelinating disorders, such as MOG-associated disease and aquaporin 4-positive neuromyelitis optica spectrum disease. For the latter CNS autoimmune disorder, antibodies are known to directly contribute to pathology and chronicity.Similarly, there are indications for a pathogenic role of antibodies in MS. In a study on early MS biopsies/autopsies, the majority of included patients showed a distinct histological profile comprising IgG and complement deposition (classified as a pattern II lesion), while this was lacking in other patients (Lucchinetti et al., 2000).In a retrospective study, only MS patients with a pattern II pathological profile at diagnostic biopsy and/or autopsy had a documented favorable clinical response to plasma exchange (Keegan et al., 2005),suggesting the involvement of antibodies.Accordingly, distinct circulating antibody signatures with a higher reactivity against Nogo-A peptides (which are expressed by oligodendrocytes and neurons)were found in patients with pattern II lesions compared to patients with other lesion patterns (Stork et al., 2020). In addition, in a myelinating culture-system,complement-dependent demyelinating IgG-antibodies were detected in 30% of MS patients versus none in controls (Elliottet al., 2012).
Despite these studies and a clear role of IgG in aforementioned autoimmune disorders, a causative role for IgG antibodies in the pathogenesis of MS remains controversial. This controversy is catalyzed by uncertainty regarding the target specificity of the oligoclonal IgG in MS patients. A broad spectrum of technical approaches has been used in studies to elucidate the target antigens of whole CSF IgG. These studies reported antibodies directed against different viruses (measles, VZV, HTLV-1 and HHV6),myelin proteins (MBP, MOG), ion channels(Kir4.1), glycolipids, and fatty acids(excellently discussed in an editorial by Winger and Zamvill (Winger and Zamvil,2016)). An elegant study by Brändle et al. combined the transcriptome of CSF B cell lineage cells as assessed by next generation sequencing, and the peptidome of oligoclonal IgG-fractions among purified IgG as identified with 2D gel electrophoresis and mass-spectrometry(Brandle et al., 2016). Matching IgG-heavy and -light chain pairs were expressed in a recombinant expression system, and produced oligoclonal band IgGs were characterized with a protein array. The four oligoclonal IgGs identified recognized non CNS-specific intracellular antigens in relapsing remitting MS patients with a median disease duration of 17.5 months. These observations collectively provide several interesting clues. First,IgG responses to intracellular antigens may be a secondary effect that is induced in response to MS-associated tissue damage. This suggests that if we want to study the most relevant antigens that are involved in the initiation of disease,we need to focus on the oligoclonal IgG that emerges in the CSF as early in the disease process as possible. Second, there may be substantial heterogeneity in the contribution of antigen-specific IgGs to disease among donors, as has been shown for the pathology of MS (Lucchinetti et al., 2000) and is known for the clinical course of MS. The presence of a B cell dominant subset of MS patients has been suggested, showing distinct profiles of IgG antibodies directed against a broad range of CNS antigens both in circulation and in supernatants ofin vitrostimulated B cells (Kuerten et al., 2020). These antigens are highly variable between patients, as illustrated by the absence of shared CSF oligoclonal IgG antigens between 20 MS patients in a phage-displayed random peptide libraries-screen (Graner et al.,2020). Therefore, an extensive analysis of CNS antigens may not lead to a single common antigen for MS. And third, it may not be the antigen-specificity that defines the role of these antibodies in MS. Of note, phagocytosed CNS antigens have been encountered in cervical lymph nodes of people with and without MS (van Zwam et al., 2009). Since cervical lymph nodes are a major site of CNS B cell maturation,not the presence of CNS antigens per se,but rather an increased responsivity and/or dysfunctional effector mechanism of the adaptive immune response could be the critical driver of intrathecal oligoclonal IgG-secreting plasma cell populations in MS.
While the (initial) target antigens could be relevant to understand the origin of MS pathogenesis, it is important to realize that not only antigen-specific,but also antigen non-specific IgG binding could promote pathology in MS patients.Notably, the key mechanism for the activation of IgG effector functions is not necessarily antigen binding, but instead the formation of IgG immune complexes.While these two events often go hand in hand, immune complex formation can also occur in an antigen-independent manner (Pryce and Baker, 2018), for example by aggregate formation or nonspecific binding to sticky compounds such as myelin. Previous studies in the last decades that used (heat-)aggregates,coated beads, plate-bound antibodies, and therapeutic Fc-containing constructs have demonstrated that these antigen nonspecific immune complexes are equally potent in activating IgG effector functions as antigen-specific immune complexes. In contrast, unbound (monomeric) IgG does not activate IgG effector functions, and can even suppress immune activation.Therefore, when discussing a potential role for oligoclonal antibodies in the pathogenesis of MS, it is critical to know whether IgG is present in unbound or complexed form. Interestingly, recent findings by us and others indeed indicate the presence of IgG immune complexes in the CNS of MS patients. For example,myelin of the majority of MS patients is bound by IgG, while these complexes are only found in a small number of non-MS controls (van der Poel et al., 2020).These data demonstrate that IgG indeed forms immune complexes in the CNS of the majority of MS patients, and therefore could contribute to (pathological) immune activation.
IgG immune complexes in the CNS of MS patients could induce pathological immune responses by activating a variety of antibody effector functions. In general,the most important IgG effector functions are complement activation, phagocytosis,antibody-dependent cellular cytotoxicity(ADCC), and cytokine induction (Figure 1B). Complement activation is indeed observed in MS patients (Lucchinetti et al., 2000) (Elliott et al., 2012). There is also evidence that the other three IgG effector functions (i.e. phagocytosis, ADCC, and cytokine induction) are activated in the CNS of MS patients, which all require the activation of Fc gamma receptors that are expressed by myeloid immune cells such as microglia. For example, binding of IgG to myelin promotes myelin uptake through phagocytosis by microglia (Hendrickx et al., 2014; van der Poel et al., 2020).In addition, IgG immune complexes can induce the production of high levels of pro-inflammatory cytokines by primary human microglia (van der Poel et al.,2020), which is in strong contrast to the general immunological tolerance of microglia to microbial stimuli. However,which of these IgG effector functions are particularly activated, and therefore are most likely to contribute to MS pathology,is still far from clear.
Importantly, the IgG effector functions that are activated upon immune complex formation in MS patients critically depend on the composition of the IgG Fc tail.The most important variables in the Fc tail composition that determine to which extent IgG effector functions are activated are (1) IgG subclass, (2) allotype, and (3)glycosylation (Figure 1C). First, IgG can be divided in into four different subclasses(IgG1–4). While it was initially thought that some subclasses are pro-inflammatory(IgG3 > IgG1) and others anti-inflammatory(IgG2 and IgG4), recent findings indicate a “division of labor”, in which every subclass is efficient in activating particular immune functions (Figure 1C) (Hoepel et al., 2020). For example, IgG3 is a very potent inducer of complement activation and phagocytosis, while IgG2 is the main subclass that promotes pro-inflammatory cytokine production (Hoepel et al., 2020).IgG1 is able to activate most effector functions, albeit at a somewhat lower level than IgG2 and IgG3 (Hoepel et al.,2020). The oligoclonal IgG that is found in CSF of MS patients is mostly of the IgG1 subclass, although also IgG3 and low levels of IgG2 have been found (Losy et al., 1990). Second, IgG subclasses can be further divided into allotypes. Particularly for IgG3, these genetic polymorphisms can affect IgG3 half-life and effector functions such as complement activation and ADCC. The potential correlation of particular IgG(3) allotypes and MS (or MS severity) is understudied and still not completely clear. Third, IgG glycosylation of a conserved glycan at position N297 has a major effect on the activation of IgG effector functions. These differences in IgG glycosylation mostly depend on the expression of glycosyltransferases and glycosidases in local B cells, which are affected by various factors such as age,hormones, inflammatory conditions, and food metabolites. The glycosylation of IgG in CSF (but not in serum) of MS patientsvs.controls is clearly different, and alterations of glycosylation coincide with MS relapses(Wuhrer et al., 2015). The glycosylation pattern of IgG from CSF of MS patients mostly differs in levels of fucose, galactose,and bisecting N-acetylglucosamine (Figure 1C), which are associated with increased inflammation and complement activation.Several dozens of different glyco-forms of IgG have already been identified, many of which have different binding affinities for Fc gamma receptors and therefore a different potential to activate IgG effector functions.
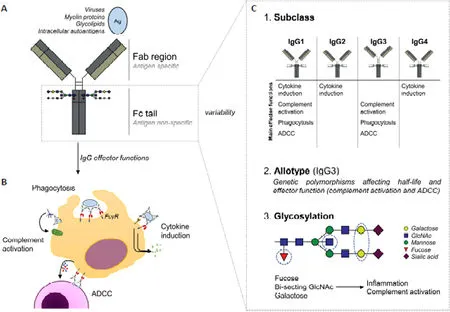
Figure 1|The Fc tail determines the pathogenicity of oligoclonal IgG in MS.
Taken together, the combination of IgG subclass, allotype, and glycosylation provide a very large variety in Fc tail composition and consequent immune activation, which ranges from practically inert to extremely inflammatory, or specifically equipped to (over-)activate particular IgG effector functions such as complement activation or cytokine production. Therefore, to truly understand how intrathecal IgG contributes to MS pathogenesis, we postulate that it is essential to fully characterize the Fc tail composition of IgG antibodies in the CNS of MS patients. Importantly, for this characterization it would not suffice to simply analyze the IgG that is present in CSF. As explained above, unbound monomeric IgG does not lead to immune activation, only IgG immune complexes do. Therefore, specifically these tissuebound IgG immune complexes hold the key to unraveling the role of IgG in MS pathogenesis. Although obtaining these antibodies will be extremely challenging,it may be possible by studying biopsies or post-mortem tissue from MS patients,or to use experimental MS (auto)antibody animal models with IgGs that have distinct Fc tail compositions. When these experiments would indeed confirm pathogenicity by IgG in MS, subsequent steps could be taken to specifically counteract these pathogenic IgG effector functions. For this, we could apply therapies that are already in practice for other antibody-related disorders,such as immune thrombocytopenia and rheumatoid arthritis, where FcγR activation is suppressed by e.g. therapeutic inhibition of the upstream kinase Syk. Although it would still be a long way to potential therapies (also because drug delivery to the CNS is always more challenging because of the blood brain barrier), the characterization of the exact composition of IgG immune complexes in the CNS of MS patients may provide the first critical stepping stone.
We sincerely thank Dr. Jörg Hamann and Prof. Dr. Inge Huitinga from the Netherlands Institute for Neuroscience and University of Amsterdam, Amsterdam, The Netherlands for their provoking thoughts,extensive feedback, and proofreading of the manuscript.
The present work was supported by ZonMw Open Competition grant(project No. 09120011910035) and ZonMw Second Wave grant (project No.10430012010008), both awarded to JdD.
Jeroen den Dunnen*, Lynn Mes,Willianne Hoepel, Joost Smolders
Amsterdam Rheumatology and Immunology Center, Department of Rheumatology and Clinical Immunology; Department of Experimental Immunology, Amsterdam Institute for Infection and Immunity, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands (den Dunnen J, Mes L, Hoepel W)Department of Medical Microbiology, Amsterdam institute for Infection and Immunity, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands (Mes L)Neuroimmunology Research group, Netherlands Institute for Neuroscience, Amsterdam; MS Center ErasMS, Departments of Neurology and Immunology, Erasmus Medical Center, Rotterdam,The Netherlands (Smolders J)
*Correspondence to:Jeroen den Dunnen, PhD,j.dendunnen@amsterdamumc.nl.https://orcid.org/0000-0002-7199-8619(Jeroen den Dunnen)
Date of submission:December 16, 2020
Date of decision:January 19, 2021
Date of acceptance:March 12, 2021
Date of web publication:April 23, 2021
https://doi.org/10.4103/1673-5374.313045
How to cite this article:den Dunnen J, Mes L,Hoepel W, Smolders J (2021) Multiple sclerosis:why we should focus on both sides of the (auto)antibody. Neural Regen Res 16(12):2422-2424.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Rogue gene networks gone awry in Alzheimer’s disease
- Corrigendum: Edible seaweed-derived constituents: an undisclosed source of neuroprotective compounds
- DNMT1-dependent regulation of cortical interneuron function and survival
- Combinatorial genetics methods for discovering high-order regulatory combinations and engineering genetic drivers for neural differentiation
- Abnormal metal homeostasis as a common drug target to combat neurodegenerative diseases
- Edible seaweed-derived constituents: an undisclosed source of neuroprotective compounds

