Nanotechnology and Nanomedicine: A Promising Avenue for Lung Cancer Diagnosis and Therapy
Wei Yin, Fen Pan, Junjie Zhu, Junwu Xu, Dieo Gonzalez-Rivas,e, Meinoshin Okumura,Zhiyon Tan, Yan Yan,*
a Key Laboratory of Oral Biomedical Engineering(Wuhan University),Ministry of Education,Hospital of Stomatology,School of Stomatology,Wuhan University,Wuhan 430079,China
b Department of Biomedical Data Science, Geisel School of Medicine, Dartmouth College, Hanover, NH 03756, USA
c Department of Thoracic Surgery, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai 200433, China
d Tus-Medical Health Technology Investment (Jiaxing) Co., Ltd., Jiaxing 314033, China
e Minimally Invasive Thoracic Surgery Unit (UCTMI), Hospital San Rafael, Coruña 15006, Spain
f Osaka Toneyama Medical Center, Osaka 560-8552, Japan
g CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190, China
Keywords:Nanomedicine Lung cancer Diagnosis Therapy
ABSTRACT Lung cancer is a leading cause of cancer-related death worldwide,with a very poor overall five-year survival rate.The intrinsic limitations associated with the conventional diagnosis and therapeutic strategies used for lung cancer have motivated the development of nanotechnology and nanomedicine approaches,in order to improve early diagnosis rate and develop more effective and safer therapeutic options for lung cancer. Cancer nanomedicines aim to individualize drug delivery, diagnosis, and therapy by tailoring them to each patient’s unique physiology and pathological features—on both the genomic and proteomic levels—and have attracted widespread attention in this field. Despite the successful application of nanomedicine techniques in lung cancer research, the clinical translation of nanomedicine approaches remains challenging due to the limited understanding of the interactions that occur between nanotechnology and biology, and the challenges posed by the toxicology, pharmacology, immunology, and largescale manufacturing of nanoparticles.In this review,we highlight the progress and opportunities associated with nanomedicine use for lung cancer treatment and discuss the prospects of this field, together with the challenges associated with clinical translation.
1. Introduction
Despite recent advances in lung cancer detection and treatment, lung cancer remains the most lethal cancer worldwide,due to the failure to detect cancer occurrence early and the lack of effective treatments for advanced-stage patients. Lung cancer is the most common newly diagnosed cancer type in North America and Asia[1,2].The primary difficulty associated with lung cancer detection is that existing detection methods, including bronchial biopsy and computed tomography (CT), depend heavily on tumor size and require specific medical equipment, which is often associated with high costs. Nanotechnology offers the promise of new detection approaches, as nanoparticle (NP) surfaces can be modified to bind to overexpressed receptors in tumor cells,which can act as cancer imaging contrast agents, to increase the sensitivity and specificity of cancer detection methods [3,4]. In addition,microfluidic arrays and array-based sensing methods that use NPs are promising and novel cancer diagnostic approaches,with ultralow detection thresholds, short assay times, highthroughput capabilities, and low sample consumption [5,6].
NPs can also be used to improve lung cancer treatment.Precision nanomedicines, which have unique properties, including nanoscale sizes, high surface-to-volume ratios, and favorable physicochemical characteristics, could potentially be used to modulate the pharmacokinetic and pharmacodynamic profiles of cancer drugs, enhancing their therapeutic indexes. These characteristics are necessary properties of current precision medicines(PMs) [7-10] because many PMs, such as nucleic acid-based therapies and antibodies, suffer from poor targeting abilities and plasma stability, suboptimal pharmacokinetic properties, and immunological toxicities, which have prohibited their clinical translation [11,12].
An increasing number of studies have indicated the potential benefits of precision nanomedicine techniques for the early diagnosis and targeted therapy of lung cancer. Therefore, a critical review is necessary to provide a more complete understanding of these new strategies.
2. Personalized diagnosis of lung cancer
2.1. Nanotechnology for the in vivo diagnosis of lung cancer
Successful early diagnosis of lung cancer can improve survival rates. Conventional medical imaging technologies, such as magnetic resonance-guided focused ultrasound surgery (MRgFUS),are currently limited by the insensitivity of magnetic resonance imaging (MRI) for the visualization of small tumors [13]. NPs can be used as imaging contrast agents to increase the resolution and improve the anatomic definition of lesions. Recently, Wang et al.[14]constructed an active-targeting nanosized,theranostic,superparamagnetic iron oxide (SPIO) platform to increase the imaging sensitivity and energy-deposition efficiency of a clinical MRgFUS system. The surfaces of these polyethylene glycol (PEG)-ylated SPIO NPs were decorated with anti-epidermal growth factor receptor (EGFR)monoclonal antibodies,for the targeted delivery of NPs to EGFR-overexpressing lung cancer cells. The researchers demonstrated that using these NPs significantly improved MRI sensitivity for the visualization of EGFR-overexpressing lung cancer cells in a rat model.
Quantum dots (QDs) are semiconductor nanocrystals (2-100 nm in size),with unique optical and electrical properties.Compared with organic dye molecules,the bright fluorescence and high photochemical endurance of QDs make them promising for use in fluorescence imaging approaches [15]. Near-infrared (NIR)-emitting QDs, which exhibit high molar excitation coefficients,are particularly well-suited for in vivo whole-body imaging techniques because NIR light penetrates the body more deeply than light in the visible spectrum. The Papagiannaros et al. [16] prepared a tumor-targeted NIR-imaging agent composed of a cancer-specific monoclonal anti-nucleosome antibody, 2C5, coupled with QD-containing polymeric micelles. They demonstrated that this fluorescent imaging molecule exhibited excellent imaging properties, with a tumor fluorescence intensity 1 h after injection that was two-fold that of nontargeted, QD-loaded PEG-polyethylene micelles.
In addition to exploring the development of a single, powerful imaging modality, the development of multimodal approaches has been attempted, through the integration of several imaging technologies, in order to overcome the individual shortcomings of each technique [17]. For example, Xiao et al. [18] incorporated gadolinium-doped mesoporous silica nanoparticles (MSNs)and gold nanoparticles (AuNPs) into a single nanosystem(Gd2O3@MCM-41@Au)and found that Gd2O3@MCM-41@Au served as an efficient MRI contrast agent during cancer imaging and successfully targeted EGFR molecules,for surface-enhanced Raman scattering (SERS) detection.
2.2. Nanotechnology for the in vitro diagnosis of lung cancer
Accurate diagnoses require the determination of how and where to collect biopsy tissue samples.Critical advances in molecular biology have allowed the capture and analysis of tumorderived substances from body fluids [19,20]. The identification of clinically relevant alterations is now possible at the DNA, RNA,and protein levels[21].Therefore,physicians have multiple biopsy options when evaluating lung cancer patients,including lesions in the lungs, peripheral blood, and pleural effusion (PE). For population screening, noninvasive analyses such as peripheral blood analyses are preferable because tissues may not be available. In recent years,nanotechnology has made great contributions toward achieving the high sensitivity and high specificity that are necessary for accurate diagnoses. NPs possess large surface areas that can be linked with multiple diagnostic agents, improving the efficiency and sensitivity of diagnoses. Compared with traditional polymerase chain reaction (PCR)-based sequencing techniques,microfluidic (lab-on-a-chip) technologies and array-based sensing that use NPs are attractive alternatives for cancer diagnoses.
2.2.1. Tumor-derived DNA in peripheral blood
Extracellular DNA detected in the peripheral blood that shares genetic information with the host is referred to as cell-free DNA(cfDNA)[22],and cfDNA that originates from tumor cells is termed circulating tumor DNA(ctDNA). Currently, the exact mechanism through which ctDNA is released into the blood remains unknown.ctDNA is thought to be released from apoptotic and necrotic tumor cells or secreted from live tumor cells[23].Cancer patients present increased amounts of both cfDNA and ctDNA compared with healthy controls [24]. Because ctDNA carries the genomic variations and heterogeneity that have emerged during tumor evolution, ctDNA monitoring presents an obvious possible avenue for monitoring tumor status,progression,and the occurrence of treatment resistance in cancer patients.
To achieve the goal of using ctDNA to monitor tumor progression,ctDNA must first be enriched from peripheral blood samples.Both cfDNA and ctDNA in peripheral blood have low molecular weights, which require improved enrichment techniques because conventional methods for DNA isolation and extraction from peripheral blood are better suited for DNA molecules with medium and high molecular weights.Furthermore,the proportion of ctDNA in cfDNA varies widely,depending on individual variations.Therefore, isolation approaches are also necessary to distinguish ctDNA from cfDNA. Fortunately, technological advances have already facilitated the enrichment of rare ctDNA [25,26]. Nanomaterials have already been developed to enrich ctDNA using specific markers, such as epithelial cell adhesion molecule (EpCAM) and cytokeratin (CK).
Biocompatible AuNPs [27], in combination with polymer beads[28] and immunomagnetic beads [29], have been investigated for this scheme. In addition, nanostructure substrates that offer increased surface areas,such as nanotubes,nanopillars,nanowires,and nanotextured surfaces, could facilitate increased interactions with biomolecules, resulting in biosensing platforms that are capable of capturing or isolating ctDNA.For example,after being functionalized with an aptamer,nanotopographic substrates were able to selectively capture more than 90% of cancer cells [30].Nanostructured electric materials [31], such as the electroactive conducting polymer polypyrrole [32] and gold (Au) nanowires coated with polypyrrole [33], have also exhibited high efficiency levels for the isolation of ctDNA, with high yields and purities.
Early lung cancer detection is just one potential application of ctDNA analysis. ctDNA can reveal genetic variations associated with lung cancer and increase the reliability of diagnoses. Thus far, several researchers have successfully enriched ctDNA for the performance of molecular genotyping analyses in lung cancer patients [34,35]. A silicon nanowire substrate (SiNS) embedded in a microfluidic chip (Fig. 1), combined with the rationally designed, cell-based, systematic evolution of ligands by exponential enrichment (SELEX)-derived aptamers, has successfully enhanced the differential capture of circulating tumor cells from non-small cell lung cancer (NSCLC) patients [36].
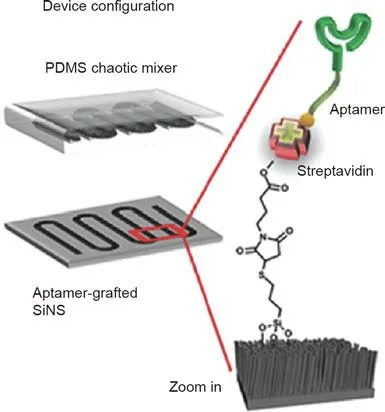
Fig.1. Schematic depiction of a microfluidic,circulating tumor cell chip,composed of an aptamer-grafted silicon nanowire substrate (SiNS) and an overlaid polydimethylsiloxane(PDMS)chaotic mixer.Reproduced from Ref.[36]with permission of Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany ©2016.
2.2.2. Tumor-derived DNA in PE
The emergence of PE is a common clinical manifestation of lung cancer. This excess fluid, which accumulates in the pleural cavity,contains tumor-derived DNA. Sampling PE is a practical approach to analyze the molecular profiles of lung cancer lesions [37]. Furthermore, in some patients, PE is the initial symptom of disease,and thus provides an early opportunity to detect tumor-derived DNA.
The enrichment of tumor-derived DNA from PE can be difficult because PE often contains various inflammatory and mesothelial cells. Existing strategies for isolating tumor-derived DNA rely on the macro-dissection, manual micro-dissection, or laser-capture micro-dissection of smears or cell blocks. Several successful cases using these strategies have been previously described [38,39].
2.2.3. Exosomes
Exosomes range from 30 to 150 nm in size and are released during the fusion between multivesicular endosomes (MVEs) and the plasma membrane [40]. Exosomes can alter the functions of various cells by regulating cell communications.A previous study indicated that exosomes may be involved in the oncogenic process,through the regulation of tumor immune responses, premetastatic niches, and chemotherapeutic resistance [41]. Thus,exosomes are potential specific targets for diagnosis and therapeutic interventions. In addition, exosomes have been identified and enriched from peripheral blood [42] and may, therefore, be clinically applicable specimens for cancer analysis.
Current exosome isolation methods, such as sucrose gradient ultracentrifugation and ultrafiltration, rely heavily on multiplestep ultracentrifugation processes, which are tedious and timeconsuming,as well as having low efficiency for discriminating exosomes from other biomaterials. Similar to existing techniques for the isolation and enrichment of ctDNA, microfluidic technologies are being developed to capture exosomes.These technologies have the advantages of small sample volume requirements, low costs,short operation times,and high sensitivity[43].The capture of targeted exosomes depends on the use of microfluidic channels that have been functionalized with antibodies,in order to enhance their affinities for targeted exosomes. For example, He et al. [44] developed an integrated microfluidic exosome analysis platform by combining a magnetic bead-based strategy with a multi-step analysis (Fig. 2). This device was able to successfully assess the total expression and phosphorylation levels of insulin-like growth factor 1 receptor (IGF-1R) in NSCLC patients by directly probing plasma exosomes. In addition to specific antibodies, aptamers[45], PEG-ylated lipids [46], and graphene oxide/polydopamine(GO/PDA) nano-interfaces [47] have demonstrated satisfactory performances as ligands. Aptamers are particularly stable in various solutions, including a wide range of salt concentrations, and under ionic and denaturing conditions. Furthermore, cancer cells can be sorted according to physical plasticity and diameter.
3. Precision nanomedicine for lung cancer
Compared with chemotherapy, which inhibits the proliferation of all cells, targeted cancer therapy specifically and accurately enacts tumor suppression by targeting the specific molecules involved in oncogenesis.The efficient and precise delivery of drugs to target lesions is a critical factor for the success of targeted treatments.
3.1. Drug delivery
The unique physicochemical properties of nanomaterials have made them premier options as both drugs and drug delivery systems (DDSs) for the targeted treatment of cancer [48]. Cancers demonstrate irregular cell growth, aided by the development of new blood vessel networks that are highly porous, with large spaces between endothelial cells. Taking advantage of the anatomical and pathophysiological differences between normal and tumor tissues,nanodrugs are designed to circulate in the blood for long periods and to accumulate at tumor sites through the enhanced permeability and retention(EPR)effect[49].In addition,nano-based DDSs have received attention for their potential to overcome problems associated with the solubility, stability,diffusivity, blood circulation half-time, and immunogenicity of chemotherapy drugs and to improve the specificity of drug release during cancer treatment[50].At present,nano-based DDSs include conventional liposomes, polymer NPs, dendritic polymers, and micelles, in addition to inorganic nanomaterials such as AuNPs,MSNs, and metal-organic frameworks. Furthermore, the favorable physicochemical, biochemical, and electrical properties of inorganic NP-based imaging contrast agents have resulted in improved sensitivity for positron emission tomography (PET), MRI, and single-photon emission computed tomography (SPECT) platforms,facilitating real-time observations of cancer progression during the treatment process.
3.1.1. Gold nanoparticles
The confirmed driver oncogene associated with NSCLC is a top potential treatment target. During recent decades, inorganic NPs have been widely investigated for use as drugs or DDSs in targeted cancer treatment [51], and AuNPs have attracted particular attention due to their unique optical properties, low toxicity, and the ease with which they can be prepared and functionalized. To achieve the maximum accumulation of NPs in tumor tissues and to increase efficiency, AuNPs have been modified with various active ligands. AuNPs bound to the anti-cancer drug methotrexate(MTX) have demonstrated high levels of tumor retention and enhanced therapeutic efficacy in a Lewis lung carcinoma mouse model, compared with an equal dose of free MTX, which may be attributed to the ‘‘concentrated effect” of MTX-AuNPs [52]. EGFR is a cell-surface receptor that is overexpressed in several tumor types, including NSCLC. In recent years, EGFR-targeted antibodies have become a popular targeting strategy for NSCLC treatment.Yokoyama et al. [53] have reported that Clone 225 antibodyconjugated hybrid plasmonic magnetic NPs (C225-AuFe NPs)exhibited enhanced antitumor activity through the induction of apoptosis and autophagy.Furthermore,a novel radioimmunotheranostic agent,131I-C225-AuNPs-PEG, was successfully synthesized(Fig. 3) and exhibited enhanced endocytosis and cytotoxicity against high-EGFR-expressing human A549 lung carcinoma cells;it also actively targeted an A549 tumor xenograft in a mouse model [54]. AuNPs have exhibited enhanced internalization via antibody-mediated endocytosis. Due to the strong and selective interaction that occurs between gold and sulfur groups, the use of sulfur-containing targeted ligands, which are chemisorbed onto the NP surface via sulfur bonds,can improve efficiency[55].AuNPs are also ideal carriers of micro-RNAs (miRNAs), which can further function as specific inhibitors [56].
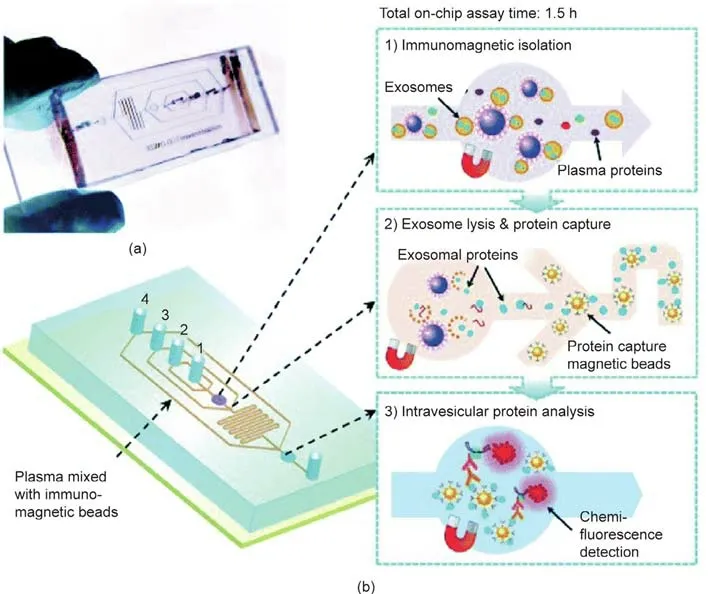
Fig. 2. Integrated microfluidic exosome analysis for NSCLC patients. (a) A PDMS chip, containing a microchannel network for exosome analysis; (b) integration of the streamlined, lab-on-a-chip, immunomagnetic isolation of exosomes, exosome lysis, protein capture, and intravesicular protein analysis. Reproduced from Ref. [44] with permission of the Royal Society of Chemistry, ©2014.
3.1.2. Mesoporous silica nanoparticles
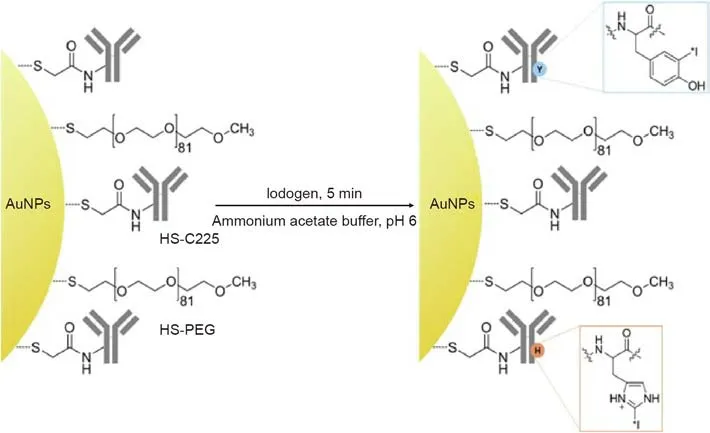
Fig. 3. Scheme depicting the radioiodination of C225-AuNPs-PEG using the iodogen method. Reproduced from Ref. [54] with permission of Elsevier Ltd., ©2013.
Due to their large surface areas, large pore volumes, and high levels of biochemical and physicochemical stability, MSNs have attracted substantial interest as potential DDSs [57,58]. MSNcoated gold nanorods (AuNRs) loaded with doxorubicin (DOX)have been designed to act as light-mediated, multifunctional,theranostic carriers for lung cancer treatments, and have been shown to result in enhanced cancer cell death,due to the synergistic effects of chemotherapy and hyperthermia-based treatments[59]. A tumor microenvironment-cascade pH-responsive DDS was constructed by functionalizing hollow MSNs (HMSNs) using β-cyclodextrin (β-CD) and adenosine deaminase (Ada)-PEG, and further loading them with DOX (HMSNs-β-CD/Ada-PEG@DOX).This approach effectively resolved the ‘‘PEG dilemma” and facilitated the specific release of loaded drugs in cancer cells, inducing cell apoptosis and inhibiting tumor growth with minimal toxic side effects(Fig.4)[60].EGFR monoclonal antibody-capped MSNs have been demonstrated to specifically target EGFR-mutant lung cancer cells and to efficiently release loaded drugs in the cancer cells.The release speed of therapeutic reagents from MSNs can also be adjusted by developing a smart nanomedicine system.
3.1.3. Nanoscale coordination polymers and nanoscale metal-organic frameworks
Nanoscale coordination polymers (NCPs)and nanoscale metalorganic frameworks(NMOFs),which are constructed from the selfassembly of metal-connecting points and organic bridging ligands,have also been developed as DDSs for cancer therapy, due to their tunable compositions, sizes, and shapes; ease of surface modification; high drug-loading capacities; and intrinsic biodegradability properties.Several reports have focused on the application of NCPs and NMOFs as DDSs. Lipid-coated and anisamide-targeted NCPs displayed enhanced cytotoxicity against human lung cancer cells,compared with as-synthesized particles or free bisphosphonates[61]. The Liu et al. [62] further constructed zinc (Zn)-bisphosphonate NCPs carrying either 48%±3% (weight percent)cisplatin prodrug or 45%±5%(weight percent)oxaliplatin prodrug.The NCPs were PEG-ylated to further stabilize the particles and to inhibit the burst release of drugs. Both NCPs demonstrated enhanced antitumor activities when compared with free drugs in three different tumor models. Folate (Fol)-targeted calcium zoledronate (CaZol) NMOFs have been fabricated by incorporating Fol-targeted ligands into CaZol NMOFs, which possess excellent chemical and colloidal stability under physiological conditions.The encapsulated zoledronate was released from the NMOFs in mid-endosomes during endocytosis and exhibited increased efficiency for the inhibition of NSCLC proliferation and the induction of apoptosis, compared with small-molecule zoledronate [63].
3.2. Nanotheranostics
Recently, the early diagnosis and targeted therapeutic results associated with the use of nanomedicines during lung cancer have encouraged scientists to explore ‘‘nanotheranostics,” which are sub-micrometer-sized carrier materials that contain both drugs and imaging agents within a single formulation [64,65].Nanotheranostics hold the potential to contribute to the development of personalized approaches to cancer management.Common diagnostic agents include SPIOs, QDs, radionuclides, and heavy elements, such as iodine. An ideal theranostic nanomedicine would recognize a specific target, bind to specific receptors on the targeted cell membrane, diagnose the cancer morphology,and provide effective therapy, while simultaneously possessing biocompatibility and biodegradability. Nanotheranostics are expected to provide practical solutions for cancer treatments and cures during the early stages of lung cancer.
The first example of theranostic nanomedicine, which combined chemotherapeutic and photothermal therapy (PTT) in vivo,was constructed by conjugating Taxol-loaded poly(lactide-coglycolic acid) (PLGA) NPs with iron oxide NPs and QDs [66]. QD/Fe3O4/Taxol-loaded PLGA NPs can potentially serve as contrast agents for MRIs, and AuNRs can convert NIR light into heat, in order to simultaneously achieve the photothermal ablation of tumor tissue and destroy spherical PLGAs, efficiently releasing encapsulated Taxol. In vivo, AuNR/QD/Fe3O4/Taxol-loaded PLGA NPs were intratumorally injected into transplanted tumors in mice,resulting in the progressive reduction of tumor volumes.

Fig. 4. Schematic illustration showing the drug delivery process of the HMSNs-β-CD/Ada-PEG system in a tumor microenvironment,in vivo. CTAB: N-cetyltrimethylammonium bromide; HepG2: human hepatocellular carcinoma. Reproduced from Ref. [60] with permission of Elsevier Ltd., ©2016.
The Jing et al. [67] successfully fabricated a robust theranostic cerasome named ICG@DPDCs-177Lu for NIR fluorescence imaging and the photothermal ablation of cancer cells by encapsulating indocyaninegreen(ICG)in1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)2000]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid monoamide(DSPE-PEG2000-DOTA) and then chelating the radioisotope177Lu.ICG@DPDCs-177Lu can be used as an attractive radionuclide cancer therapy; in vivo, the ICG@DPDCs effectively ablated cancer cells through photothermal effects (Fig. 5).
4. Improvements in traditional lung cancer diagnosis methods using nanomedicine
Traditionally, histological examinations of resected tumors have been necessary in order to make reliable diagnoses. For preclinical diagnoses, CT and MRI are the most commonly used approaches. However, the use of nontargeted contrast agents during diagnosis has intrinsic limitations, such as low sensitivity and specificity, which can affect accurate tumor localization. NPs have been synthesized to overcome these problems.By conjugating NPs with other moieties, which act as markers, the morphologies of tumors can be more clearly delineated [68].
Inorganic nanomaterials have long been used for DDSs,imaging,tumor treatment, diagnosis, and prognosis. The most commonly employed materials are gold, silver (Ag), silica, and iron oxides.Several studies have focused on AuNPs,due to their biocompatible,multifunctional, and theranostic properties. For example, silicagold nanoshells,modified with PEG,were synthesized for use during PTT,which can be applied to solid tumors using NIR light[69].Knights and McLaughlan [70] demonstrated the size-dependent effects of AuNRs on both the photoacoustic(PA)imaging response and pulsed-wave photothermal therapeutic efficacy. A study demonstrated the in vivo lung cancer antitumor activity of silver nanoparticles (AgNPs) against lung cancer cells and a xenograft mouse model[71].However,the toxicity of inorganic nanomaterials can result in harmful impacts on normal cells. Because the mononuclear phagocyte system(MPS)is responsible for the clearance of drugs, non-biodegradable inorganic NPs and toxic macromolecules can be difficult to clear from the human body.Cytotoxicity tests are usually performed using trypan blue and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT)assays.
To overcome the difficulties associated with inorganic NPs,biocompatible organic nanomaterials and ultrasmall biodegradable nanomedicines have been developed [72,73]. Ultrasmall SPIOs(USPIOs),for example,are biodegradable NPs that are smaller than 50 nm,which allows them to pass through even the smallest blood vessels and remain in circulation[74,75].In addition,these NPs are nontoxic to major MPS organs, such as the bone marrow, spleen,and kidney,and are easily cleared from the body;thus,these novel nanomaterials have a potentially bright future.
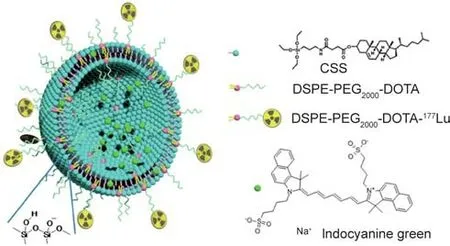
Fig.5. Structural illustration of 177Lu-labeled cerasomes encapsulating indocyanine green (ICG@DPDCs-177Lu). CSS: cholesteryl succinyl silane. Reproduced from Ref.[67] with permission of American Chemical Society, ©2015.
5.Improvements in traditional lung cancer treatment methods,using nanomedicine
Several lung cancer treatments are currently available, including surgical resections, chemotherapy, and radiation; however,none of these options are optimal for lung cancer therapy [76].Although the surgical resection of lung cancer is a relatively effective treatment method, by the time most lung cancers are diagnosed, the patients have often reached an advanced stage of lung cancer or metastasis has occurred,which can make operations difficult and sometimes unfeasible to perform. The differentiation between tumors and surrounding normal tissues can be vague,which poses a great challenge during surgery and increases the likelihood of either overtreatment or relapse. Multiple-drug resistance (MDR) is a major problem associated with chemotherapy,and most chemotherapeutic drugs are toxic to both tumor cells and normal tissues. Radiation can cause serious side effects in patients and reduce their quality of life.
The surfaces of NPs can be modified with fluorescent dyes,hydrophilic ligands, and specific molecules that have affinities for unique proteins that are only expressed on the tumor surface;these fluorescently tagged nanomaterials can then be used to guide surgery [77,78]. NPs can increase the concentration of chemotherapeutic drugs near tumors, either through the EPR effect or through active-target delivery [79,80]. Angiogenic blood vessels found in tumors differ from their normal counterparts due to the presence of gaps between adjacent endothelial cells,which are large enough to induce the EPR effect. Active targeting involves the conjugation to the NP surface of either a targeting ligand or antibodies that specifically recognize tumor cells. Many factors, including the size, charge, surface modifications, and angiogenesis of the tumor, the tumor microenvironment, and the half-lives of NPs, can affect the final accumulation of NPs in tumors. Several liposomal NPs have been used during clinical treatments, including the most popular liposome formulation,DOX; some are available on the market, including liposomal daunorubicin and stealth liposomal DOX [81-83]. However, the majority of DOX-encapsulated liposomes remain in the clinical trial phase. By modifying the liposome surface, NPs can extend the circulation time of liposomes [84]. Moreover, chemotherapy drugs can be loaded into the nanoliposome capsule, thereby combining chemotherapy with NPs [85]. Liposomal paclitaxel is another example of a liposomal NP that has been used for the clinical treatment of solid tumors [86-88].
NPs can enhance the response to radiation and improve immunotherapeutic efficacy by regulating the tumor microenvironment [89,90] or integrating chemotherapies with other neoadjuvant therapies or adjuvant treatments [91-93]. Some nanomaterials possess unique properties that can be used to attack cancer cells. Photodynamic therapy (PDT) [94,95] and PTT [96,97]are based on nanomaterials with high efficacy during light-toheat conversions. PDT and PTT can be used as alternative treatments for patients who are unable to undergo surgical resections.The administration of both therapies requires photosensitizers(PSs), which have low efficacies [98,99]. Coupling PSs with targeting molecules or encapsulating them within the cores of nanomaterials can improve the efficiencies of both PDT and PTT.Because of their light-to-heat conversion properties,these NPs can also be utilized to perform photoacoustic imaging(PAI)[100,101].Combining therapy with imaging is consistent with the theory of developing multifunctional materials.Other novel therapies,such as ion interference and chemo-dynamic treatments, are also NP-dependent.
6. Perspective
Tumorigenesis is a complicated process that is associated with multiple changes in molecular biology. To date, functionalized nanomaterials and nanotechnologies,such as microfluidic devices,have achieved great success in improving the efficiency and specificity of ctDNA, tumor-derived DNA, and exosome isolation and detection during the diagnosis of lung cancer. However, single biomarkers may not be adequate for every cancer patient. As an alternative, PM, using a panel of molecular biomarkers identified by genomic and proteomic studies, may be more effective for the early screening of lung cancer.In the future,identifying additional biomarkers and developing a new generation of biosensors, with the help of microfluidics, will facilitate decreases in the cancer mortality rate.
In addition,precision nanomedicine is a promising tool for cancer treatment. Several precision nanomedicine platforms have been developed and used during clinical cancer care. However,concerns regarding the safety of nanomedicines persist. Thus, the comprehensive characterization of nanomedicine products and standards, using both in vitro and in vivo models, remains necessary to predict the performance of nanomedicines when translated to clinical applications.
Acknowledgments
This work was supported by the National Program on Key Basic Research Project (2020YFA0211100), National Natural Science Foundation of China(51872205,51922077,and 81602412),Fundamental Research Funds for the Central Universities, Training Plan of Outstanding Young Medical Talents, Shanghai Municipal Commission of Health and Family Planning (2017YQ050), Scientific Research Project of Shanghai Municipal Commission of Health and Family Planning (2016Y0121), Natural Scientific Foundation of Shanghai (134119b1002), and Outstanding Young Scientific Researcher of Shanghai Pulmonary Hospital.
Compliance with ethics guidelines
Wei Yin,Feng Pan,Junjie Zhu,Junwu Xu,Diego Gonzalez-Rivas,Meinoshin Okumura, Zhiyong Tang, and Yang Yang declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- PVC Formulation of Anastrepha suspensa Pheromones Suitable for Field Studies
- Handheld Ultrasound Advances Diagnosis
- Nuclear Energy Seeks Revival with Advanced Fuel Options
- Mars Helicopter Exceeds Expectations
- Toward Systemic Thinking in Managing Environmental Risks
- The Experimental Advanced Superconducting Tokamak

