Effects of moxibustion on the P2X7R/STAT3/VEGF pathway in rats with colitis-associated colon cancer
Lin Ya-ying (林亚莹), Wang Di (汪迪), Wu Huan-gan (吴焕淦), Gu Mu-en (顾沐恩), Li Qi (李琪), Ma Zhe (马喆),Huang Yan (黄艳), Lu Yuan (陆嫄), Li Kun-shan (李昆珊), Wu Lu-yi (吴璐一)
1 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
2 Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine,Shanghai 200437, China
3 Key Laboratory of Acupuncture and Immunological Effects, Shanghai University of Traditional Chinese Medicine, Shanghai 200030,China
Abstract
Objective: To observe the effects of herb-partitioned moxibustion and ginger-partitioned moxibustion on the growth of colon tumors in rats with colitis-associated colon cancer (CACC), and explore the mechanism of moxibustion intervening CACC through the purinergic receptor P2X ligand-gated ion channel 7 (P2X7R)/signal transducer and activator of transcription 3 (STAT3)/vascular endothelial growth factor (VEGF) pathway.
Methods: A total of 26 male Sprague-Dawley rats were selected. According to the random number table method, 6 rats were selected as the normal group. The remaining 20 rats were injected intraperitoneally with azoxymethane (AOM)combined with oral dextran sodium sulfate (DSS) to prepare the CACC model. After the model was successfully established, 2 rats were randomly selected for model identification. The remaining 18 rats which were successfully modeled were randomly divided into a model group, a herb-partitioned moxibustion group and a ginger-partitioned moxibustion group, with 6 rats in each group. Moxibustion intervention was performed in the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group at Qihai (CV 6) and bilateral Tianshu (ST 25).Moxibustion was performed twice at each point each time, once a day, at a 1-day interval after 6 consecutive interventions, for a total of 30 interventions. After intervention, the colon tumor load, pathological change and histopathological score were observed. Immunohistochemistry was used to detect the expressions of VEGF, P2X7R,phospho-STAT3 (p-STAT3), and nuclear factor-kappa B p65 (NF-κB p65) proteins in rat colon tissue. Western blot was used to detect the levels of p-STAT3 and NF-κB p65 proteins in rat colon tissue.
Results: Compared with the normal group, the colon tumor load and histopathological score in the model group were significantly increased (both P<0.001), and different grades of dysplasia were observed in colon tissue from the model group, reaching the degree of adenocarcinoma; the expression level of P2X7R protein in colon tissue was significantly decreased (P<0.001), and the expression levels of p-STAT3, NF-κB p65 and VEGF proteins were significantly increased (all P<0.001) in the model group. Compared with the model group, the colon tumor load, colon histopathological score and the levels of p-STAT3, NF-κB p65 and VEGF proteins in colon tissue were significantly decreased (all P<0.05) in the herbpartitioned moxibustion group and the ginger-partitioned moxibustion group while the expression levels of P2X7R protein in colon tissue were significantly increased (both P<0.05).
Conclusion: Both herb-partitioned moxibustion and ginger-partitioned moxibustion can reduce the colon tumor load in CACC rats and delay the progression of colon adenomas. The mechanism may be mediated by the P2X7R/STAT3 pathway to inhibit STAT3 phosphorylation, thereby reducing VEGF protein expression.
Keywords: Moxibustion Therapy; Medicinal Cake-partitioned Moxibustion; Ginger-partitioned Moxibustion; Colitisassociated Neoplasms; Vascular Endothelial Growth Factor; Receptors, Purinergic P2X7; STAT3 Protein; NF-kappa B
Colitis-associated colon cancer (CACC) is a type of colorectal cancer. Its pathogenesis is characterized by chronic inflammation or damage to the colorectal mucosa, which gradually evolves into mild dysplasia,then to severe dysplasia, and finally to cancer[1]. CACC is a typical model manifesting the pathological process of colitis-induced carcinoma, and it has become one of the hot spots in the study of tumorigenesis[2-3]. Angiogenesis plays an important role in the development of tumor and metastasis. Tumors release vascular growth factors to stimulate blood vessel growth, thereby providing oxygen and nutrients to promote tumor growth[4].
As an important angiogenic factor, vascular endothelial growth factor (VEGF) and its receptors promote the formation of new blood vessels through autocrine or paracrine pathways and are considered one of the key indicators of malignancy of CACC[5]. VEGF requires signal transducer and activator of transcription 3 (STAT3) to promote CACC colonic epithelial cell proliferation and tumor growth[6]. STAT3 is regulated by purinergic receptor P2X ligand-gated ion channel 7(P2X7R) and nuclear factor kappa-B (NF-κB)[7-8].
Clinical studies have found that moxibustion relieves the pain associated with colon cancer, promotes the recovery of gastrointestinal function, and improves the quality of life of patients with colorectal cancer[9-11]. Basic research has found that moxibustion may reduce tumor cell proliferation by promoting tumor cell differentiation and apoptosis, and interfering with tumor cell nucleic acid metabolism[12]. Our team found that moxibustion may inhibit the growth of CACC tumors by regulating the abnormal expression of P2X7R and cellular myelocytomatosis oncogene (c-Myc) proteins[13].However, no study has determined whether moxibustion inhibits CACC tumor angiogenesis. Therefore, this study explored the effect of moxibustion on the P2X7R/STAT3/VEGF pathway in CACC model rats from the perspective of angiogenesis to provide a scientific basis for moxibustion to treat colorectal cancer.
1 Materials and Methods
1.1 Experimental animals and groups
Twenty-six clean grade male Sprague-Dawley rats weighing (100±20) g were provided by Shanghai Slack Laboratory Animal Co., Ltd., China. The animal production license number was SCXK (Hu) 2017-0005.The rats were raised in a 12 h/12 h day and night environment at a temperature of 18-22 °C and a relative humidity of 50%-70%. After 1 week of adaptive feeding,rats were randomly divided into 6 in the normal group and 20 for modeling (2 rats were used for model identification) using the random number table method.After successful modeling, 18 model rats were randomly divided into the model group, herb-partitioned moxibustion group and ginger-partitioned moxibustion group, with 6 rats in each group. All experiments were conducted in accordance with the requirements of the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine.
1.2 Main reagents and instruments
Fully automatic tissue dehydrator (Le ica, Germany);tissue embedding machine (Thermo Fisher, USA); optical microscope (Olympus, Japan); glue rack, transfer instrument, electrophoresis tank and gel imaging system(Bio-Rad, USA).
Five percent bovine serum albumin (BSA) blocking solution (Shanghai Weiao Biotechnology Co., Ltd., China);anti-VEGF antibody, anti-P2X7R antibody and antiphospho-STAT3 (p-STAT3) antibody (Abcam, UK); anti-NF-κB p65 antibody (Millipore Sigma, USA); radio immunoprecipitation assay (RIPA) lysate (Shanghai Weiao Biotechnology Co., Ltd., China); RNA enzyme inhibitor (Thermo Fisher, USA); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) internal reference and universal immunohistochemical kit for pika(Shanghai Weiao Biotechnology Co., Ltd., China).
1.3 Preparation of CACC model
Based on previous research, intraperitoneal injection of 2 mg/mL azoxymethane (AOM) was administered at 10 mg/(kg·bw), and after 1 week of feeding, dextran sodium sulfate (DSS) was administered via drinking water for 3 cycles of inflammatory stimulation at concentrations of 3%, 2%, and 2%, respectively[13]. In the first cycle, after drinking 3% DSS water for 4 d, rats were provided with normal drinking water for 17 d. In the 2nd and 3rd cycles, rats were provided with 2% DSS in the drinking water for 4 d followed by normal drinking water for 17 d (see Figure 1 for details). After the model was established, 2 model rats were anesthetized and euthanized, and colon tissues were removed to observe the presence or absence of tumors and to judge whether the model was successfully established.

Figure 1. CACC model preparation
1.4 Intervention methods
On the day before moxibustion intervention and after model establishment, the hair near the acupoints on the abdomen of rats in each group was shaved.
1.4.1 Herb-partitioned moxibustion group
Acupoints: Qihai (CV 6) and Bilateral Tianshu (ST 25)were chosen[14-16]. Tianshu (ST 25) is located 5 mm lateral to the rat's navel (on the midline of the abdomen, the intersection of the upper 2/3 and the lower 1/3 of the line between the xiphoid process and the upper edge of the symphysis pubis). Qihai (CV 6) is located at the midline of the rat's abdomen, 12.5 mm below the navel.
Methods:Fu Zi(Radix Aconiti Lateralis Praeparata)10 g,Mu Xiang(Radix Aucklandiae) 2 g,Rou Gui(Cortex Cinnamomi) 2 g,Dan Shen(Radix Salviae Miltiorrhizae et Rhizoma) 3 g,Hong Hua(Flos Carthami) 3 g and other herbs were grinded into powder, and an appropriate amount of rice wine was added to make medicinal mash.The medicinal mash was shaped into a medicinal cake with a diameter of 0.8 cm and a thickness of 0.4 cm by a brass mold. Medicinal cakes were placed at Tianshu(ST 25) and Qihai (CV 6), and moxa sticks weighing approximately 90 mg each were placed on the medicinal cakes. Moxibustion was performed twice at each acupoint each time, once a day, 6 times a week for a total of 30 treatments.
1.4.2 Ginger-partitioned moxibustion group
Acupoints: Same as in the herb-partitioned moxibustion group.
Methods:Fresh ginger was cut into ginger slices of approximately 0.8 cm in diameter and 0.4 cm in thickness. The ginger slices were placed on the acupoints of the rats. Moxa sticks of the same specification were placed on the ginger slices as in the herb-partitioned moxibustion group for moxibustion. The intervention parameters were the same as those in the herbpartitioned moxibustion group.
1.4.3 Normal group
Rats in the normal group only received the same fixation as in the above groups without other interventions.
1.4.4 Model group
Rats in the model group only received the same fixation without other interventions.
1.5 Sampling of animal specimens
After treatment, rats in each group were fasted for 24 h and anesthetized by intraperitoneal injection[2% sodium pentobarbital, dose 40-50 mg/(kg·bw)].After the neck was severed, about 5 cm long colon from the pubic symphysis to the ileocecal area was removed.The colon was cut longitudinally along the mesentery.The feces and blood were cleaned with normal saline.The number of colon tumors was observed with the naked eye, and the tumor diameter was measured. A section of the colon (including the tumor) approximately 1 cm long was cut and fixed with 4% paraformaldehyde.The remaining colon tissue was cut into pieces, placed into cryopreservation tubes, incubated in liquid nitrogen for 1 h, and stored in a -80 °C freezer.
1.6 Observation indicator and test methods
1.6.1 Number, size and tumor load (TL) of tumors in rats[17]
The tumor number (TN) (diameter greater than 1 mm),visible to the naked eye in the colon, was counted. The diameter of each tumor was measured, and the average diameter was calculated. TL meant the sum of the long diameter of all tumors.
1.6.2 Observation of histopathology of rat colon by hematoxylin-eosin (HE) staining
The slices were routinely deparaffinized to water;stained with hematoxylin for 2 min, rinsed with tap water for 10 min, 1% hydrochloric acid alcohol for 2 s, and tap water for 5 min; stained with eosin for 2 min before dehydration in a gradient of 70%, 80%, 90%, and 100%alcohol solutions for 2 s each, followed by soaking in xylene I and II for 15 min to make the slices transparent;mounted with neutral gum. Three different fields were randomly selected from each tissue slice to score colon cancer pathology, referring to the method of Rose AH[18]to score colon cancer pathology. See Table 1 for details.
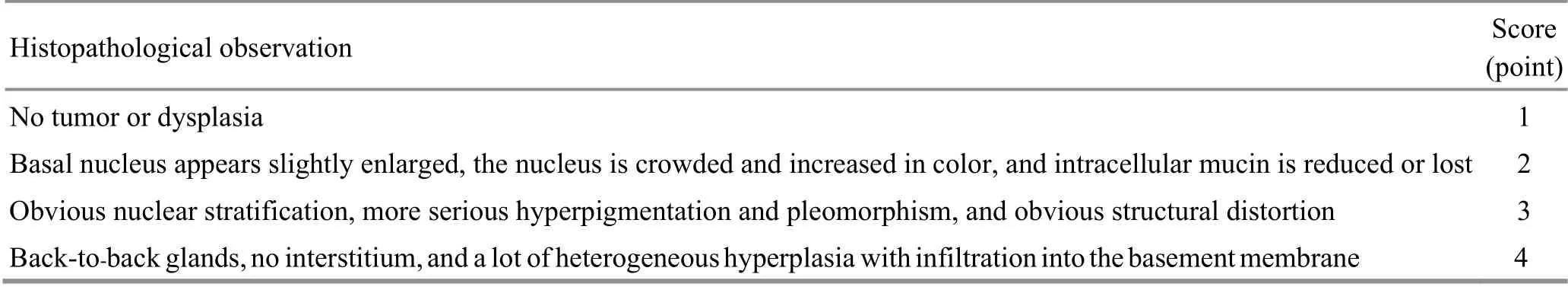
Table 1. Scoring criteria for colonic histopathology
1.6.3 Immunohistochemistry (IHC) to detect the expressions of VEGF, P2X7R, p-STAT3, and NF-κB p65 proteins
The slices were routinely deparaffinized to water;soaked in 3% H2O2for 10 min and then rinsed with tap water for 10 min; immersed in microwave-preheated antigen retrieval solution, heated in a microwave oven with moderate-high heat for 10 min, cooled to room temperature, repaired for 12 min, and rinsed with tap water for 10 min; immersed in the antigen retrieval solution for 10 min, cooled to room temperature,repaired for 12 min, cooled to room temperature, and rinsed with PBS; 5% BSA blocking solution was added to the slices dropwise and incubated for 35 min before the dropwise addition of the primary antibody [diluted P2X7R (1:10 000), p-STAT3 (1:200), NF-κB p65 (1:1 000),and VEGF (1:50)], incubated in a 4 °C refrigerator overnight, and washed with PBS. The secondary antibody was added dropwise and the mixture was incubated at 37 °C for 35 min. The slices were washed with PBS; the DAB color developing solution was added dropwise. Hematoxylin was added dropwise, incubated for 2 min and then rinsed; routine dehydration and mounting were performed; 3 to 5 fields of view were randomly selected from each tissue slice, images were captured, and the pictures were imported into Image-PlusPro 6.0 software to calculate the positive target area(area) in each image and the integrated optical density(IOD). The average optical density (AOD) of the positive target was also calculated, AOD = IOD ÷ Area.
1.6.4 Western blot (WB) to detect the expressions of p-STAT3 and NF-κB p65 proteins
The colon tissue was cut into small pieces, mixed with RIPA lysis buffer, grinded and centrifuged; the BCA protein concentration determination kit was used to determine the protein concentration; the gel was prepared using standard methods; after the electrophoresis samples had been prepared, and electrophoresis at a constant voltage of 80 V for 1 h was provided, when the bromophenol blue indicator entered the separation gel, electrophoresis was performed at a constant voltage of 120 V. When the indicator reached approximately 0.5 cm from the lower end of the gel,electrophoresis was stopped and the gel was removed; a transfer membrane sandwich was prepared and transfer membrane buffer solution was added. The sandwich was placed on ice and proteins were transferred to the membrane at a constant current of 200 mA for 50-70 min according to different target proteins; the PVDF membrane was blocked with 5% BSA at room temperature for 2 h and then washed with PBST on a shaker. The primary antibodies [dilutions for p-STAT3(1:20 000), NF-κB p65 (1:2 000) and β-actin (1:1 000)]were added to the membrane and incubated overnight at 4 °C, and the membrane was washed with PBST on a shaker. Horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:2 000) was incubated with the membrane on a shaker for 2 h at room temperature, and then the membrane was washed with PBST on a shaker;the membrane was immersed in the chemiluminescence detection reagent and placed in the gel imaging system for automatic exposure after 2 min; images were saved for later use. A gel image processing system was used to digitally analyze the gray value of the sample band.
1.7 Statistical analysis
SPSS version 26.0 statistical analysis software was used for statistical analysis of data. If the measurement data conformed to a normal distribution, they were presented as mean ± standard deviation (±s). The comparison between groups was performed using oneway analysis of variance, and the pairwise comparison used the least significant difference (LSD) method or the Tamhane method; if the measurement data didn’t conform to a normal distribution, they were presented as median (lower quartile, upper quartile) [M (QL, QU)],the rank sum test was used for comparison between groups. The test level wasα=0.05, andP<0.05 indicated that the difference was statistically significant.
2 Results
2.1 Observation of the effect of moxibustion in inhibiting tumor growth in rats with CACC
2.1.1 Comparison of colon tumor formation in rats from each group
Macroscopically, rats in the model group, herbpartitioned moxibustion group, and ginger-partitioned moxibustion group had tumors in the colon. The tumors were located in the distal two-thirds of the colon, mainly near the rectum. Compared with the normal group, the average number of tumors in the model group increased significantly (P<0.001), and the average tumor diameter and TL increased significantly (bothP<0.001). Compared with the model group, the average numbers of tumors of rats in the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group were significantly reduced (bothP<0.01), and the average tumor diameter and TL were significantly reduced (allP<0.01). No significant differences in the average number of tumors,average tumor diameter, or TL were observed between the herb-partitioned moxibustion group and the gingerpartitioned moxibustion group (P>0.05). See Table 2 for details.
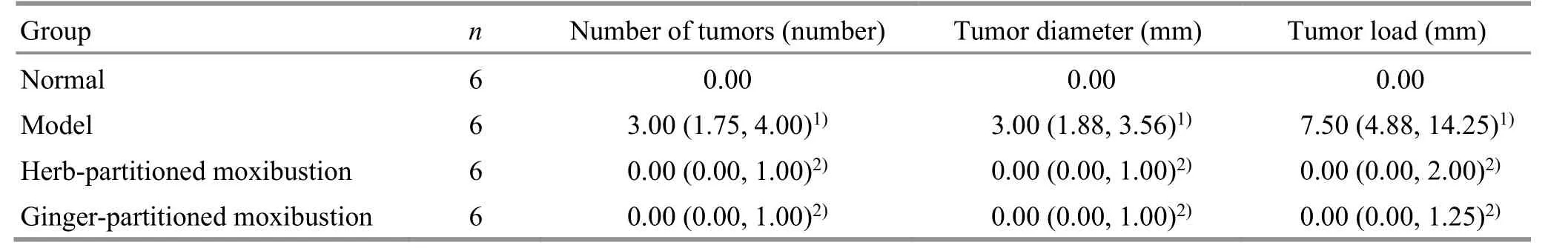
Table 2. Comparison of colon tumor formation in rats from each group [M (QL, QU)]
2.1.2 Observation of histopathology in rats from each group
In the normal group, clear mucosal structure,complete epithelial tissue, and orderly-arranged glands were observed in the colon. Capillaries and scattered lymphocytes were observed in the lamina propria of the mucosa. No congestion, edema, or inflammatory cell infiltration was observed. In the model group, the size and shape of the glands were very irregular, with the arrangement disordered, goblet cells lost, glandular epithelial cells in the glands missing their polar orientation and closely overlapped, and multiple layers(marked by the red arrow) visible. The nucleus was large and deeply stained. The ratio of nucleus to cytoplasm increased, mitosis increased, interstitial congestion and edema were observed, and part of the gland cavity substantially expanded and became cystic (marked by red arrow). In the herb-partitioned moxibustion group,colonic epithelial cells proliferation, lymphocyte infiltration, inherent gland atrophy and dysplasia were all lower than in the model group. In the ginger-partitioned moxibustion group, colonic epithelial cell proliferation,disordered gland arrangement, deep nuclear staining inflammatory cell infiltration, and interstitial congestion and edema were less compared with the model group.See Figure 2 for details.
The colonic histopathological score of the model group was significantly higher than that of the normal group (P<0.001). Compared with the model group, the colonic histopathological scores of rats in the herbpartitioned moxibustion group and ginger-partitioned moxibustion group were significantly reduced (bothP<0.05). No significant difference in the histopathological score was observed between the herbpartitioned moxibustion group and the gingerpartitioned moxibustion group (P>0.05). See Table 3 for details.
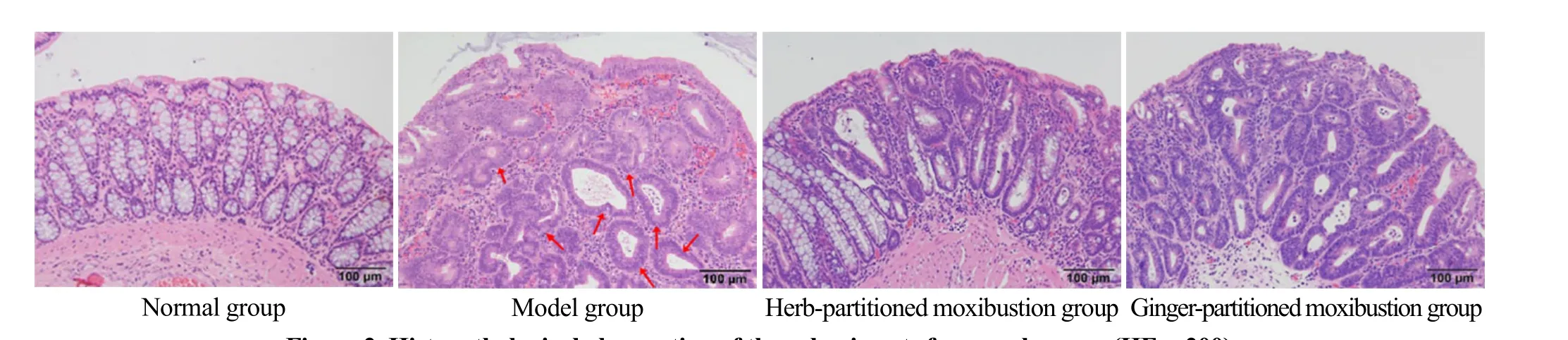
Figure 2. Histopathological observation of the colon in rats from each group (HE, ×200)

Table 3. Histopathological score of rat colon [M (QL, QU), point]
2.2 Effects of moxibustion on the P2X7R/STAT3/VEGF pathway in CACC model rats
2.2.1 Expression of VEGF protein in the colon tissue of rats in each group
VEGF protein was mainly expressed in the cytoplasm of epithelial cells and glandular epithelial cells, and the positive expression displayed a brown-yellow granular pattern. In the normal group, only a small amount of VEGF protein was detected in the cytoplasm of the abovementioned cells. In the model group, the cytoplasm of these cells was deeply stained brownish yellow and the staining was densely distributed,suggesting a large amount of VEGF protein expression.Compared with the model group, the positive expression in the herb-partitioned moxibustion group and gingerpartitioned moxibustion group decreased to different degrees. The quantitative analysis of AOD showed that the expression of VEGF protein in colon tissue of the model group was significantly increased compared with the normal group (P<0.001). Compared with the model group, the expressions of VEGF protein in colon tissue of rats in the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group were significantly down-regulated (bothP<0.001). A statistically significant difference in the expression of VEGF protein in colon tissue was not observed between the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group (P>0.05). See Figure 3 and Figure 4 for details.

Figure 3. VEGF protein expression in colon tissue from rats in each group (IHC, ×200)

Figure 4. Comparison of VEGF protein expression in colon tissue from rats in each group (IHC)
2.2.2 Expression of P2X7R protein in colon tissue from rats in each group
P2X7R protein was mainly expressed in the cytoplasm of epithelial cells and glandular epithelial cells, and the positive expression was brown-yellow. In the normal group, the cytoplasm of the abovementioned cells was deeply stained brownish yellow and the staining was densely distributed, suggesting a large amount of P2X7R protein expression. In the model group, only a small amount of P2X7R protein was detected in the cytoplasm of these cells. Compared with the model group, the positive expression in the herb-partitioned moxibustion group and ginger-partitioned moxibustion group increased to different degrees. The quantitative analysis of AOD showed that the expression of the P2X7R protein in rat colon tissue in the model group was significantly lower than in the normal group (P<0.001). Compared with the model group, the expressions of the P2X7R protein in colon tissue of rats in the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group were significantly increased, and the differences were statistically significant (bothP<0.001).A significant difference in the expression of the P2X7R protein in colon tissue was not observed between the herb-partitioned moxibustion group and the gingerpartitioned moxibustion group (P>0.05). See Figure 5 and Figure 6 for details.

Figure 5. P2X7R protein expression in colon tissue from rats in each group (IHC, ×200)
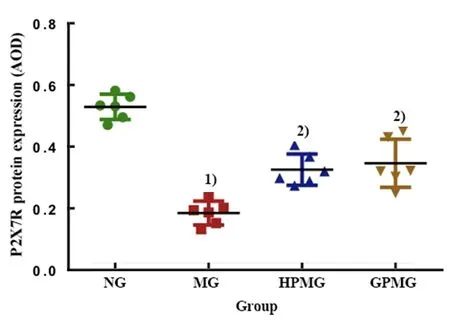
Figure 6. Comparison of P2X7R protein expression in colon tissue from rats in each group (IHC)
2.2.3 Expression of p-STAT3 protein in colon tissue from rats in each group
p-STAT3 protein was mainly expressed in the nuclei of epithelial cells and glandular epithelial cells, and the positive expression was visible as brown staining. In the normal group, only a small amount of p-STAT3 protein was expressed in the nuclei of the abovementioned cells.In the model group, the nuclei of these cells were deeply stained brownish-yellow and densely distributed,suggesting a large amount of p-STAT3 protein expression.Compared with the model group, the positive expression in the herb-partitioned moxibustion group and gingerpartitioned moxibustion group decreased to different degrees. The quantitative analysis of AOD showed that the expression of p-STAT3 protein in the colon tissue of the model group was significantly increased compared with the normal group (P<0.001). Compared with the model group, the expressions of p-STAT3 protein in colon tissue of rats in the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group were significantly down-regulated (bothP<0.001). A statistically significant difference in the expression of p-STAT3 protein in colon tissue was not observed between the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group (P>0.05). See Figure 7 and Figure 8 for details.
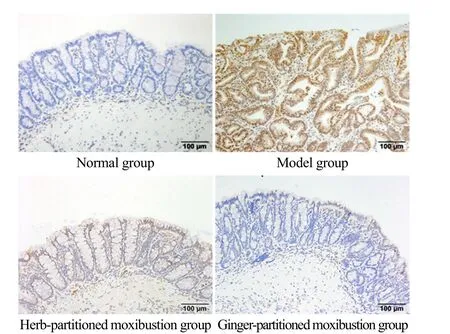
Figure 7. p-STAT3 protein expression in colon tissue from rats in each group (IHC, ×200)

Figure 8. Comparison of p-STAT3 protein expression in colon tissue from rats in each group (IHC)
WB was performed for further verification. Compared with the normal group, the level of the p-STAT3 protein in the colon tissue of the model group was significantly increased (P<0.001). Compared with the model group,the levels of p-STAT3 in the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group were significantly down-regulated (bothP<0.001). A statistically significant difference in the level of the p-STAT3 protein in the colon tissue was not observed between the herb-partitioned and ginger-partitioned moxibustion groups (P>0.05). See Figure 9 for details.
2.2.4 Expression of NF-κB p65 protein in colon tissues from rats in each group
NF-κB p65 protein was mainly expressed in the nuclei and cytoplasm of epithelial cells and glandular epithelial cells, and the positive expression was visible as brownyellow staining. In the normal group, only a small amount of the NF-κB p65 protein was detected in the cytoplasm of the abovementioned cells. In the model group, the cytoplasm of these cells was deeply stained brown and the staining was densely distributed, suggesting a large amount of NF-κB p65 protein expression. Compared with the model group, the positive expression in the herbpartitioned and ginger-partitioned moxibustion groups increased to different degrees. The quantitative analysis of AOD showed that the expression of the NF-κB p65 protein in colon tissue of the model group was significantly increased compared with the normal group,and the difference was statistically significant (P<0.001).Compared with the model group, the expressions of the NF-κB p65 protein in colon tissue of rats in the herbpartitioned moxibustion group and the gingerpartitioned moxibustion group were significantly downregulated (bothP<0.05). No significant difference in the expression of NF-κB p65 protein in colon tissue was observed between the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group(P>0.05). See Figure 10 and Figure 11 for details.

Figure 9. Comparison of p-STAT3 protein expression in colon tissue from rats in each group (WB)
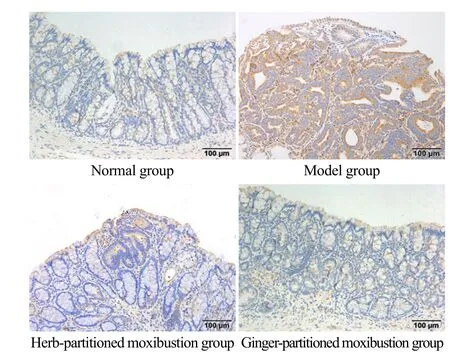
Figure 10. NF-κB p65 protein expression in colon tissue from rats in each group (IHC, ×200)

Figure 11. Comparison of NF-κB p65 protein expression in colon tissue from rats in each group (IHC)
WB was used for further verification. Compared with the normal group, the expression of NF-κB p65 protein in the colon tissue of the model group increased significantly (P<0.001). Compared with the model group,the expressions of NF-κB p65 protein in colon tissue of rats in the herb-partitioned moxibustion group and the ginger-partitioned moxibustion group were significantly down-regulated (bothP<0.001). A significant difference in the expression of NF-κB p65 protein in the colon tissue was not observed between the herb-partitioned and ginger-partitioned moxibustion groups (P>0.05). See Figure 12 for details.

Figure 12. Comparison of NF-κB p65 protein expression in colon tissue from rats in each group (WB)
3 Discussion
CACC belongs to the category of 'accumulation and gathering’ in traditional Chinese medicine (TCM). TCM proposes that accumulation occurs because the external pathogens take advantage of the deficiency under the state of imbalance of yin-yang and qi-blood, and deficiency of Zang-fu organs in human body, which cause qi stagnation, blood stasis, phlegm coagulation,dampness retention, and accumulation of toxins. Longterm interaction among the pathogens leads to CACC[19].During treatment, doctors should focus on tonifying qi,invigorating the spleen and dredging the intestine to promote the recovery of damaged organs. In the early stage of CACC, acupuncture can be used as a complementary or alternative therapy. It can be used in conjunction with surgery, radiotherapy, and chemotherapy to reduce the adverse effects of radiotherapy and chemotherapy, improve the efficacy of radiotherapy and chemotherapy, and promote the recovery of the patient’s body after surgery. In the advanced stage of CACC, acupuncture can be used as one of the main treatment methods which can relieve symptoms, improve the quality of life, and prolong the survival time of patients[20-23].
Tianshu (ST 25) and Qihai (CV 6) were selected in this study. Front-Mu points are often used to treat Fu-organ diseases, and Tianshu (ST 25), the Front-Mu point of the large intestine, is located near the intestine, can invigorate the spleen and stomach and regulate the intestine. The location of Qihai (CV 6) covered by the Conception Vessel is exactly where the intestine resides,and Qihai (CV 6) is located in the lower abdomen, thus having the ability to treat local diseases. Therefore, Qihai(CV 6) was selected to tonify and regulate qi. The combined use of Tianshu (ST 25) and Qihai (CV 6) can tonify qi, regulate the spleen and intestine, and strengthen the body.
In recent years, the role of angiogenesis in tumor growth and metastasis has attracted increasing attention from researchers. In 1971, Folkman J proposed that angiogenesis should be the key factor to the growth of tumors larger than 2 mm[24]. Blood vessels are mainly composed of vascular endothelial cells and surrounding parietal cells. When vascular endothelial cells are activated by factors such as vascular growth factors, they migrate and proliferate to form new blood vessels[25]. As classic proangiogenic factors, VEGF and its receptors are key molecules that promote angiogenesisin vivoandin vitroand actively participate in tumor growth and metastasis[26]. VEGF stimulates the proliferation and differentiation of endothelial cells to promote new blood vessel formation[5]; meanwhile it enhances vascular permeability and promotes the infiltration of fibroblasts and endothelial cells, which is conducive to the formation of tumor stroma and promotes tumor metastasis[27]. VEGF plays a role in promoting neovascularization mainly by binding to VEGF tyrosine kinase receptors on endothelial cells and initiating intracellular signal transduction pathways related to angiogenesis[26].
VEGF receptor signal links CACC inflammation and tumorigenesis through STAT3. VEGF signal requires STAT3 to promote the proliferation of CACC colonic epithelial cells and tumor growth in CACCin vivo[6]. As a DNA binding protein, STAT3 is the main central molecule in cellular signal transduction and transcription signaling pathways. It plays important roles in cell proliferation,invasion and migration[28]. Activated STAT3 mainly regulates transcription by transmitting activated receptors in the cytoplasm or intracellular kinase signals to the nucleus[29-30]. In normal cells, activated STAT3 is quickly inactivated by cytokine signaling inhibitors, and thus the phosphorylation and activation of STAT3 occur rapidly and are maintained for a short period. However,in colon tumor cells, the function of the tumor cell cytokine signaling inhibitor is damaged and the upregulation of tyrosine kinases results in the abnormally increased activation of STAT3 for a long time,which directly binds to specific sites in the DNA of cells,regulates downstream target gene expression, promotes abnormal cell proliferation, inhibits apoptosis, and finally leads to colon cancer[31]. At the same time, STAT3 has been shown to play a key role in inflammation-induced tumor development by recruiting white blood cells[32].P2X7R antagonists increase the expression of p-STAT3 in colon tumor tissues from CACC mice, and P2X7R agonists inhibit the expression of p-STAT3 in colon tumor tissues of CACC mice, suggesting that P2X7R may play a role in inhibiting the progression of CACC by inhibiting p-STAT3 in mouse colon tumor tissues[33]. NF-κB and STAT3 interact with each other during tumorigenesis and development. Grivennikov S,et al[34]found that the carcinogenic effect of NF-κB on the tumor epithelial cells of CACC model mice is mainly mediated by the B cell lymphoma XL protein (Bcl-XL), and the expression of Bcl-XL is regulated by STAT3. Activated STAT3 in the intestinal epithelial mediates the proliferation of interleukin (IL)-6, but inactivation of IL-6 reduces the diversity and diameter of colon adenomas in CACC mice[35]. Therefore, STAT3 participates in the regulation of NF-κB and cytokine IL-6 in the signaling pathway.Moreover, the effects of IL-6 are correlated with IL-11,IL-22 and epidermal growth factor (EGF). IL-11, IL-22 and EGF also act on intestinal cells and activate STAT3[36].
The microenvironment of tumors contains high concentrations of adenosine and adenosine triphosphate (ATP), which may affect inflammatory cell infiltration, immune cell response and tumor progression. The natural ligand of P2X7R is ATP, and many of the effects of extracellular ATP on tumor and inflammatory cells are mediated by P2X7R. P2X7R activates the NF-κB signaling pathway, which induces the production and release of tumor necrosis factor (TNF)-α,IL-1β, and IL-6 and participates in immune regulation and tumor occurrence[6,33]. In subjects with inflammatory bowel disease, NF-κB induces the production of the inflammatory factors TNF-α, IL-1β, and IL-6 through the RelA signaling pathway. These inflammatory factors further promote the activation of NF-κB signal[36-38]. This activation cycle exerts an anti-apoptotic effect on intestinal epithelial cells and a pro-inflammatory effect on immune cells in the lamina propria of intestinal mucosa, promoting abnormal cell proliferation and VEGF-driven angiogenesis in tumor tissue[39].
In this study, two moxibustion methods, herbpartitioned and ginger-partitioned moxibustion were used. Because they are clinical treatments for inflammatory bowel diseases[40-44], and studies have found that herb-partitioned moxibustion and gingerpartitioned moxibustion are commonly used moxibustion methods for treating cancer-related diseases[45]. We found that the intervention with herbpartitioned moxibustion and ginger-partitioned moxibustion at Tianshu (ST 25) and Qihai (CV 6) in CACC model rats reduced the number of tumors, average tumor diameter and TL. Benign tumors and malignant tumors are distinguished mainly by pathomorphological changes. Among these changes, cell atypia is regarded as the main point of difference. Unlike benign tumors,malignant tumors show a low differentiation rate and high atypia, which are very different from the original tissue morphology. Changes such as nuclear fission and hemorrhage are common[46]. The results of HE staining in this study showed that the cancer cells of the model group formed adenoid structures of different sizes and shapes, and with irregular arrangements. The cells were often arranged irregularly into multiple layers, with different sizes of nuclei, mitoses and other typical adenocarcinoma lesions[46]. Although the herbpartitioned and ginger-partitioned moxibustion groups also exhibited histomorphological changes such as tumors and dysplasia, inflammatory cell infiltration,mucosal hyperemia and edema, carcinogenesis was not observed, indicating that moxibustion therapy inhibited inflammation-mediated tumor growth to different degrees. Furthermore, IHC was used to observe the expression of VEGF protein in colon tissue from rats in each group. Both herb-partitioned moxibustion and ginger-partitioned moxibustion reduced the expression of VEGF protein, indicating that these treatments may reduce the formation of cancer-related new blood vessels by inhibiting the proliferation and differentiation of endothelial cells. VEGF signal requires STAT3 to promote the proliferation of CACC colonic epithelial cells and tumor growth[6], and P2X7R inhibitors activate STAT3 by promoting the phosphorylation of STAT3. NF-κB also activates STAT3, and STAT3 promotes NF-κB signal to play a role in cancer through Bcl-XL. Therefore, IHC and WB were used to observe the expression of related proteins in the P2X7R/STAT3/VEGF pathway in colon tissue of rats in each group. Both detection methods revealed that herb-partitioned moxibustion and ginger-partitioned moxibustion promoted the expression of P2X7R protein and inhibited the expressions of p-STAT3 and NF-κB p65 proteins, indicating that herb-partitioned and gingerpartitioned moxibustion may promote the expression of P2X7R protein and reduce the expression of NF-κB p65
protein, thereby inhibiting STAT3 activity and blocking the effect of VEGF in promoting tumor growth. Based on the results of the present study, the two moxibustion methods, herb-partitioned and ginger-partitioned moxibustion, did not show obvious differences in inhibiting the progression of tumors in CACC model rats and the P2X7R/STAT3/VEGF pathway, indicating that the two types of moxibustion may have exerted certain therapeutic effects through the P2X7R/STAT3/VEGF pathway. However, whether this pathway is the direct target of herb-partitioned and ginger-partitioned moxibustion requires further research.
Conflict of Interest
There is no potential conflict of interest in this article.
Acknowledgments
This work was supported by National Natural Science Foundation of China ( 国家自然科学基金项目,No. 81774405, No. 81973953); Scientific Research Project of Shanghai Municipal Commission of Health and Family Planning (上海市卫生和计划生育委员会科研课题, No. 20174Y0001); Special Clinical Research Project in the Health Industry of Shanghai Municipal Commission of Health and Family Planning (上海市卫生和计划生育委员会卫生行业临床研究专项, No. 20184Y0038, No.20184Y0106); Training Program of the Excellent Talents of Shanghai Municipal Health System (上海市卫生系统优秀人才培养计划, No. 2018YQ11); Natural Science Foundation of Shanghai (上海市自然科学基金课题, No.20ZR1453200).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 14 November 2020/Accepted: 3 February 2021
 Journal of Acupuncture and Tuina Science2021年2期
Journal of Acupuncture and Tuina Science2021年2期
- Journal of Acupuncture and Tuina Science的其它文章
- Effects of auricular point sticking on dry eye in myopia patients after SMILE surgery: a prospective randomized controlled clinical trial
- Effects of acupoint thread-embedding therapy on serum apelin and GLP-1 in type 2 diabetes mellitus patients with obesity due to dampness-heat encumbering spleen
- Nie-pinching the spine, puncturing Sifeng (EX-UE 10)plus Chinese herbs for pediatric anorexia due to dysfunction of spleen in transportation:a randomized controlled trial
- Evaluation of the prevention and treatment effects of acupuncture-moxibustion for Alzheimer disease based on various mouse models
- Warm needling moxibustion plus PKP for vertebral compression fracture due to kidney deficiency and blood stasis: a randomized controlled trial
- Clinical observation of electroacupuncture with different frequencies for migraine without aura
