Machine Learning and Medical Devices: The Next Step for Tissue Engineering
Hannah A. Pearce, Antonios G. Mikos
Department of Bioengineering, Rice University, Houston, TX 77030, USA
The pathway for creating and seeing a medical device to market is time-intensive, costly, and demanding [1,2]. This is particularly true of devices developed with tissue engineering components within the device[1,3].Machine learning and artificial intelligence have expedited optimization and engineering design in many other engineering disciplines [4,5]. By developing and using machine learning algorithms, novel drugs and enzymes have been discovered, innovative synthetic pathways and new chemistries have been identified, parameters for the three-dimensional printing of tissue engineering scaffolds have been elucidated, and better predictors for the success of electronics and robotics have been demonstrated [6–9]. Although machine learning and artificial intelligence have begun to be integrated into engineering and science research, medical device research and tissue engineering have not kept pace with this trend[1,4].A wealth of underutilized data is available in the form of research studies, clinical studies,and medical device and patent applications [1,4]. There are also enormous costs and complex regulatory pathways in seeing medical devices—particularly tissue engineering devices—to market [1,2,10]. Utilizing machine learning will enable the design of innovative, cost-efficient, and effective tissue engineering medical devices while minimizing the time to market. Harnessing the power of machine learning is the next step in the evolution of medical device development and is key for the continued success of tissue engineering.
Tissue engineering and biomedical engineering are convergent sciences, born from the fields of biology, engineering, chemistry,physics,and medical science.While tissue engineering is still relatively new at around 40 years old,the discipline has become a bed of discovery for new knowledge in the fundamental and applied sciences [1]. From its initial goal of creating replacement organs to address the organ donor shortage, the field has expanded to encompass the creation of replacement tissues such as bone, skin,and cartilage; targeted and enhanced drug delivery; disease modeling platforms; and high-throughput screening devices. The field of tissue engineering has benefited from its highly interdisciplinary nascence, and there is a wealth of information and data that currently requires extensive searches and digestion when designing new tissue engineering strategies.This large dataset of tissue engineering and biomaterials strategies has yet to be compiled into easily usable formats,which has contributed to the delay in seeing tissue engineering solutions actualized in clinical settings [1,3,4].Utilizing machine learning to comb through this data and identify important trends and relationships in biomaterials selections, cell types,induction strategies,and loaded factors is necessary to keep pace with other engineering disciplines and allow tissue engineering’s translation into medical devices.
In creating medical devices with tissue engineering components, there are many factors for consideration [4]. The intended use of the device, the materials and biomaterials that will come into contact with the body, any cell types that might be included,the drugs or active agents that will be administered,and the physical implantation or implementation strategies must all be taken into account [4]. At present in the United States, medical devices require approval from the Food and Drug Administration (FDA),and may be reviewed through 510(k) regulatory pathways that allow for more rapid time to market (3–6 months), or as novel devices that require a much more intensive evaluation before being released to the market (3–7 years) [2]. The 510(k) pathway is faster, as the devices that are approved through this pipeline are demonstrated to be similar to existing approved medical devices.This regulatory hurdle has been a barrier to many medical devices that incorporate tissue engineering strategies [1]. Medical devices that harness tissue engineering components in their design are often not similar enough to existing medical devices to fall into the 510(k) pathway, and thus require a novel device route that takes much longer and is much more costly to see to market.Taking a new drug as an example device, the cost of developing the drug and seeing it through the US FDA approval and release to the market has been estimated to cost 800 million USD [2].Tissue engineering devices are especially costly,as they often contain finely tuned and uniquely designed materials strategies, cells,growth factors, or other active agents. These regulatory and financial barriers pose a significant challenge to the realization of impactful medical devices incorporating tissue engineering solutions reaching the clinic [3]. Utilizing machine learning to better predict which technologies would be most impactful while simultaneously possessing the easiest path to market would help to pave the way for more tissue engineering solutions to be implemented in the clinic.This endeavor should be partnered with coordination from regulatory agencies to disrupt the current regulatory pipelines, which do not allow the easing of tissue engineering devices into the clinic, in order to create novel channels for these innovative devices.
Recently, the US FDA has set a precedent for establishing novel pathways by piloting an approval pipeline for artificial-intelligence-incorporating devices. They have also issued a directive to permit some machine learning technologies within medical devices [10]. These actions have resulted in the development of the first personalized cardiogram created from electrocardiograms(ECGs). This technology has already been implemented in smartwatches to monitor people’s cardiovascular health [11,12].Through machine learning, the technology builds an individual profile to understand the wearer’s cardiovascular behaviors that fall within a normal and healthy range for that person. The device is then capable of alerting the wearer when her or his cardiovascular signals begin to fall outside of this range. This technology has successfully detected atrial fibrillation,changes in respiratory rate,and Lyme disease, and was even able to detect pre-symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)infections during coronavirus disease 2019 (COVID-19) in a retrospective study [11,12]. The advances made possible through machine learning within medical devices hold enormous value.The utilization and adoption of machine learning to design better medical devices from the existing knowledge and data we have are absolutely vital.
Perhaps one of the greatest barriers to more widespread adoption and use of machine learning and artificial intelligence is public trust[13].Integration of and education on machine learning within the public sector is crucial if these technologies are to be used for the design and creation of improved medical devices[14].Technology is advancing rapidly in the modern world and, with the increased use and widespread availability of machine learning and artificial intelligence, the pace of technological development will only quicken. Machine learning and deep learning must be taught in schools,so students are better suited to begin interfacing with this technology in college or in their future careers [14]. Two hundred years ago, the concept of including science education in grade school or high school in the United States was rejected,as scientific topics were viewed as advanced subjects only required for those attending academic institutions of higher education. Now,science education is required within the United States and most other nations due to the fundamental framework it builds in understanding and interfacing with the world around us.Engineering education in high school settings is underway in many schools but is not required. It is time for kindergarden through twelfh grade (K–12) students to begin their introduction to computer science, engineering, machine learning, and artificial intelligence early on, if we want our next generation of students to be able to keep pace with the changing world around them[13,14]. Familiarity with artificial intelligence and machine learning will encourage machine learning to be further integrated into engineering and the conception of medical devices. It will also increase familiarity with the associated issues of explainability and bias, and encourage the thoughtful adoption and use of these machine-learning-inspired medical devices when they reach the market.
Machine learning and artificial intelligence will play a critical role in scientific development in the years to come. These technologies hold the power to work with large datasets to improve engineering design and serve as better predictors of experimental outcomes. Many other engineering disciplines have recognized the value of these technologies and have already begun adopting them. Tissue engineering and medical device development have fallen behind this trend. Using machine learning for designing tissue engineering schemata for medical device development, as shown in Fig. 1, would enable the delivery of better, safer, and more effective medical devices to market in a timelier manner,since those with the most promising outcomes would be the only devices working their way through the regulatory pipeline.Machine learning algorithms could use the known and reported data of the cell types proposed, the materials in question, and any active agents, as well as factoring in the predicted time to market and the costs associated with seeing the device to market,in order to output the most effective and economical tissue engineering device for the desired application. Tissue engineering is a convergent science,and the next generation of tissue engineers must harness machine learning to improve the development of tissue engineering medical devices so that more of these technologies can be realized in a clinical setting.
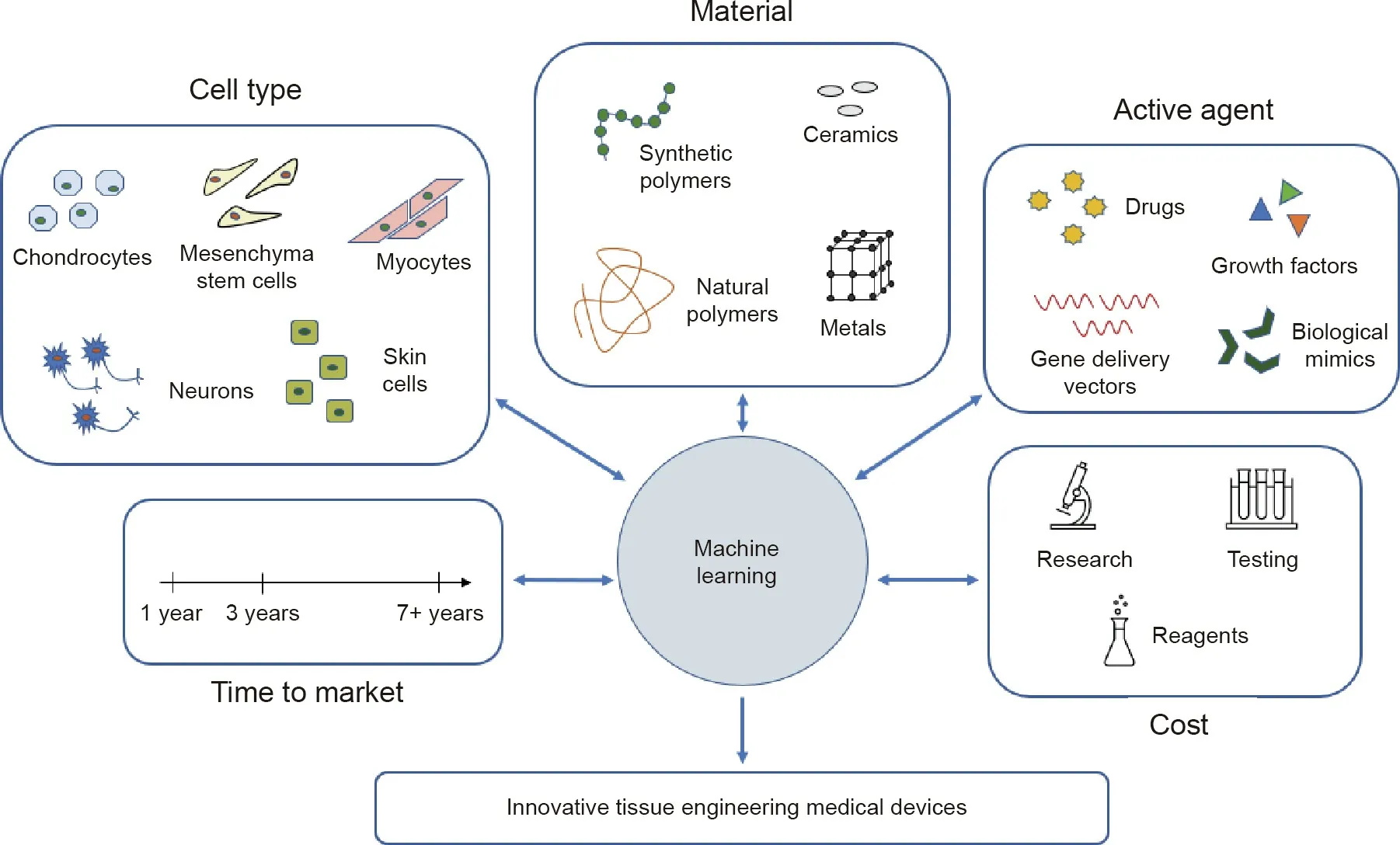
Fig. 1. Utilizing machine learning to design novel tissue engineering medical devices.
Acknowledgments
The authors acknowledge support from the National Institutes of Health (P41 EB023833 and R01 AR068073). We also acknowledge support from the National Science Foundation Graduate Research Fellowship Program, USA.
- Engineering的其它文章
- The Intelligent Beijing–Zhangjiakou High-Speed Railway
- Mechanisms of Steatosis-Derived Hepatocarcinogenesis: Lessons from HCV Core Gene Transgenic Mice
- Microneedle Makers Seek to Engineer a Better Shot
- Battery Recycling Challenge Looms as Electric Vehicle Business Booms
- Global Top Ten Engineering Achievements 2021
- Biomedical Engineering: Materials, Devices, and Technological Innovation Continue to Build a Better Future for Humankind

