An Outlook on Implantable Biosensors for Personalized Medicine
Rita Rebelo, Ana Isabel Barbosa, Vitor M. Correlo, Rui L. Reis
a Research Institute on Biomaterials, Biodegradables, and Biomimetics, University of Minho, Headquarters of the European Institute of Excellence on Tissue Engineering and Regenerative Medicine, Barco, Guimarães 4805-017, Portugal
b ICVS/3B’s–PT Government-Associated Laboratory, Braga, Guimarães 4710-057, Portugal
Biosensors are a fast-growing field,as they have been shown to be very helpful in our daily life, playing roles in industries such as agriculture, food safety, homeland security, bioprocessing, environmental monitoring, and industrial monitoring. Beyond these,the application of biosensing in medicine and biomedical engineering may have the highest potential for growth and for affecting human quality of life in the near future. This potential is driven by the need for new and improved devices and technologies with improved sensitivity, specificity, reliability, and biocompatibility,which can solve and manage medical and health problems such as heart diseases, cancer,or diabetes,among others[1]. For example, since tumors present a unique microenvironment, an implantable biosensor near a tumor would allow precise monitoring of the disease’s progression. The first biosensor reported for use in vivo was based on magnetic nanoparticles and was used to detect soluble cancer biomarkers in mice [2]. Since then, the research field of implantable biosensors has become a hot topic in the scientific community [3].
Implantable biosensors are the most challenging type of biosensors. The first appearance of biosensors in medicine was reported in 1962 by Clark and Lyons [4], who invented a glucose biosensor based on an oxygen electrode with immobilized enzymes as probes. This technology was followed by the development of another biosensor capable of urea measurements in 1969 using similar principles [5]. Since then, numerous biosensors involving immobilized enzymes,antibodies,and aptamers, and using a variety of detection modes such as electrochemistry,optical detection,piezoelectricity,and so forth,have been developed for the monitoring of several diseases, resulting in significant improvements in miniaturization, selectivity, sensitivity, and multiplexing. For example, ZnO nanoflowers were developed to quantify beta amyloids using fluorescent detection method [6–10]. However, these developments are small in comparison with the idea of placing biosensors inside the human body that permit the continuous monitoring of in vivo conditions,and thus provide physiology readings that can be used for the early diagnosis of health conditions.Such devices could also be programmed to simultaneously detect a disease and perform a defined treatment, thereby combining diagnostics and therapeutics in what is known as theranostics.Implantable biosensors can revolutionize not only medical care,but also the way in which people connect with their own health.From the perspective of healthcare providers, practitioners would receive daily information on their patients’ physiology, and alert systems would ensure that no important deviation in standard values was overlooked.This could significantly reduce the number of patients at overcrowded hospitals and health centers, and therefore improve health service quality. Even more importantly, such devices would diagnose health conditions and start adequate treatments earlier, thereby improving patients’ quality of life and reducing death rates. Implantable biosensors would also be vital for personalizing medicine, since the amount of information obtained from patients would lead to patient stratification, resulting in more efficient treatments and allowing the development of a predictive, preventative, and participatory medical follow-up system. Patient information could also be used for big data analysis,through which variables such as socio-demographics,medical conditions, genetics, and treatments would be analyzed and new trends would be discovered, leading to more efficient medical treatments based on patients’ physiological, genetic, and demographic characteristics.From the perspective of the general public,people would know first-hand which specific behaviors would negatively affect their health,which would increase self-awareness and behavioral change.Lastly,such devices would allow people to feel more in control of their health, which would decrease their level of stress—a known cause of a series of chronic conditions.
Despite the huge efforts that have been made in this area, the development of implantable biosensors still presents major challenges,such as the foreign-body response,the biosensor’s response and stability,continuous monitoring,power supply,and data transmission [11]. In order to overcome these limitations, specific requirements must be met for implantable biosensors,such as the use of more flexible and biocompatible biomaterials(biodegradability and/or bioresorbability may also be required in some contexts),miniaturization,and reliability.Thus,the following design parameters are crucial in the development of implantable biosensors.
1. Flexibility and biocompatibility
In recent years,several strategies have been used to reduce the inflammatory response after implantation. These include:
(1)Minimizing the mismatch between the implantable hard surface and the soft living tissue[1].It has been realized that the electrode’s modulus of elasticity plays a major role in the inflammatory process.Electrodes that are too stiff cause a greater inflammatory response and become encapsulated to a greater extent,diminishing the in vivo sensor performance [1,12–14]. Therefore,promoting the correct integration of the device with the surrounding tissue can enhance the biocompatibility and,consequently,the in vivo biosensor functionality.Hence,for implantable applications,it is important to develop flexible biosensors by replacing traditional silicon wafers with flexible, soft, and biocompatible materials, in order to minimize the foreign-body issue and suppress fibrotic tissue encapsulation.
(2) Using biocompatible coatings [15–17]. Biocompatible coatings such as hydrogels have been engineered to recreate cell microenvironments,due to their similarities with the extracellular matrix environment of human tissues in terms of high water content and other physical properties [18,19]. For example, a glucose subcutaneous implanted hydrogel-based sensor was developed to reduce biofouling and therefore improve sensor longevity;it lasted functioned for 21 days in the body [18].
(3) Performing chemical surface modifications [17,20,21]. It has been reported that chemical surface modification of layers that allow water penetration results in improved anti-fouling properties and thus improves the diffusion of the analyte to the sensor[22].
(4)Using angiogenic and anti-inflammatorydrugs[15,17,20].It has been demonstrated that the incorporation of an anti-inflammatory drug into biocompatible alginate nanoparticles glucose sensors made it possible to achieve 28 days of biosensor functionality[23].
(5) Using biodegradable materials [24]. The use of biodegradable materials for the development of implantable biosensors eliminates the need for extraction or reoperation,and can minimize chronic inflammatory responses. Biodegradable materials are usually polymers, which are normally employed as substrates, or metals, which are used as electrodes; both can be dissolved or disintegrated by body fluids,thereby minimizing the foreign-body response [14].
Strategies to reduce the foreign-body response must be carefully selected so that the analyte can still be transported to the biosensors’ surface, enabling its measurement [16]. In addition,properties such as the thickness, porosity, and hydrophilicity of the biosensor surface will determine the biosensor’s functionality[25]. A combination of such strategies for eliminating the foreign-body response is often used, so that biosensor functionality and biocompatibility can be simultaneously achieved for a specific clinical application [16].
Nevertheless, none of these strategies completely overcomes the negative foreign-body response and its cascade of events—such as acute and chronic inflammation and the formation of fibrotic tissue—for long periods of time. So far, most of the biosensors reported in the literature present an in vivo functionality of up to one month,with commercial glucose sensors lasting for 6–14 days[26].This sensor duration is not acceptable for most of the clinical needs that currently exist, such as cancer early detection and cancer treatment monitoring [27].
2. Miniaturization
At present, the investigation of implantable sensors and the constant search for a minimal foreign-body response and less invasive techniques lead to a continuous pursuit of the following technologies: miniaturization; the integration of electronic components, data processing, and wireless transmission; and the absence of re-calibration [28]. Ideally, biosensors should also be implanted without the need for major or complicated surgery. To achieve this aim,a biosensor should be as small as possible,which demands the miniaturization of the biosensor’s components—namely, sensing electrodes, power generation, data communication, and their integration or packaging. Miniaturization is often achieved through nanotechnology techniques; the use of nanobiosensors (1–100 nm) improves signal density, decreases background signals, and enhances the signal-to-noise ratio. However,it is essential to take into consideration that the miniaturization of an implantable biosensor affects not only the limit of its detection,but also its response time [29]. Thus, there is an important trade-off between enhanced efficiency in signal transduction and the longer time it takes for a miniaturized biosensor to collect target analytes at its surface,due to the increase in mass transport times.Therefore,depending on the specific application,microscale biosensors often offer a good alternative to nanobiosensors, since they possess some advantages of miniaturization while avoiding the challenges mentioned above [30].
3. Power supply and data transmission
Current implantable devices often use bulky batteries;although such batteries (e.g., lithium batteries) are often compatible with flexible electronics, they introduce problems such as the need for replacement,an increased risk of infections,body rejection,discomfort,and restriction of the patient’s movements[31].Fuel cells provide a possible alternative for powering implantable biosensors.Enzymatic fuel cells exploit redox enzymes to harvest electrical energy from the chemical energy stored in biomolecules, which makes them self-powered sensors for a specific biomarker. However, technical issues in the use of enzymatic flow cells must still be overcome,such as the enzymes’short life and the weak electrical contact between the redox center of the enzyme and the electrode current[32].‘‘Bio-galvanic”cells can also be used for this purpose,since they can be activated by body fluids such as urine,saliva,blood,or plasma.These cells usually include a sacrificial anode and a cathode, with the anode being consumed during the electrochemical reactions.Thus,the main disadvantage of this type of cell is its lifetime,which depends on the corrosion rate of the anode[33,34].
In a different approach, piezoelectricity can be used to charge implanted biosensors, since body movements can provide the mechanical stress necessary for piezoelectric materials to generate electric current.Although this type of power supply source presents high output power,it can only be placed in specific body locations to generate electricity.Finally, systems that have external units to transfer energy continuously are becoming popular,since the need to find a power supply is coupled with the importance of transmitting and storing data. For example, in inductive coupling, mutual inductance between two coils(one outside the body and the other integrated in the implanted biosensor)without wires is simultaneously used as a power supply and for data transmission. Inductive coupling is the most commonly used wireless technology and can provide a high data rate and high level of power transmission without the need for a battery;however,this method has a limited carrier frequency due to tissue absorption [35–38], Performing continuous measurements requires the transmission and storage of a significant amount of data, which makes data output a real challenge for implantable biosensors. Currently, most approaches use inductive-coupling wireless technology to send information to an external platform with good storage capacity[38–41].
4. Tissue engineering strategies
Tissue engineering (TE) combines cells, biomaterials, and growth factors that function to regenerate, maintain, and heal different types of biological tissues and organs. TE approaches use this triad to generate tissues in the laboratory that can then be easily transplanted into the human body with minimum foreignbody response, since the cells used in TE can be isolated from the patient’s own tissues. In addition, by embedding electronic systems within tissue engineered constructs composed of biocompatible scaffolds and tissue-specific cells, bio-integration is improved due to a lower host immune response.Another approach is to produce flexible, biocompatible scaffolds that can simultaneously act as sensors. This strategy is exemplified by the production of biocompatible inks that can be used to manufacture implanted electrodes by means of inkjet printing [42]. However, TE approaches present a few challenges in turn, which still need to be overcome.Developing scaffolds that are capable of reproducing complex structures is still difficult and is translated into the biggest drawbacks of TE approaches: limited oxygen and nutrient supply to the innermost implanted tissue constructs, and inefficient waste elimination [43]. These drawbacks directly affect the implanted biosensor in tissue constructs,since they can affect the analyte diffusion to the sensor and thereby compromise the biosensor’s functionality.Nevertheless,using TE‘‘know-how”to embed biosensors in living tissues (Fig. 1) that have been engineered in vitro using a patient’s own cells can greatly impact the development of implanted biosensors, since it will drastically improve their biocompatibility, reduce their foreign-body response, and therefore allow them to maintain in vivo functionality for longer. Nevertheless, the main developments of biosensors in TE applications thus far consist of miniaturized microfluidic platforms with integrated biosensors[44]that make it possible to predict the body’s physiological response in a rapid and real-time manner. Still, it is necessary to overcome the challenges of in vitro applications and achieve in vivo implantable biosensors in order to create breakthrough innovation in medical diagnostics and disease follow-up.The combination of flexible and miniaturized biosensors with TE can be a real game-changer in the design of implantable biosensors.
To summarize,implantable biosensors are not yet at a developmental phase where they can be vital for personalized medicine,which would deconstruct healthcare as we know it today.However,the obstacles preventing such development have been identified,and multidisciplinary teams of researchers worldwide are working to overcome the main technical problems that hamper the clinical application of implantable biosensors.TE strategies for implantable biosensors seem to be the best way to tackle the body’s immune rejection, since such strategies can minimize the body’s response by increasing biosensor biocompatibility and reducing tissue mismatch, thus maintaining biosensor functionality for longer.Nevertheless, before commercialization can occur, implantable biosensors must be subjected to a long, rigorous, time-consuming evaluation process, which would include clinical trials. Medical device clinical trials are performed worldwide and are fundamental for product safety,since they evaluate the effectiveness and safety of a medical strategy, treatment, or device in humans. These research studies need the approval of a health ethics committee before they are performed; they also follow strict scientific standards,which are designed to produce reliable results while protecting patients.However,clinical trial results can only come at the end of a careful and long progression that begins in the laboratory,is followed by animal testing,and only then reaches clinical trials as the final stage,under the restrictive guidance of the US Food and Drug Administration(FDA),or CE marking in Europe[45].
In the future, the successful implantation of biosensors will transform medicine as we know it, creating new opportunities for studying disease states, improving surgical procedures, monitoring health and wellness, and establishing human–machine interfaces. In this way, implantable biosensors will be both industrially and clinically relevant for creating economical value, useful therapies, and products with significant impacts on patients’healthcare and quality of life.
Acknowledgments
This research was funded by the Foundation for Science and Technology(FCT-Fundação para a Ciência e a Tecnologia),Portugal(PTDC/EMD-EMD/31590/2017 and PTDC/BTM-ORG/28168/2017).
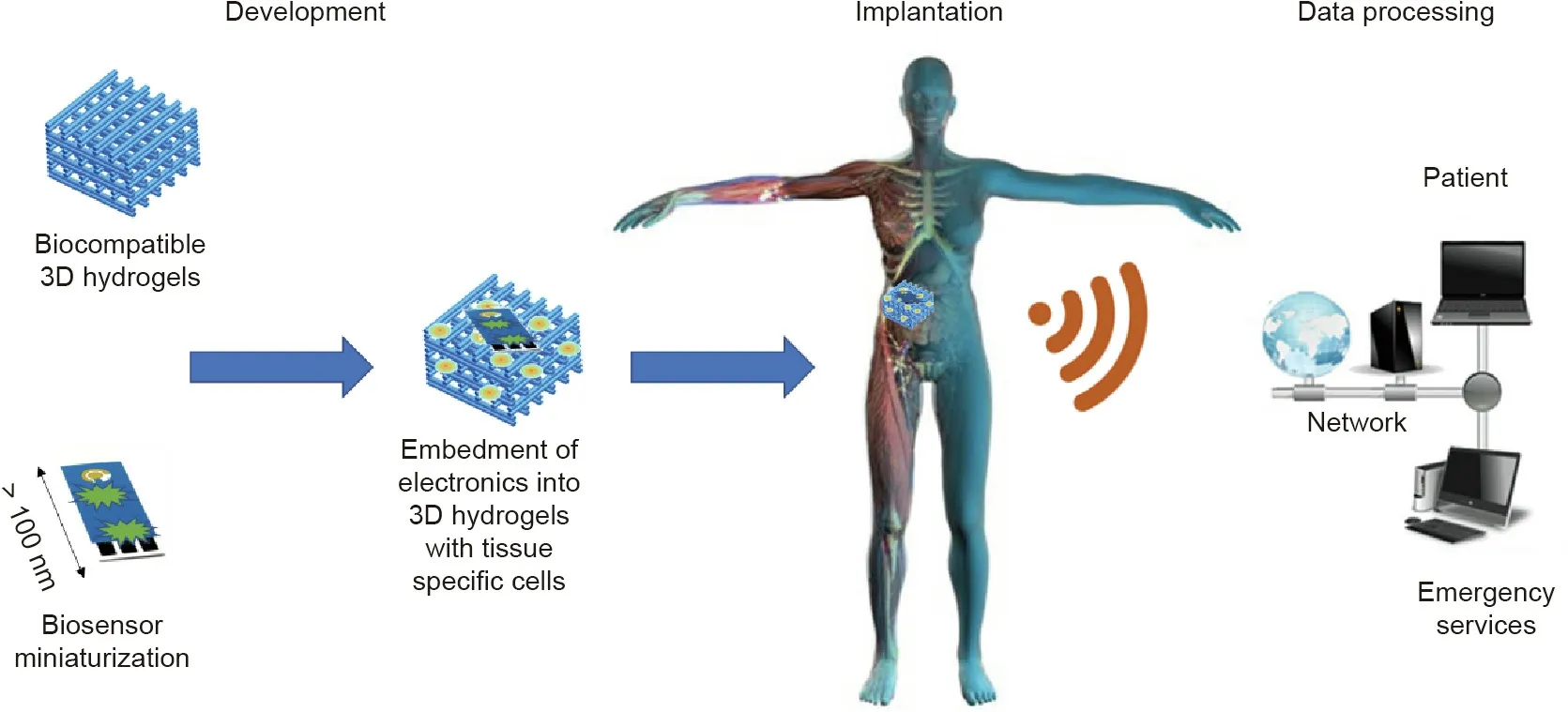
Fig. 1. Biosensor embedment: a tissue engineering approach for implantable biosensors. 3D: three-dimensional.
- Engineering的其它文章
- The Intelligent Beijing–Zhangjiakou High-Speed Railway
- Mechanisms of Steatosis-Derived Hepatocarcinogenesis: Lessons from HCV Core Gene Transgenic Mice
- Microneedle Makers Seek to Engineer a Better Shot
- Battery Recycling Challenge Looms as Electric Vehicle Business Booms
- Global Top Ten Engineering Achievements 2021
- Biomedical Engineering: Materials, Devices, and Technological Innovation Continue to Build a Better Future for Humankind

