Effect of liver inflammation on accuracy of FibroScan device in assessing liver fibrosis stage in patients with chronic hepatitis B virus infection
Ling-Ling Huang, Xue-Ping Yu, Ju-Lan Li, Hui-Ming Lin, Na-Ling Kang, Jia-Ji Jiang, Yue-Yong Zhu, Yu-Rui Liu,Da-Wu Zeng
Abstract
Key Words: Liver stiffness measurement; Fibrosis stage; Liver inflammation; Hepatitis B virus; FibroScan; Predictive model
INTRODUCTION
Approximately 248 million individuals worldwide have been infected with chronic hepatitis B virus (HBV)[1], which can develop into hepatic failure, cirrhosis, and tumorigenesis, causing nearly 650000 deaths every year[2]. Hepatic fibrosis is an intermediate stage in the progression of chronic hepatic disease from mild hepatitis to decompensated cirrhosis[2,3]. Therefore, timely and accurate assessment of hepatic fibrosis stage is helpful to determine the optimal treatment plan, so as to minimize and delay the progression of liver injury[3,4]. Although liver biopsy is the gold standard for evaluating the stage of liver fibrosis, it is invasive, expensive, and accompanied by potential complications and sampling errors[5]. Transient elastography (FibroScan) is a new non-invasive test[3,6]that can replace biopsy, and it has been widely recommended by the guidelines on HBV management for assessing the stage of hepatic fibrosis[4].Therefore, considering liver biopsy only in patients at a high fibrosis stage could minimize unnecessary biopsies.
The Society of Radiologists in Ultrasound consensus statement on liver elastography indicated that liver stiffness measurement (LSM) obtained using ultrasound elastography is associated with the degree of hepatic fibrosis[7]. However, increased LSM values as per transient elastography in acute hepatitis do not actually reflect the grade of liver fibrosis. During an acute attack of chronic liver disease, LSM values are affected by liver inflammatory activity indices such as serum total bilirubin (TBIL) and alanine aminotransferase (ALT), which may overestimate the liver fibrosis stage. The 2019 Chinese guidelines for chronic hepatitis B and the non-invasive liver fibrosis guidelines of the European Society and Latin American Society of Hepatology indicated that the diagnostic cutoffs of LSM should be adapted to ALT levels that assess the stage of HBV-related fibrosis[8,9]. In clinical practice, elevated ALT levels in many patients with chronic hepatic disease reflect hepatic inflammatory injury. Many studies have suggested that the cutoff value of LSM tends to increase and its diagnostic accuracy tends to decrease with elevated ALT level[10,11]; however, whether pathological hepatic inflammation would similarly affect cutoff values and the diagnostic accuracy of LSM in assessing the stage of hepatic fibrosis remains unclear.
In this study, we aimed to investigate in detail the impact of liver inflammation on LSM values and the diagnostic performance of FibroScan in assessing the stage of fibrosis in patients with chronic HBV infection.
MATERIALS AND METHODS
Research population
The study protocol was approved by the Institutional Review Board of Fujian Medical University, Fuzhou, China, and the need for written informed consent from patients was waived owing to the retrospective nature of the study. As shown in Figure 1, a total of 416 patients aged 18 years and above with chronic HBV infection who consented to undergo FibroScan and liver biopsy were enrolled in The First Affiliated Hospital of Fujian Medical University, and The First Hospital of Quanzhou Affiliated to Fujian Medical University between January 2014 and December 2019. Chronic HBV infection was defined as the persistent presence of hepatitis B surface antigen (HBsAg)and HBV-DNA in the serum for more than 6 mo. Patients with other types of hepatitis virus infections; those with body mass index (BMI) > 28 kg/m2; those with fatty liver disease, alcoholic liver disease, drug-induced liver disease, autoimmune liver disease,genetic, or metabolic disease; those with decompensated cirrhosis, malignant tumors,or severe extrahepatic disease or pregnancy; and those with unreliable LSM values by FibroScan were excluded. Patients with hepatic steatosis by histology of liver biopsy were also excluded. All patients were examined using FibroScan, and fasting venous blood samples were collected for routine clinical examination within 1 wk of liver biopsy.
Clinical and laboratory parameters
Information regarding the following clinical parameters was collected: Patient age, sex,weight, height, status of alcohol consumption, and history of HBV infection. The BMI was calculated as weight (kg)/height2(m2). Serum samples were collected after the patients fasted for 8 h at night, for the following measurements: HBsAg, hepatitis B envelope antigen (HBeAg), HBV-DNA, TBIL, ALT, aspartate aminotransferase (AST),albumin (ALB), prothrombin time (PT), platelet (PLT), and alpha-fetoprotein.
Liver stiffness measurement by FibroScan
LSM was performed using FibroScan 502 (Echosens, Paris, France). The detection method was followed as per the user manual, and the monitoring points were selected from the right anterior axillary line to the axillary midline 7, 8 or 8, 9 intercostals of the patient. The LSM values could be considered reliable when at least 10 valid measurements yielded a success rate of more than 60% and the interquartile range/median was less than 30%. The median value was determined as the final result of liver stiffness, and its unit was kPa. FibroScan was performed by an expert certified technician.

Figure 1 Flowchart of patient enrolment. BMI: Body mass index; HBV: Hepatitis B virus; LSM: Liver stiffness measurements; FibroScan: Transient elastography.
Liver histology assessment
Percutaneous liver biopsy was performed using 16-gauge modified aspiration needles(ACUSON; Siemens, United States) under ultrasound guidance. Qualified liver specimens with a minimum length of 1.5 cm and having more than six portal veins were fixed in 4% neutral formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E) and Masson’s trichrome by two experienced pathologists who were blinded to the LSM values of FibroScan and clinical data. The pathological diagnosis was graded according to the METAVIR score standard[12], as follows: F0, no fibrosis; F1, fibrous enlargement in the manifold area without septa; F2,fibrous enlargement in the manifold area and few septa; F3, plentiful septa without cirrhosis; and F4, early cirrhosis. Significant fibrosis was defined as ≥ F2; advanced fibrosis, as ≥ F3; and cirrhosis, as F4. Hepatic inflammation activity according to the degree of piecemeal necrosis (PN) was graded as A0, none; A1, mild PN; A2, moderate PN; and A3, severe PN[12].
Statistical analysis
Measurement and enumeration data were expressed as the means with standard deviation or median and ratio or composition ratio, respectively. Student’s t-test, Chisquared test, and Mann-Whitney U test were performed for comparative analysis, and the Spearman test was performed for correlation analyses. Receiver operating characteristic (ROC) curves were used to analyze the diagnostic performance and obtain the optimal cut-off value of FibroScan for assessing the stage of liver fibrosis.Multivariate regression analyses were employed to select the independent risk factors related to the misdiagnosis of the stage of fibrosis using FibroScan, and a non-invasive risk prediction model was constructed. To compare the area under the curves (AUCs)of the prediction model with that of other single related factors, the DeLong test was applied. Statistical analyses were performed using SPSS v23.0 (SPSS Inc. Chicago, IL,United States) and MedCalc v19.1 (MedCalc Software Bvba, Ostend, Belgium). A twosided P < 0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics
In total, 416 patients were enrolled in this study (Table 1). All patients were HBsAg positive, and most of them were male (73.3%) and HBeAg positive (57.0%). The mean age, BMI, TBIL, ALB, ALT, AST, PLT, HBV DNA, PT, and LSM values were 38.67 years, 22.90 kg/m2, 17.11 μmoL/L, 42.50 g/L, 95.25 IU/L, 58.46 IU/L, 187.46 × 109/L,4.98 log IU/mL, 12.20 s, and 9.83 kPa, respectively. According to the METAVIR score,the distribution of the stage of liver fibrosis was as follows: F0-F1 = 175 (42.1%), F2 =106 (25.5%), F3 = 67 (16.1%), and F4 = 68 (16.3%). The distribution of liver inflammation activity was as follows: A0 = 17 (4.1%), A1 = 236 (56.7%), A2 = 119(28.6%), and A3 = 44 (10.6%).
Diagnostic value of FibroScan for staging of liver fibrosis
Using hepatic pathology and METAVIR fibrosis stages as a reference, the LSM values of FibroScan were positively associated with hepatic fibrosis (r = 0.732). In the overall cohort, the optimal diagnostic LSM values of FibroScan for significant fibrosis (≥ F2),severe fibrosis (≥ F3), and cirrhosis (F4) were 7.3 kPa (AUC = 0.863), 9.7 kPa (AUC =0.911), and 11.3 kPa (AUC = 0.918), respectively (Table 2).
Discordance in stage of liver fibrosis between FibroScan and pathological scores
Misdiagnosis of the stage of fibrosis using FibroScan was defined when at least one stage of liver fibrosis was discordant with that observed using pathological staging in the METAVIR scoring system. The 416 patients were accordingly divided into the concordance group (n = 274) and discordance group (n = 142). Figure 2 shows the distribution of predicted fibrosis stage by FibroScan in different pathological stages of liver fibrosis. The rate of misdiagnosis using FibroScan was 34.1% (142/416 patients),and 8.2% (34/416) of the patients showed a discordance between the values obtained using the two methods for two stages. In total, 81 patients showed discordance (19.5%)attributed to overstaging by FibroScan, and the remaining 61 patients showed discordance (14.7%) attributed to understaging. There were no significant differences in the demography, HBV virology, and LSM values obtained using FibroScan between the two groups. However, in the discordance group, ALT and AST levels, the proportion of liver inflammation activity over 2, and significant fibrosis were significantly higher than the levels in the concordance group (P < 0.001) (Table 1).
Factors related to misdiagnosis of liver fibrosis stage by FibroScan
Univariate analysis revealed that ALT levels ≥ 5 times the upper limit of normal (5 ULN), AST levels ≥ 2 ULN, and liver inflammation activity over 2 (A ≥ 2) were significantly related to misdiagnosis of the stage of liver fibrosis by FibroScan (P <0.001). Subsequently, these variables were subjected to multiple regression analyses.Finally, liver inflammation activity ≥ 2 (OR = 3.53, 95%CI: 2.11-5.92, P < 0.001) was considered an independent risk factor for mis-staging of liver fibrosis using FibroScan(Table 3).
Effect of liver inflammation on diagnostic accuracy of FibroScan staging
Figure 3 shows the effect of liver inflammation on LSM values obtained using FibroScan for different stages of fibrosis. Within each fibrosis stage, namely F0-1, F2,F3, and F4, the LSM values of patients with inflammation activity ≥ 2 (A ≥ 2) were significantly higher than those of patients with inflammation activity < 2 (A < 2) (all P< 0.05).
Figure 4 shows the prevalence of misdiagnosis of the stage of liver fibrosis using FibroScan staging in patients with different liver inflammation activities. Patients with inflammation activity ≥ 2 had higher rates of FibroScan mis-staging (55.8% vs 20.2%, P< 0.001), over-staging (36.8% vs 8.3%, P < 0.001), and under-staging (19.0% vs 11.9%, P= 0.044), compared with patients with inflammation activity < 2.
Figure 5 shows the effect of liver inflammation activity on the diagnostic performance of FibroScan for different fibrosis stages. In patients with inflammation activity < 2, the diagnostic performance of FibroScan for significant fibrosis (≥ F2),advanced fibrosis (≥ F3), and cirrhosis (F4) were significantly better than that in patients with inflammation activity ≥ 2 (0.831 vs 0.702, 0.903 vs 0.815, and 0.941 vs 0.836, all P < 0.05), as observed by comparing the AUCs.

Table 1 Demographic characteristics and clinical features of our patient cohort
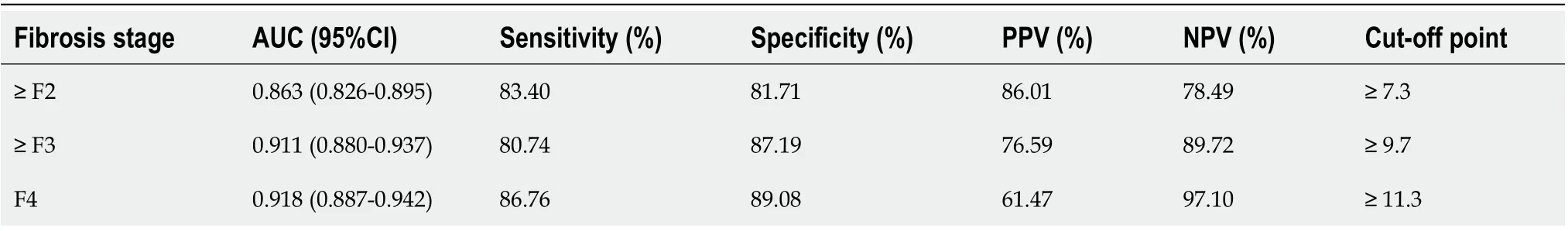
Table 2 Accuracy of liver stiffness measurement values by transient elastography in diagnosing ≥ F2, ≥ F3, and F4, as measured by area under the receiver operating characteristic curve (n = 416)
Development of a non-invasive prediction model for misdiagnosis of liver fibrosis stage using FibroScan
The ALT and AST levels were positively correlated with hepatic inflammation (r =0.534 and 0.527, P < 0.001) by the Spearman’s test, and these were significantly related with misdiagnosis of fibrosis stage using FibroScan (all P < 0.001) (Table 3). Usingthese related factors, a non-invasive prediction model was developed to identify the risk of misdiagnosis using FibroScan, as follows: logit (P) = -1.477 + (0.139, 0.732) ×ALT levels (2-5, ≥ 5 ULN) + 1.310 × AST levels (> 2 ULN) + (1.056, 0.815, −0.154) ×FibroScan-predicted fibrosis staging (F2, F3, and F4).

Table 3 Univariate and multivariate regression analyses of risk of misdiagnosis of fibrosis stage by transient elastography in all patients
We compared the prediction performance of the model with that of other single related factors to evaluate the misdiagnosis of the stage of liver fibrosis using FibroScan (Figure 6). The AUC value of the prediction model was 0.701 (95%CI: 0.655-0.745), which was significantly higher than that of ALT levels (0.636, 95%CI: 0.588-0.683), AST levels (0.639, 95%CI: 0.590-0.685) and FibroScan-predicted fibrosis stages(0.611, 95%CI: 0.562-0.658) (all P < 0.001). The cut-off point, sensitivity, and specificity of the model were 0.340, 63.38%, and 67.52%, respectively.
DISCUSSION
Accurate evaluation of the stage of hepatic fibrosis is important in patients with chronic HBV infection for determining the initiation of antiviral therapy and is an important index for evaluating the efficacy of antiviral therapy. FibroScan is a recommended non-invasive test for evaluation of liver fibrosis in patients with chronic HBV infection[4,13]. In the present study, we confirmed that LSM values obtained using FibroScan were positively correlated with hepatic fibrosis and demonstrated the good performance of FibroScan in predicting the stage of liver fibrosis. We found that the optimal diagnostic LSM values of FibroScan for significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4) were 7.3 kPa (AUC = 0.863), 9.7 kPa (AUC = 0.911),and 11.3 kPa (AUC = 0.918), respectively. Our results are consistent with those of previous studies[6,14,15].

Figure 2 Distribution of predicted fibrosis stages by transient elastography according to different METAVIR liver fibrosis stages.FibroScan: Transient elastography.

Figure 3 Comparison of liver stiffness measurement values by transient elastography in patients with different liver inflammation activities in different METAVIR fibrosis stages. LSM: Liver stiffness measurements.
Although LSM values measured by ultrasound elastography are related to the stage of fibrosis, they could be affected by acute hepatitis, high ALT and/or AST levels,obstructive cholestasis, and infiltrative hepatic disease[7,16,17]. We explored the relationship between various anthropometric, biochemical, and pathological parameters and the diagnostic accuracy of FibroScan for determining the stage of liver fibrosis. A discordance between the fibrosis stage determined using FibroScan and that determined by pathological examination was observed in 34.1% of the patients(142/416), with 19.5% of patients (81/416) over-staged and 14.7% of patients (61/416)under-staged in our study. Compared with patients who showed concordance between values obtained using the two methods, those who showed discordance had significantly higher ALT and AST levels, and a higher proportion of moderate to severe liver inflammatory activity. Furthermore, multivariate analysis showed that liver inflammatory activity over 2 was an independent risk factor for misdiagnosis of fibrosis stage using FibroScan.

Figure 4 Prevalence of misdiagnosis of stage of liver fibrosis by transient elastography in patients with different inflammatory activities.
However, the bias caused by liver inflammation in the assessment of liver fibrosis stage using FibroScan is still unclear. The changes occurring in liver enzymes during inflammatory degeneration, necrosis, and fibrosis of hepatic cells are strong indicators of inflammation, in which ALT and AST are the most valuable serum biochemical indices for the detection of liver injury. Many studies have shown that elevated LSM values were related to increased ALT levels, and have proposed a variety of dual cutoffs of LSM values adapted to ALT levels, which may improve the diagnostic performance of FibroScan in evaluating the stage of hepatic fibrosis in patients with chronic HBV infection[10,11,18]. The elevated baseline LSM values due to liver inflammation in patients with elevated ALT levels could lead to inappropriate overestimation of the stage of liver fibrosis. We found that patients with inflammation activity ≥ 2 had higher LSM values in each fibrosis stage among F0-1, F2, F3, and F4(all P < 0.05), and a higher percentage of mis-staging (55.8% vs 20.2%, P < 0.001), overstaging (36.8% vs 8.3%, P < 0.001), and under-staging (19.0% vs 11.9%, P = 0.044) using FibroScan, compared with patients with inflammation activity < 2. Other studies reported a lack of these correlations and indicated that mildly increased ALT levels did not affect the performance of LSM in assessing hepatic fibrosis in patients with chronic HBV infection[19,20]. A recent study reported that the sensitivity and specificity of LSM values for assessing the stage of liver fibrosis were significantly lower in patients with ALT levels ≥ 2 times the ULN[11]. Our study findings are consistent with this result. We found that FibroScan was significantly better in predicting significant fibrosis (≥ F2), advanced fibrosis (≥ F3), and cirrhosis (F4) in patients with inflammation activity < 2 than in patients with inflammation activity ≥ 2, by comparing the AUCs (0.831 vs 0.702, 0.903 vs 0.815, and 0.941 vs 0.836, all P < 0.05).Therefore, we concluded that the diagnostic accuracy of LSM was mainly influenced by significantly elevated ALT levels (ALT > 2 ULN), acute viral hepatitis, HBV flares,and the severity of liver fibrosis.
At present, many non-invasive models have been developed to diagnose liver fibrosis. The WHO guidelines on chronic HBV infection recommended that LSM and APRI are the most helpful detection methods to evaluate hepatic fibrosis with limited resources[21]. The accuracy of LSM values could be affected by inflammation and other influencing factors. FibroScan may yield low LSM values and underestimate or misdiagnose the stage of liver fibrosis in patients with mild hepatic inflammation, and it may show elevated LSM values and overestimate or misdiagnose cirrhosis in patients with severe inflammation. In our study, the severity of liver inflammation was an independent risk factor for misdiagnosis of the stage of liver fibrosis using FibroScan; however, the measurement of severity entailed an invasive procedure.Therefore, we used other relevant non-invasive factors to predict the risk of misdiagnosis using FibroScan, which may be of great significance in determining the fibrosis stage or performing liver biopsy, and may guide the diagnosis of and therapy of chronic HBV infection. Our model consisted of three routinely assessed parameters(ALT levels, AST levels, and FibroScan-predicted fibrosis staging), which showed better performance than those of other single related factors in predicting the risk of misdiagnosis of the stage of hepatic fibrosis using FibroScan staging by ROC analysis.According to this model, more attention should be paid to patients at a high risk of being misdiagnosed using FibroScan, a comprehensive evaluation of the degree of hepatic fibrosis should be conducted, and further liver biopsy should be performed, if necessary, to determine whether antiviral therapy needs to be initiated immediately.
This study has several limitations. First, the effects of controlled attenuation parameters and histological steatosis on the diagnostic performance of FibroScan were not discussed. Second, the sample size of the study was very small. An extensive liver biopsy database should be established to comprehensively evaluate the reliable cut-off value of FibroScan for assessing the stage of liver fibrosis. Third, the results of our study warrant further verification in large-scale, multicenter cohort studies.
CONCLUSION
In conclusion, liver inflammation is an independent risk factor that affects the accuracy of FibroScan in assessing the stage of HBV-related liver fibrosis. A combination of other related non-invasive factors can help predict the risk of misdiagnosis of the stage of liver fibrosis using FibroScan, which may help to decide whether liver biopsy is required and guide the diagnosis of and therapy of chronic HBV infection.

Figure 6 Comparison of receiver operating characteristic curves in prediction model and single related factors with regard to misdiagnosis of the stage of liver fibrosis using transient elastography. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ROC:Receiver operating characteristic; FibroScan: Transient elastography.
ARTICLE HIGHLIGHTS
Research background
Transient elastography (FibroScan) is a new and non-invasive test, which can replace biopsy and has been widely recommended by the guidelines of chronic hepatitis B virus (HBV) management for assessing hepatic fibrosis staging. Liver stiffness measurement (LSM) by FibroScan is associated with the degree of hepatic fibrosis, but can also be confounded by liver necroinflammation, alanine aminotransferase (ALT),cholestasis, portal hypertension, hepatic congestion, and body mass index (BMI) and other factors, which may affect the diagnostic accuracy of the FibroScan device in fibrosis staging.
Research motivation
Many studies suggested that the cutoff value of LSM tends to increase with elevated ALT level, and its diagnostic accuracy tends to decrease with elevated ALT level, but it is not clear whether pathological hepatic inflammation would similarly affect LSM values and diagnostic accuracy of FibroScan assessing hepatic fibrosis.
Research objectives
We aimed to evaluate the diagnostic value of FibroScan and the effect of hepatic inflammation on the accuracy of FibroScan assessing liver fibrosis staging in patients with chronic HBV infection, and to develop a predictive model combining other related non-invasive confounders to predict the risk of FibroScan staging misdiagnosis.
Research methods
The data of 416 patients with chronic HBV infection who accepted FibroScan, liver biopsy, clinical, and biological examination were retrospectively collected between January 2014 and December 2019 from two affiliated hospitals of Fujian Medical University. Receiver operating characteristic (ROC) curves were used to analyze the data. The diagnostic performance of FibroScan for the stage of liver fibrosis was analyzed using ROC curves. Any discordance in fibrosis staging by FibroScan and pathological scores was statistically analyzed. The accuracy of FibroScan in assessing the stage of fibrosis in patients with different degrees of liver inflammation was analyzed using Logistic regression and ROC curves. A non-invasive model was constructed to predict the risk of misdiagnosis of fibrosis stage using FibroScan.
Research results
We confirmed that LSM values obtained using FibroScan were positively correlated with hepatic fibrosis and demonstrated the good performance of FibroScan in
predicting the stage of liver fibrosis. However, discordance between the fibrosis stage determined using FibroScan and that determined by pathological examination was observed in some patients. Furthermore, we found that liver inflammatory activity over 2 was an independent risk factor for misdiagnosis of fibrosis stage using FibroScan. Patients with liver inflammation activity ≥ 2 showed higher LSM values using FibroScan and higher rates of misdiagnosis of fibrosis stage, whereas the diagnostic performance of FibroScan for different fibrosis stages was significantly lower than that in patients with inflammation activity < 2. A non-invasive prediction model was established to assess the risk of misdiagnosis of fibrosis stage using FibroScan, and the area under the curve was 0.701, which was superior to that observed using other single related factors.
Research conclusions
Liver inflammation was an independent risk factor affecting the diagnostic accuracy of FibroScan for HBV-related fibrosis staging. The combination of other related noninvasive factors can predict the risk of misdiagnosis of fibrosis staging using FibroScan, and may be helpful for making decisions on liver biopsy and guiding the diagnosis and therapy of chronic HBV infection.
Research perspectives
This multi-center cross-sectional study developed and evaluated a noninvasive model to predict the risk of misdiagnosis of fibrosis staging using FibroScan, thus an extensive liver biopsy database should be established to comprehensively evaluate the reliable cut-off value of FibroScan for assessing the stage of liver fibrosis and further verify the diagnostic performance of this model in future prospective studies.
 World Journal of Gastroenterology2021年7期
World Journal of Gastroenterology2021年7期
- World Journal of Gastroenterology的其它文章
- Helicobacter pylori: Commensal, symbiont or pathogen?
- Simultaneous partial splenectomy during liver transplantation for advanced cirrhosis patients combined with severe splenomegaly and hypersplenism
- Clinicopathological features and prognostic factors associated with gastroenteropancreatic mixed neuroendocrine non-neuroendocrine neoplasms in Chinese patients
- Quantitative multiparametric magnetic resonance imaging can aid non-alcoholic steatohepatitis diagnosis in a Japanese cohort
- Sinapic acid ameliorates D-galactosamine/lipopolysaccharideinduced fulminant hepatitis in rats: Role of nuclear factor erythroidrelated factor 2/heme oxygenase-1 pathways
- Xiangbinfang granules enhance gastric antrum motility via intramuscular interstitial cells of Cajal in mice
