Adjusting energy level alignment between HTL and CsPbI2Br to improve solar cell efficiency
Zihan Zhang , Jia Li , Zhimin Fang Haipeng Xie, Yongbo Yuan, Chuantian ZuoLiming Ding , and Bin Yang
1College of Materials Science and Engineering, Hunan University, Changsha 410082, China
2Center for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China
3Hunan Key Laboratory of Super Microstructure and Ultrafast Process, College of Physics and Electronics, Central South University,Changsha 410083, China
Lead halide perovskites are at the forefront of optoelectronic materials due to their high absorption coefficient, tunable bandgap, long carrier diffusion length, and small exciton binding energy[1−3], yielding high-performance optoelectronic devices.The power conversion efficiency (PCE) of organic–inorganic hybrid perovskite solar cells has exceeded 25%[4].But the organic–inorganic hybrid perovskites present low thermal stability.All-inorganic perovskites (CsPbX3, X = I, Br,Cl) prepared by replacing the organic ions (MA+, FA+) with inorganic Cs+show better thermal stability[5].But the photoactive phaseα-CsPbI3is easy to change to the non-photoactive phase (δ-CsPbI3) at room temperature[6,7].The bromide-containing inorganic perovskite CsPbI2Br is becoming popular due to better phase stability[8−10].
Compared with the organic–inorganic hybrid perovskite solar cells, CsPbI2Br solar cells exhibited a relatively large energy loss (>0.8 eV) (Eloss=Eg–qVoc)[11−13], which is mainly caused by defects in the perovskite film and the large energy level offset between the perovskite and charge-transport layers[14,15].A feasible strategy is to modify the chargetransport materials to reduce the energy level offset.Modification of PTAA hole-transport layer (HTL) was performed to obtain suitable energy levels and improve the hole-transporting capability[16].Seoet al.developed a series of F-containing PTAA derivatives with the highest occupied molecular orbital (HOMO) energy levels from –5.14 to –5.63 eV to match the energy levels of perovskites[17].However, these derivatives need tedious chemical synthesis.In this work, the energy level offset was reduced from 0.79 to 0.60 eV by blending 7.5% PVK into PTAA layer.Significantly improvedVoc(from 1.10 to 1.19 V) and PCE (from 11.1% to 13.6%) were obtained.
CsPbI2Br films were characterized by SEM (Fig.S1), XRD(Fig.S2), and optical measurements (Fig.S3).Solar cells with a structure of ITO/SnO2/CsPbI2Br/HTL/MoO3/Ag (Figs.1(a)and 1(b)) were fabricated.The performance of perovskite solar cells changing with PVK content in PTAA is summarized in Figs.S4 and S5 and Table S1.The device with pristine PTAA exhibits aJscof 14.4 mA cm–2, aVocof 1.10 V, a FF of 70%, and a PCE of 11.1%.The device with PTAA (7.5% PVK) gave a PCE of 13.6%, with aJscof 14.5 mA cm–2, aVocof 1.19 V, and a FF of 79% (Fig.1(c)).The integrated current density from EQE spectrum is 13.8 mA cm–2, which is consistent with theJscfromJ–Vmeasurements (Fig.S6).The cells presented slightJ–Vhysteresis (Fig.S7).The steady-state current and stabilized PCE at 0.99 V are 13.2 mA cm–2and 13.0%, respectively(Fig.S8).
The difference in charge extraction for pristine PTAA and PTAA (7.5% PVK) was investigated by conducting the transient photocurrent (TPC) measurements under the short circuit condition (Fig.1(d)).The TPC decay curves were fitted with a single exponential function ofI0exp(−t/τ), whereI0is steady-state photocurrent andτrepresents the charge extraction time.τwas estimated to be 0.26 and 0.83μs for the cells with PTAA (7.5% PVK) and pristine PTAA, respectively.The decrease ofτindicates that adding 7.5% PVK into PTAA layer increases the charge extraction efficiency and reduces the charge recombination, which is consistent with the electrochemical impedance spectroscopy (EIS) measurements(Fig.S9 and Table S2).
The changes ofJ–Vcurves,VocandJscwith light intensity were investigated (Figs.S10–S12).The slope of the semilogarithmic plot forVocversus light intensity is equal tonkT/q,wherenis the diode quality factor,kis the Boltzmann constant,Tis the temperature, andqis the elementary charge.nwas calculated to be 1.72 and 1.21 for the solar cells with pristine PTAA and PTAA (7.5% PVK), respectively.The reduction ofnindicates reduced trap-induced recombination.Linear fitting forJscversus light intensity plots gave slopes of 0.94 and 0.97 for the cells with pristine PTAA and PTAA (7.5%PVK), respectively.The slope for the cell with PTAA (7.5%PVK) is closer to 1, indicating less bimolecular recombination[18].
The energy levels for pristine PTAA and PTAA (7.5% PVK)were measured by ultraviolet photoelectron spectroscopy(UPS) (Fig.1(e)).The workfunction (WF) for pristine PTAA is–3.92 eV, with a HOMO level of –5.29 eV, which is consistentwith the reported value (–5.23 eV)[19].The HOMO level for PTAA (7.5% PVK) film is –5.48 eV, which is closer to the valence band maximum (VBM) (–6.08 eV) of CsPbI2Br[20], leading to a better energy level alignment at CsPbI2Br/HTL interface.The energy level offset being reduced from 0.79 to 0.60 eV (Fig.1(f)) accounts for the increase ofVoc.
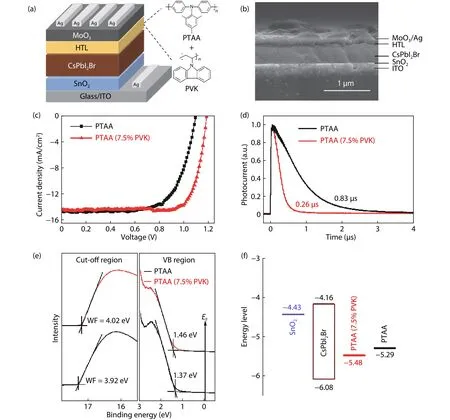
Fig.1.(Color online) (a) The device structure and chemical structures for PTAA and PVK.(b) Cross-sectional SEM image of the device.(c) J–V curves for the solar cells with pristine PTAA and PTAA (7.5% PVK).(d) Transient photocurrent measurements for the solar cells.(e) UPS spectra for pristine PTAA and PTAA (7.5% PVK).(f) The energy level diagram.
In summary, PVK was introduced into PTAA HTL to optimize the energy level alignment at the CsPbI2Br/HTL interface.The energy level offset decreased from 0.79 to 0.60 eV.The resulting solar cells delivered a PCE of 13.6% and a highVocof 1.19 V.This work provides an effective approach for developing efficient all-inorganic perovskite solar cells.
Acknowledgements
B.Yang thanks National Natural Science Foundation of China (62004066) and Hunan Provincial Science and Technology Department (2019GK2101) for financial support.L.Ding thanks the National Key Research and Development Program of China (2017YFA0206600) and the National Natural Science Foundation of China (51773045, 21772030, 51922032,21961160720) for financial support.
Appendix A.Supplementary materials
Supplementary materials to this article can be found online at https://doi.org/1674-4926/42/3/030501.
 Journal of Semiconductors2021年3期
Journal of Semiconductors2021年3期
- Journal of Semiconductors的其它文章
- Renaissance of tin halide perovskite solar cells
- White light-emitting diodes from perovskites
- Blade-coated organic solar cells from non-halogenated solvent offer 17% efficiency
- Self-assembled monolayers enhance the performance of oxide thin-film transistors
- I ndium–gallium–zinc–oxide thin-film transistors: Materials,devices, and applications
- L ow-dimensional materials for photovoltaic application
