Expert consensus of the Chinese Association for the Study of Pain on pain treatment with the transdermal patch
Ke Ma, Wei Jiang, Yun-Xia Wang, Lin Wang, Yan Lv, Jin-Feng Liu, Rong-Guo Liu, Hui Liu, Li-Zu Xiao, Dong-Ping Du, Li-Juan Lu, Xiao-Qiu Yang, Ling-Jie Xia, Dong Huang, Zhi-Jian Fu, Bao-Gan Peng, Yan-Qing Liu
Ke Ma, Department of Algology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China
Wei Jiang, Department of Anesthesiology, Third Medical Center of People’s Liberation Army General Hospital, Beijing 100039, China
Yun-Xia Wang, Department of Algology, The Third People’s Hospital of Hubei Province, Hubei Zhongshan Hospital, Wuhan 430033, Hubei Province, China
Lin Wang, Department of Algology, Affiliated Hospital of Guizhou Medical University,Guiyang 550004, Guizhou Province, China
Yan Lv, Department of Algology, Xijing Hospital, Air Force Medical University, Xi'an 710032,Shaanxi Province, China
Jin-Feng Liu, Department of Algology, The Second Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, China
Rong-Guo Liu, Department of Algology, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China
Hui Liu, Department of Algology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China
Li-Zu Xiao, Department of Algology, Shenzhen Sixth People’s Hospital (Nanshan Hospital),Shenzhen 518000, Guangdong Province, China
Dong-Ping Du, Department of Algology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiaotong University, Shanghai 200233, China
Li-Juan Lu, Department of Algology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing 210008, Jiangsu Province, China
Xiao-Qiu Yang, Department of Algology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
Ling-Jie Xia, Department of Algology, Henan Provincial People’s Hospital, Zhengzhou 450000,Henan Province, China
Dong Huang, Department of Algology, The Third Xiangya Hospital of Central South University, Changsha 410013, Hunan Province, China
Zhi-Jian Fu, Department of Algology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan 250021, Shandong Province, China
Bao-Gan Peng, Department of Orthopedics, The Third Medical Center, General Hospital of the Chinese People’s Liberation Army, Beijing 100039, China
Yan-Qing Liu, Department of Algology, Beijing Tiantan Hospital, Capital Medical University,Beijing 100050, China
Abstract Chronic pain lasting more than 3 mo, or even several years can lead to disability.Treating chronic pain safely and effectively is a critical challenge faced by clinicians. Because administration of analgesics through oral, intravenous or intramuscular routes is not satisfactory, research toward percutaneous delivery has gained interest. The transdermal patch is one such percutaneous delivery system that can deliver drugs through the skin and capillaries at a certain rate to achieve a systemic or local therapeutic effect in the affected area. It has many advantages including ease of administration and hepatic first pass metabolism avoidance as well as controlling drug delivery, which reduces the dose frequency and side effects. If not required, then the patch can be removed from the skin immediately. The scopolamine patch was the first transdermal patch to be approved for the treatment of motion sickness by the Food and Drug Administration in 1979. From then on, the transdermal patch has been widely used to treat many diseases. To date, no guidelines or consensus are available on the use of analgesic drugs through transdermal delivery. The pain branch of the Chinese Medical Association, after meeting and discussing with experts and based on clinical evidence, developed a consensus for promoting and regulating standard use of transdermal patches containing analgesic drugs.
Key Words: Transdermal drug delivery systems; Pain; Transdermal patches; Topical;Nonsteroidal anti-inflammatory drugs; Analgesics
INTRODUCTION
According to the International Association for the Study on Pain, “Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” Chronic pain refers to pain lasting or recurring for more than 3 mo[1]. Chronic low back pain is one of the leading causes of disability in the Chinese population[2,3]. Hence, treating chronic pain safely and effectively is one of the important clinical problems. In recent years, the research and development of new drugs for chronic painviaoral, intravenous and intramuscular delivery are not satisfactory, but transdermal delivery of analgesic drugs has made great progress[4].
Currently, no international guidelines or consensus are available on the use of transdermal analgesic drugs. Therefore, this article summarizes China’s expert consensus on “transdermal pain treatment in China,” with a view to regulate and promote the standardized use of the transdermal patch containing analgesic drugs.The consensus was formulated by the pain branch of the Chinese Medical Association with the help of experts and using recent clinical evidence.
OVERVIEW OF TRANSDERMAL DRUG DELIVERY SYSTEM
In the transdermal drug delivery system (TDDS), the drug enters systemic circulation through the skin and capillaries at a certain rate to achieve a systemic or local therapeutic effect[5,6]. TDDS in the broad sense includes all topically administered formulations such as ointment, patch, cataplasm, aerosol, coating,etc., whereas TDDS in the narrow sense refers to the transdermal patch[7]. The history of transdermal drug delivery dates back thousands of years. In ancient China, leaves and grass stalks were used to smear the skin and wounds. More than 1500 prescriptions are available in the treatise on external treatment of emergency Guangsheng in the Ming and Qing dynasties[8]. In the modern era, the scopolamine patch for the treatment of motion sickness was the first transdermal patch to be approved by the Food and Drug Administration (FDA) in 1979. From then on, the transdermal patch has been widely used to treat many diseases[9].
CLINICAL CHARACTERISTICS OF TDDS
Advantages of TDDS
Compared with other routes of administration, transdermal administration has many advantages, as summarized in Table 1[10,11].
Absorption and factors influencing skin absorption
Traditionally, transdermal drugs are absorbed by passive diffusion. The stratum corneum, the outermost layer of the skin, is the main rate-limiting barrier for transdermal drug transport. Factors affecting transdermal absorption include physicochemical properties (molecular weight, solubility, partition coefficient and dissociation constant, PKA), carrier properties and skin conditions (Table 2)[10,12]. The rate and extent of drug absorption through skin can be calculated using the following formula:
LogP= −2.7 + 0.71 × log Ko/w − 0.0061 × M
Where, Ko/w = oil-water partition coefficient and M = molecular weight[9-11].
Systemic and local effects of TDDS
In TDDS for systemic delivery, the drug from TDDS gets transported to the subcutaneous capillaries through the skin without accumulating in the dermis. Once the drug reaches systemic circulation, it exhibits its therapeutic action. Examples of such TDDS include fentanyl transdermal patch, buprenorphine transdermal patch and scopolamine transdermal patch[10,13].
In TDDS, the drug is transported to the subcutaneous tissue through the skin and then to the deeper tissue to exert local action. Some of the TDDS for local action include nonsteroidal anti-inflammatory drugs (NSAIDs) transdermal patch, capsaicin patch and lidocaine patch[10,13].
Structure and development of the transdermal patch
According to the characteristics of its dosage form, the transdermal patch can be roughly divided into three generations (Table 3). The first generation of transdermal patch is the most representative transdermal patch[14].
The structure of a TDDS includes four layers: (1) an impermeable backing layer to protect the system from invasion of external substances and to prevent the loss ofdrugs or evaporation of skin moisture; (2) a drug storage or framework system to store and release drugs; (3) a liner that protects the patch during storage, which has to be removed prior to use; and (4) an adhesive layer to keep the patch in contact with the skin[10]. The adhesive layer can be divided into peripheral type and surface type.Peripheral type refers to applying a circle of pressure-sensitive adhesive on the periphery of the TDDS drug part. In surface type, TDDS is completely covered with finger-sensitive adhesive coating. Of the two types, the surface type of adhesive layer is the most common[10].

Table 1 Advantages of transdermal drug delivery system administration

Table 2 Factors influencing drug percutaneous absorption[10,12]

Table 3 Development of transdermal patch[14]
Only two types of TDDS were available before 1990: reservoir type and matrix type.After 1990, the adhesive dispersion type was introduced. In the adhesive dispersion type, the drug is dispersed in the adhesive layer itself; this helps to reduce the layers to two or three[15]. Since 1999, the FDA has not approved a reservoir type because of the risk of uncontrolled drug release from the reservoir. Most of the existing patches in the United States market (72%) are of the adhesive dispersion type[16].
Complications with application of patch
Dose adjustment is the main challenge faced in the use of a transdermal patch because only fixed dosages are commercially available[17]. In theory, an alternative option to reduce the dose is cutting the patch. However, cutting the patch may result in altering the structure of the patch, which may result in altering drug release, especially controlled release, and the quality of the adhesive layer[18]Cutting the microdrug reservoir of the patch can damage the microparticles of the drug, which leads to inaccuracy of dose evaluation[17,18]. A comparative study on the use of the clonidine patch (Catapres TTS) in sections and as a whole reported difficulty in predicting absorption degree and rate of drug release after cutting the patch, and the incidence of abnormal (too high or too low) blood concentration increased significantly with sections of patch. For most analgesic drugs, it is recommended to refer to the relevant instructions provided by the manufacturer[19].
Application site of patch: transdermal analgesic drugs can be applied at two application sites. Opioid transdermal patch is generally fixed on the chest, abdomen or upper arm because of its systemic effect. Topical patches with local effects such as topical NSAID patches, 8% capsaicin patch, 5% lidocaine patch and other patches are generally applied to the pain site[17,18].
COMMON CLINICAL TDDS
Transdermal NSAIDs
NSAIDs act by inhibiting prostaglandin synthesis and reduce persistent hyperalgesia by inhibiting cyclooxygenase activity to play an analgesic and anti-inflammatory role[20,21]. Compared with NSAIDs administered systemically, topical administration of NSAIDs reach therapeutic concentrations at the pain/inflammation site while maintaining low serum levels and potentially minimizing adverse effects related to high systemic absorption[20-22].
The advantages of topical NSAID application depend on the ability of the drug to penetrate the skin and reach the site of action. Different NSAIDs have different penetration abilities. Table 4 summarizes the pharmacokinetic properties of different topical NSAIDs[23-29]. According to studies reported, for a drug to be formulated as a TDDS, it should have ideal properties such as a partition coefficient (logP) of 1 to 4,molecular weight of < 400 Da, lipophilic, highly potent, low melting point and short half-life[6,30,31]. The peak plasma concentrations of NSAIDs vary greatly, but their concentrations in synovial fluid were much more stable than those in the plasma[32].The plasma concentration of NSAIDs through topical delivery is about 1% to 10% of that of systemic administration. However, whether the concentration of NSAIDs in the local tissue of the application site is higher than that of systemic administration remains uncertain[32]. Most studies show that the concentration of NSAIDs in the deep tissue of the application site (such as the skeletal muscle and synovium) is equivalent to that of systemic administration. In addition, topical administration of NSAIDs in elderly patients may result in increased plasma concentration, which may be due to reduced drug clearance and thin skin[31,32].
Topical NSAIDs can be used in different diseases such as acute sprain/strain, low back pain, chronic musculoskeletal pain and neuropathic pain[13,21,22,31-33]. A review showed similar efficacy with topical and oral NSAIDs in the treatment of chronic skeletal muscle pain[34,35]. When NSAIDs alone are not effective in treating moderate to severe pain or multisite pain, combination therapy with other analgesic drugs helps in effective treatment. When a combination of topical and oral NSAIDs is used, attention should be paid to avoid an overdose[36-40].
NSAIDs for topical application can be in the form of ointment, gel, gel paste and patch. Some studies compared topical NSAID patches with ointments and gelatin formulations. The results showed that the permeability of patches was better than that of gelatin and ointments. Compared with ointments, patches had better adherence[41-44].
Adverse effects with topical NSAIDs can be cutaneous and systemic adverse reactions. The incidence of cutaneous adverse reactions is about 1% to 2%, which include erythema, pruritus, irritation, fever or burning sensation and contact dermatitis. Most of the cutaneous adverse reactions were reported to be mild and disappeared after drug withdrawal[45-47].

Table 4 Pharmacokinetic characteristics of topical nonsteroidal anti-inflammatory drugs patches[23-29]
Transdermal opioids
The commonly used opioid transdermal patches in China are the fentanyl transdermal patch and buprenorphine transdermal patch[13]. Table 5 represents the pharmacology of opioid transdermal patches[48-52].
Fentanyl transdermal patch
Fentanyl is a strong opioid analgesic. Its analgesic intensity is about 100 times that of morphine, and its transdermal penetration ability is 43 times that of morphine.Because of its ideal properties such as small molecular weight (337 Da), highly lipophilic properties and no biotransformation in the process of transdermal penetration, it was the first analgesic drug formulated as a TDDS[49]. The fentanyl transdermal patch was approved by the FDA in 1990 for the treatment of chronic pain[13]. Other than chronic pain, it is also used for neuropathic pain and cancer pain[50].
Buprenorphine transdermal patch
Buprenorphine, a semisynthetic derivative of dimethylmorphine, is a partial agonist of the μ opioid receptor and antagonist of the κ receptor. The buprenorphine transdermal patch was approved by the FDA in 2010 for pain treatment. It is used for moderate to severe cancer pain, skeletal muscle pain, neuropathological pai, and visceral pain. As with fentanyl, buprenorphine is also not recommended in the treatment of acute and breakthrough pain[4,13,51,52].
Compared with fentanyl and other opioids, buprenorphine transdermal patch has lower neurotoxicity, especially in the elderly or patients with Alzheimer’s disease.Another significant advantage of the transdermal buprenorphine patch is that no dosage adjustment is needed in patients with renal insufficiency[13].
Opioid transdermal patches can significantly reduce pain and gastrointestinal related adverse effects such as nausea, vomiting and constipation and reduce the proportion of patients discontinuing treatment due to adverse reactions. However, in the treatment of chronic low back pain and knee and hip arthritis opioids (including opioid transdermal patches) were reported to have no significant difference in relieving pain and improving body function, while more adverse reactions were reported when compared with NSAIDs[53-57].
Capsaicin transdermal patch
Capsaicin is a selective transient receptor potential vanilloid receptor, subtype 1 agonist that activates the nociceptive sensory nerve fibers (C- and Ad-fibers) of transient receptor potential vanilloid receptor, subtype 1 in the skin, resulting in enhanced sensitivity to stimuli, burning sensation and erythema. Exposure to a single high dose or repeated exposure to low dose of capsaicin can lead to the nonfunctioning of nociceptors[13,58].
Studies reported absorption of 1% capsaicin into the epidermis and dermis after an hour of patch application. The absorption of capsaicin is directly proportional to the surface area and time of application. Skin temperature was also found to have an influence on the absorption of capsaicin. The results of a population pharmacokinetics study showed that peak plasma concentration (1.38 ng/mL) of an 8% capsaicin patch was attained after 1.46 h. Moreover, capsaicin has a high protein-binding capacity(93%-94%). After absorption, it is mainly metabolized in the liver by P450, and the elimination half-life is 1.64 h[59,60]. The transdermal patch available is 8% capsaicin at a dosing frequency of one patch per day. Capsaicin is used for the treatment of neuropathic pain. Better results were reported with capsaicin than placebo in the treatment of post herpetic neuralgia and diabetic peripheral neuralgia[61,62].

Table 5 Pharmacology of fentanyl and buprenorphine transdermal patches[48-52]
Lidocaine transdermal patch
Lidocaine is a voltage-gated sodium channel blocker (mainly Nav1.7 and 1.8). It can reduce the ectopic negative charge, increase the threshold of peripheral ectopic discharge and reduce the pain transmission by stabilizing the membrane potential of neurons on abnormal excitation of Aδ and C fibers to have an analgesic effect. In addition, the lidocaine patch also has an analgesic effect on pain from injurious sources[13,63].
Transdermal lidocaine majorly penetrates local tissues through the skin, and very little is absorbed into systemic circulation (about 3% ± 2%); hence, adverse reactions related to systemic administration can be avoided. Patients with severe impairment of heart, kidney and liver function should be cautious with lidocaine use. It is contraindicated in pregnant women and patients allergic to local anesthetics[13,64,65].
The lidocaine patch is generally well tolerated even after long term use. The most common adverse events include erythema, pruritus, rash, burning sensation,dermatitis, edema and other skin reactions. The lidocaine patch is used in the treatment of neuropathic pain, but there is no high-quality evidence to prove its clinical efficacy[64-69].
Other transdermal patches
The ketamine transdermal patch and dextromethorphan transdermal patch are common nonbarbiturate anesthetics. They are peripheral N-methyl-D-aspartate receptor inhibitors and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor inhibitors. They also inhibit voltage-gated Na+and K+channels[63].
Ketamine transdermal patch (25 mg/24 h) is used to relieve postoperative pain. The 4% dextromethorphan hydrochloride is formulated with 4% lidocaine and 10%trolamine salicylate (Permavan) and used to treat pain, but its efficacy and safety have not been determined. At present, neither of the two transdermal patches have been approved for marketing[70,71].
The bupivacaine transdermal patch is also under development. Compared with the lidocaine patch for 12 h, the bupivacaine patch can be applied once every 3 d and can be used for post herpetic neuralgia treatment. The United States bupivacaine transdermal patch (Eladur) is under investigation[71].
Rotigotine and amitriptyline are commonly used in the treatment of neuropathic pain. At present, the rotigotine transdermal patch (listed in Europe and the United States in 2007 and in China in 2018) and amitriptyline transdermal patch are available.The rotigotine transdermal patch can improve chronic pain in Parkinson’s disease[72].However, neither of the drugs has been approved for pain treatment[73].
Clonidine, a α2adrenergic receptor agonist and imidazoline receptor agonist, is used in antihypertension, acute and chronic pain management and sedation. However, the clonidine transdermal patch is currently approved only in the treatment of hypertension. The analgesic effect of the clonidine patch is related to the α2receptor in the skin and the imidazoline receptor in the peripheral nerve endings, but clonidine use in the treatment of neuropathic pain is insufficient[71,74-77].
CONCLUSION
Based on expert opinions and careful assessment of the existing evidence, the classification and definition of grade evidence were summarized in Tables 6 and 7,while the consensus recommended by experts was summarized in Table 8. This consensus can help clinicians to use transdermal patches in pain management more effectively.

Table 6 Quality classification and definition of grade evidence

Table 7 Grade recommended strength classification and definition
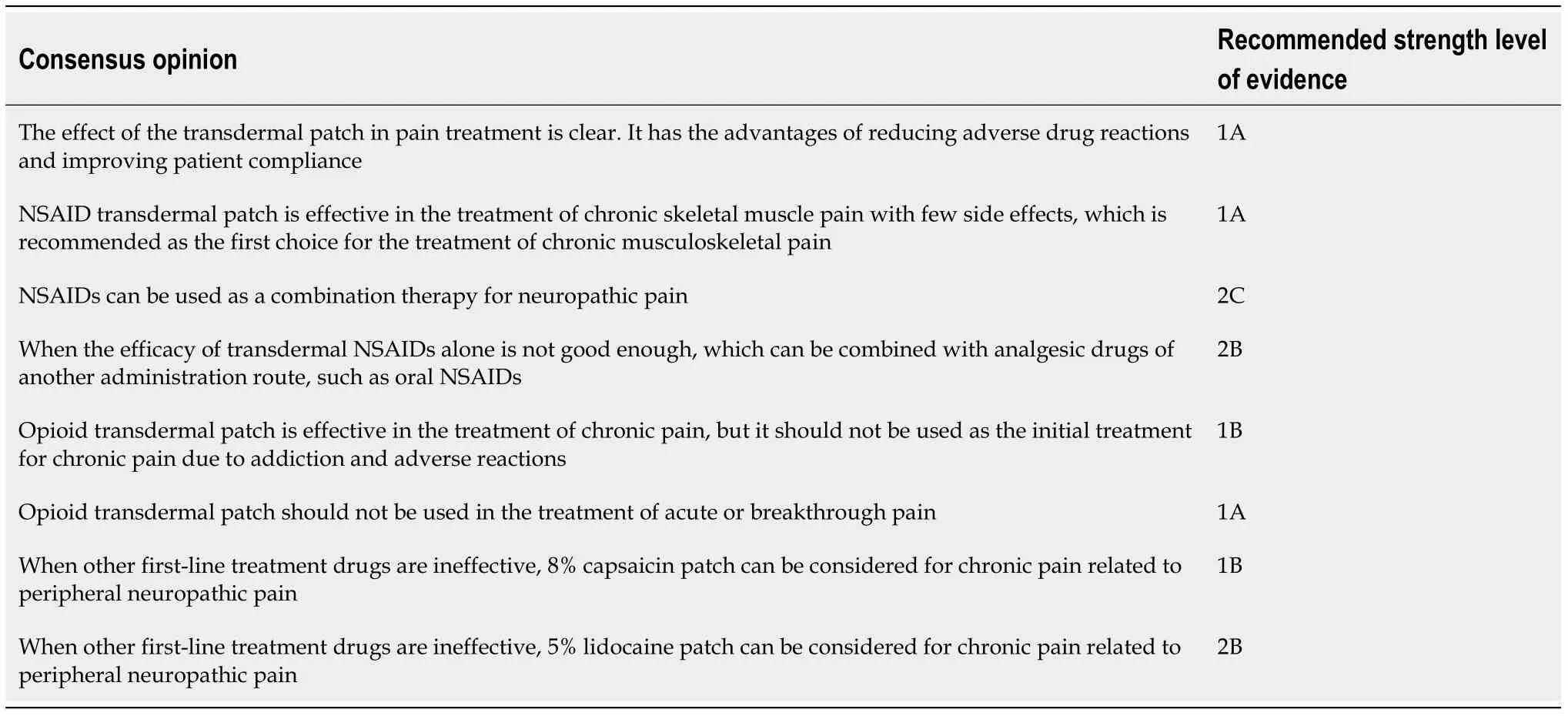
Table 8 Consensus statement of Chinese experts on pain treatment with transdermal patch
ACKNOWLEDGEMENTS
The authors would like to thank Daiichi-Sankyo Co., Ltd for the medical support of the manuscript.
 World Journal of Clinical Cases2021年9期
World Journal of Clinical Cases2021年9期
- World Journal of Clinical Cases的其它文章
- Cervical intervertebral disc degeneration and dizziness
- Contributions of aversive environmental stress to migraine chronification: Research update of migraine pathophysiology
- Expert consensus of Chinese Association for the Study of Pain on the non-opioid analgesics for chronic musculoskeletal pain
- Editorial for the special issue of the Chinese Association for the Study of Pain
- Clinical efficacy of ultrasound-guided pulsed radiofrequency combined with ganglion impar block for treatment of perineal pain
- Expert consensus of Chinese Association for the Study of Pain on the radiofrequency therapy technology in the Department of Pain
