Genotype 3-hepatitis C virus' last line of defense
Dorota Zarebska-Michaluk
Abstract Chronic infection with hepatitis C virus (HCV) is one of the leading causes of liver disease globally, affecting approximately 71 million people. The majority of them are infected with genotype (GT) 1 but infections with GT3 are second in frequency. For many years, GT3 was considered to be less pathogenic compared to other GTs in the HCV family due to its favorable response to interferon (IFN)-based regimen. However, the growing evidence of a higher rate of steatosis, more rapid progression of liver fibrosis, and lower efficacy of antiviral treatment compared to infection with other HCV GTs has changed this conviction. This review presents the specifics of the course of GT3 infection and the development of therapeutic options for GT3-infected patients in the era of direct-acting antivirals (DAA). The way from a standard of care therapy with pegylated IFNalpha (pegIFNα) and ribavirin (RBV) through a triple combination of pegIFNα + RBV and DAA to the highly potent IFN-free pangenotypic DAA regimens is discussed along with some treatment options which appeared to be dead ends. Although the implementation of highly effective pangenotypic regimens is the most recent stage of revolution in the treatment of GT3 infection, there is still room for improvement, especially in patients with liver cirrhosis and those who fail to respond to DAA therapies, particularly those containing inhibitors of HCV nonstructural protein 5A.
Key Words: Hepatitis C virus; Genotype 3; Antiviral treatment; Interferon; Direct-acting antivirals; Pangenotypic
INTRODUCTION
Chronic infection with hepatitis C virus (HCV) is assumed to be one of the leading causes of liver disease globally, affecting approximately 71 million people[1]. Due to the high genetic diversity of the viral nucleic acid sequence, six major genotypes (GT), differing from each other by 30% at the nucleotide level, comprising multiple subtypes of HCV, have been identified[2]. The majority of patients worldwide are infected with GT1, but infections of GT3 are also common in some regions. GT3 is defined by a higher rate of steatosis, increased risk of liver cirrhosis, and different response to antiviral drugs compared to other GTs. In the era of treatment with pegylated interferon alpha (IFNα) and ribavirin (RBV), patients infected with GT3 were considered "easy to treat" due to an efficacy rate of 70%, compared to less than 50% in GT1 and GT4 infected patients. The introduction of direct-acting antivirals (DAA) has changed the management of HCV infection, allowing a substantial increase in the treatment efficacy, however, the improvement for GT3 infection was not as pronounced as for other GTs, particularly in treatment-experienced patients and those with liver cirrhosis.
THE GLOBAL SPREAD OF GT3
GT3 infections are widely distributed worldwide and are estimated to be the second in frequency accounting for approximately 25%-30% of all HCV cases globally[2]. The distribution of GT3 varies between different countries and continents. The highest global prevalence (exceeding 70%) is found in South Asian countries, while a rate of approximately 20% is reported in Central and Southeast Asia, a few percent in East Asia, and only 0.4% in the Asia-Pacific region. A relatively high frequency of 36% is documented in Australia[3]. GT3 infection accounts for 14.2% of HCV cases in South America, 2.1% in Central America, and 15.7% in North America, ranging from 8.9% in the United States to 22.3% in Canada[3]. In Europe, the distribution of GT3 infections is also heterogeneous with the highest frequency exceeding 40% in Scandinavia and England, over 30% in Ireland, Greece Russia, and Slovenia, and more than 20% of infections in Germany, Switzerland, Montenegro, Belgium, Bosnia, and France[4-6]. The rate of a dozen percent is reported from Spain, Poland, Portugal, Bulgaria, and Croatia, whereas the lowest prevalence, below 10%, are documented in Italy, Albania, Hungary, and Romania[7-10]. The lowest proportional frequency of GT3 infections is found in Africa at an average of 5.3%, with the highest frequency at 7.4% in East Africa through 6.3% in North Africa to 0.8% in the central part of the continent[3].
GOOD OR BAD IN THE HCV FAMILY
For many years, GT3 was considered to be less pathogenic compared to other GTs in the HCV family due to its favorable response to an IFN-based regimen. However, the growing evidence of a higher rate of steatosis and more rapid progression of liver fibrosis compared to infection with other HCV GTs has changed this conviction.
Liver steatosis is a frequent histological finding in patients with chronic hepatitis C (CHC). Although this feature is common among all HCV infected individuals, with an average rate of 50%, the highest prevalence exceeding 70% is observed in patients infected with GT3[11-14]. In in-vitro studies, GT3 is also demonstrated to be much more likely to induce liver steatosis than other HCV GTs[15]. The pathogenesis of hepatic steatosis is complex and related to host and viral factors, as well as to alcohol consumption. Metabolic steatosis, which is associated with host risk factors including high body mass index, obesity, dyslipidemia, metabolic syndrome, insulin resistance and type 2 diabetes, is commonly found in patients infected with non-GT3 HCV, whereas in the GT3-infected individuals, virus-related hepatic steatosis is described as predominantly being induced by the direct cytopathic effect of the HCV[16-20]. Although the exact mechanism remains unknown, several pathways are linked to the pathogenesis of GT3-induced steatosis. A central role is played by the inhibition of microsomal triglyceride transfer protein function by HCV core protein, resulting in overall decreased hepatocyte lipid export with intracellular triglycerides accumulation. This effect is documented to be amplified with HCV-3 core proteins[21]. Another mechanism through which the virus modulates the host lipid metabolic pathways is inhibition of the peroxisome proliferator associated receptor-α (PPAR-α), a transcription factor inducing hepatic fatty acid oxidation and ketogenesis. A decrease in PPAR-α level leads to hepatic lipid collection. In vitro studies document inhibition of PPAR-α observed in the GT3 infections as being more efficient than in infections with GT1 HCV[22]. Viral-induced hepatic steatosis results not only from the reduction of the lipid excretion with subsequent intracellular lipid accumulation but also from the promotion of the neolipogenesis with fatty acid synthesis. This activity is proposed to be a consequence of an increase in function of sterol regulatory element-binding protein-1c activated by the HCV-3 core protein, however, the exact mode of activation is unknown[23]. The hypothesis of a pathogenic link between GT3 infection and steatosis is supported by a significant correlation of the steatosis score and intrahepatic titer of HCV RNA only in patients infected with GT3[16]. The improvement in liver steatosis in GT3-infected patients after successful antiviral therapy, which is not observed in patients infected with GT1, seems to indirectly confirm this association[24,25]. GT3 HCV was also identified as an independent predictor for the accelerated progression of liver fibrosis in addition to established risk factors including the age of infection, male gender, coinfection with hepatitis B virus and human immunodeficiency virus, insulin resistance, iron overload, alcohol and drugs intake[26]. Precise analysis of the influence of HCV GT on the more advanced liver disease is difficult due to the coexistence of the aforementioned predictors and the previously discussed higher prevalence of liver steatosis in GT3 infection, which contributes to more rapid progression of hepatic fibrosis[12,27,28]. However, the pooled analysis conrmed a signicantly more severe liver disease in single-biopsy studies and a trend towards the faster progression of fibrosis in GT3 patients compared with the other GTs[29]. The strong association between GT3 infection and end-stage liver disease was documented in HCV-infected drug abusers in France and confirmed by a populationbased study in a cohort of native Alaskans with CHC[30,31].
An increased risk not only of liver cirrhosis but also of hepatocellular carcinoma (HCC) among GT3 infected individuals compared to those infected with other GTs was reported in a large cohort (> 110000) of American patients from the Veterans Affairs Registry[32,33]. Consistent results of significantly higher incidence of HCC in GT3 patients were also obtained in French and Korean populations[34,35]. Nevertheless, the effect of HCV GT3 infection on the higher prevalence of liver cancer remains controversial because of the data demonstrated for GT1b as a major risk factor for HCC development[36,37].
IFN AND RBV COUPLE
The standard of care therapy of pegylated (peg) IFNα and RBV established in 2000 has resulted in a sustained virologic response (SVR) of approximately 70% in GT3-infected patients[38-42]. Such a high effectiveness compared to the SVR below 50% achieved by patients with GT1 and GT4 infection was the basis for the GT3 being deemed "easy to treat" and has led to attempts to shorten the treatment course to 16, 14, and even 12 wk. However, the reduction in the SVR rate was reported in patients who did not achieve the so-called rapid virologic response (RVR) defined as undetectability of HCV RNA after 4 wk of therapy[38,39,42-44]. The meta-analysis of twelve clinical trials performed by Andriulli et al[45]documented a wide variance in response to pegIFNα and RBV in GT3-infected patients depending on the baseline viral load. Individuals with a high baseline level of HCV RNA demonstrated a significantly lower SVR rate of 58% compared to 75% in those with a low baseline viral load. The strongest predictive factor for treatment response was RVR and this finding provided the basis for the conclusion that patients without RVR may need a longer therapy duration. The negative impact of cirrhosis on treatment response among subjects infected with GT3, leading to poor antiviral effectiveness was documented by Powis et al[46], suggesting that such patients also require an alternative management strategy. However, the extension of the treatment course did not result in higher effectiveness, nor was there an improvement in the SVR rate on increasing the dose of RBV[47-49].
THE COUPLE WITH A LITTLE HELP OF DAA
The registration of the first DAAs in 2011, which were inhibitors of the HCV serine protease (nonstructural protein 3/4A, NS3/4A), started a revolution in the treatment of CHC. The combination of telaprevir or boceprevir with pegIFNα and RBV significantly increased the SVR rate, but only in patients infected with GT1[50,51]. Those infected with other GTs, including GT3, were still treated with pegIFNα and RBV because no significant improvement was demonstrated after the addition of telaprevir or boceprevir[52,53]. Therefore, at the beginning of the DAA era GT3 emerged as a "difficult-to-treat" GT. New hopes for higher effectiveness were raised with the introduction of the next-generation DAAs for possible combination with pegIFNα and RBV. Unfortunately, clinical trials demonstrated that simeprevir, a second-wave protease inhibitor with documented in-vitro pangenotypic activity has limited efficacy in GT3-infected patients, and the effectiveness of daclatasvir (DCV), acting through inhibition of the HCV nonstructural protein 5A (NS5A), has proven to be also disappointing, with SVR rates of 45% and 74% in GT3 patients with and without liver cirrhosis, respectively[54,55]. However, the expectations of a higher response rate among GT3-infected patients have been met by sofosbuvir (SOF), a new DAA class representative, HCV polymerase (NS5B) inhibitor. The addition of SOF to pegIFNα and RBV (SPR) leads to better outcomes when compared to standard of care therapy, regardless of liver fibrosis and history of previous antiviral therapy. Phase 2 clinical trials documented a response of 83% among treatment-experienced patients with liver cirrhosis participating in the LONESTAR-2 study, while non-cirrhotic, treatment-naïve individuals treated in the QUANTUM study responded in 92% of cases[56,57].
Patients included in the BOSON phase 3 clinical trial achieved an SVR of 93%, specifically 88% among individuals with liver cirrhosis and 95% in those without; the lowest efficacy of 86% was demonstrated for patients with liver cirrhosis who failed to respond to previous antiviral therapy[58]. An open-label clinical study evaluating the outcome of SOF-containing treatments reported 100% efficacy among GT3-infected treatment naïve patients without cirrhosis treated with triple therapy[59].
Those results from clinical trials were supported by real-world experience (RWE) data that documented an SVR rate higher than that following dual therapy. The best response of 93% was reported for non-cirrhotics among Americans treated in the Veterans Affairs health care system[60]. Scandinavian patients responded in 96% of cases, an efficacy of 98% was obtained in the Polish EpiTer-2 study and an SVR reached 99% in a German cohort[6,61,62]. The effectiveness of the SPR regimen in cirrhotic individuals in these RWE studies was also promising, reaching 92%, 81%, 91%, and 88%, respectively[6,60-62].
RWE data revealed a failure of previous therapy, with a history of treatment with IFN and RBV shown to be a negative prognostic factor of the response to SPR treatment. This triple regimen was still recommended by the guidelines of the European Association for the Study of the Liver in 2015 for GT3-infected non-cirrhotic patients and those with compensated liver cirrhosis, regardless of treatment history[63]. Irrespective of the high effectiveness of SPR, accompanied by reasonable tolerability due to the short treatment period, any IFN-based therapy was refused by patients[61]. Therefore, further research on the treatment of GT3 infections has focused on highly efficient IFN-free therapeutic options.
DAA HOME ALONE
The first available IFN-free regimen, SOF and RBV, used in GT3-infected patients for 12 wk or 16 wk, did not result in increased efficacy when compared to standard of care therapy, which demonstrated significantly lower response rates in patients with liver cirrhosis, especially in those who had previously failed IFN and RBV therapy (Table 1)[40,58,64].
The extension of treatment duration to 24 wk enabled effectiveness of up to 95%,however, the difference in response rates between patients without and with liver cirrhosis was significant[58,65]. The results of phase 3 clinical trials confirmed by RWE data became a basis for treatment guidelines, according to which IFN-ineligible GT3-infected patients should receive SOF/RBV for 24 wk. However, this regimen was recognized as suboptimal, due to unsatisfactory effectiveness for those with liver cirrhosis who had previously failed IFN and RBV therapy[66-68].
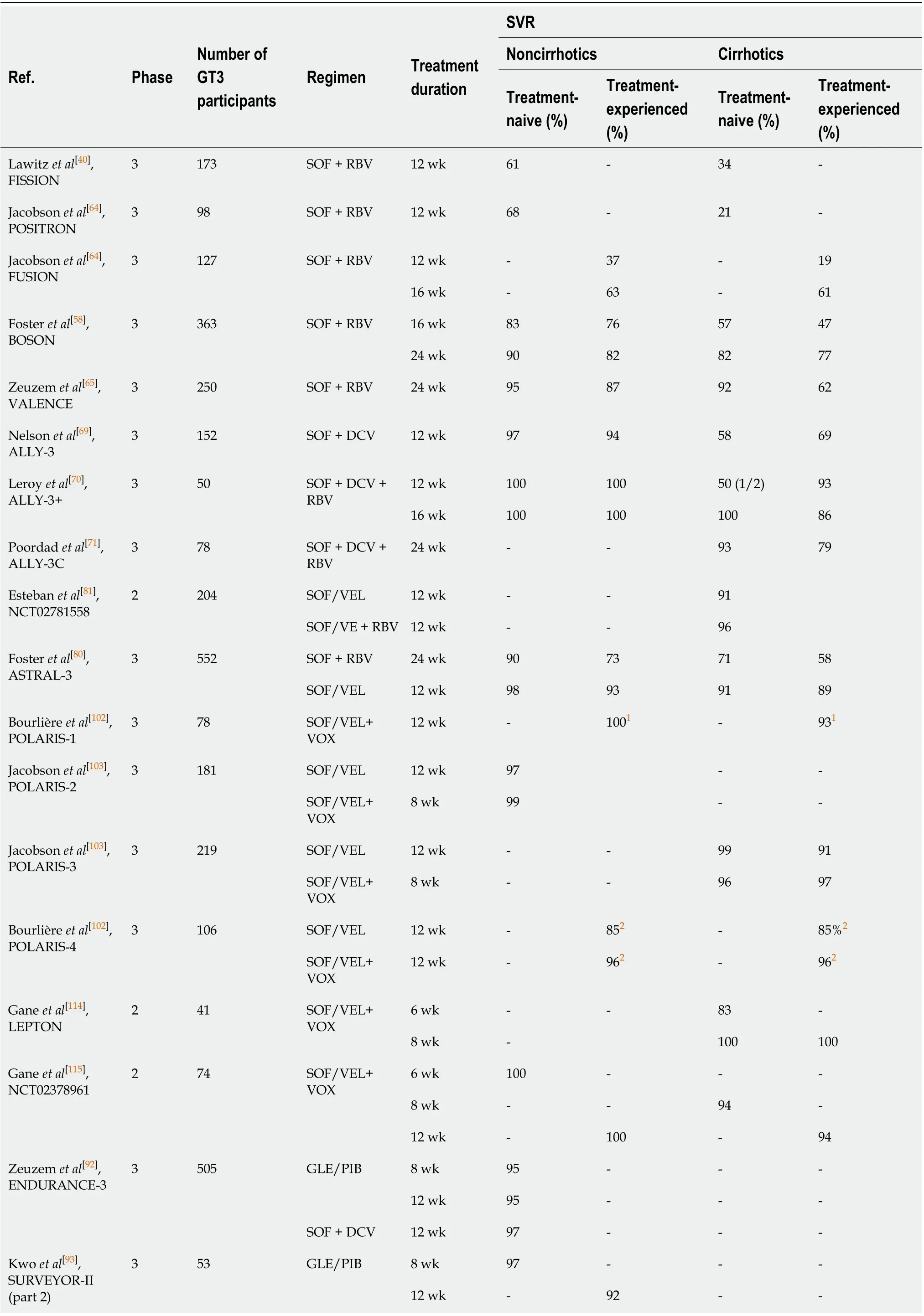
Table 1 Efficacy of interferon-free regimens in genotype 3 patients in clinical trials
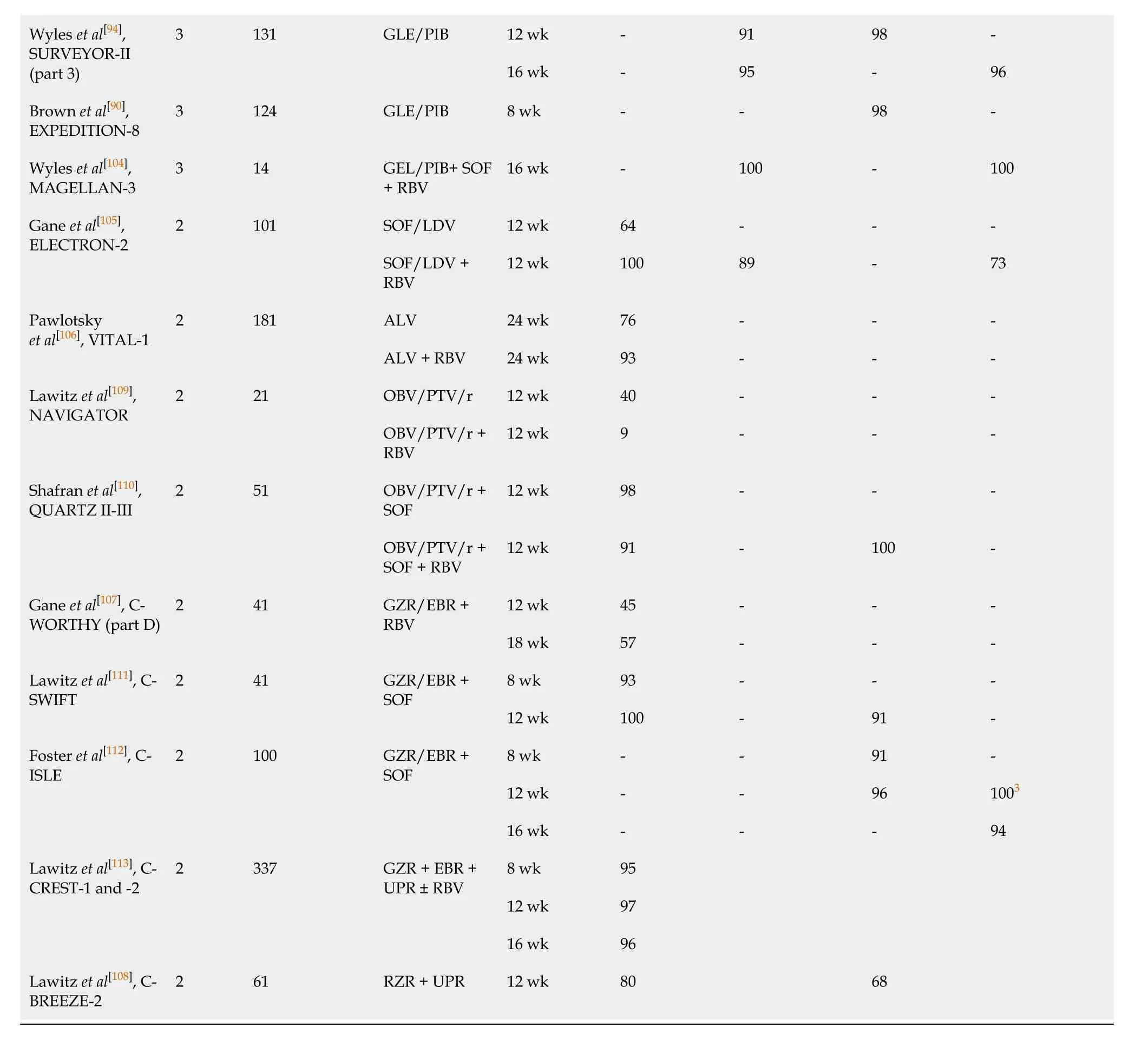
1NS5A-inhibitor-experienced.2No detailed information on the response rate in patients with and without liver cirrhosis.3Recommended by American Association for the Study of Liver Diseases/Infectious Diseases Society of America as an alternative option for pegylated interferon + ribavirin-experienced patients with compensated liver cirrhosis. SVR: Sustained virologic response; GT: Genotype; SOF: Sofosbuvir; RVB: Ribavirin; DCV: Daclatasvir; VEL: Velpatasvir; VOX: Voxilaprevir; GLE: Glecaprevir; PIB: Pibrentasvir; LDV: Ledipasvir; ALV: Alisporivir; OBV: Ombitasvir; PTV/r: Paritaprevir boosted by ritonavir; GZR: Grazoprevir; EBR: Elbasvir; UPR: Uprifosbuvir; RBV: Ribavirin; RZR: Ruzasvir.
Searching for the optimal antiviral regimen, the combinations of SOF and another DAA with a different mechanism of action were studied. Promising results were obtained with a SOF and DCV combination administered for 12 wk and 24 wk, with or without RBV[69-71]. Although the difference in response rates with SOF + DCV ± RBV between non-cirrhotic and cirrhotic patients was still noticeable, this regimen was recommended for the treatment for GT3-infected patients regardless of liver fibrosis and history of previous therapy and has been widely used in RWE settings[66,72-74]. High efficacy and good tolerability demonstrated in both clinical trials and real-world cohorts have made this regimen a reasonable choice for therapy for GT3 infection as long as highly potent pangenotypic options became broadly available[75-77].
BETTER IS THE ENEMY OF GOOD ENOUGH
The final stage of the revolution in antiviral treatment which improved the outcome and simplified the management of GT3-infected patients was the implementation of potent pangenotypic regimens (Table 1).
According to the most recent guidelines, two basic options of DAAs are currently approved for the treatment of GT3 infection; a fixed-dose combination of SOF and velpatasvir (VEL), and dual treatment with glecaprevir (GLE), an NS3/4A protease inhibitor, and pibrentasvir (PIB), an NS5A inhibitor (Table 2)[78,79]. A single-tablet regimen containing SOF and VEL was registered based on the results of the ASTRAL-3 study, which confirmed effectiveness exceeding 93% in non-cirrhotic and 89% in cirrhotic patients[80]. The 12-wk regimen is recommended for non-cirrhotics and patients with compensated cirrhosis, regardless of the previous treatment history. The addition of RBV may be considered in compensated individuals.
It is noteworthy that SOF/VEL combined with RBV is the only option recommended for patients with decompensated liver cirrhosis[81,82]. Data on the high efficacy and favorable safety profile of SOF/VEL achieved in clinical trials were supported by RWE studies reporting comparable SVR rates. Prior treatment-experience, as well as advanced liver fibrosis, were significant predictors of reduced effectiveness[83-87]. As resistance-associated substitutions at the NS5A position can be responsible for a reduction in the efficacy of the NS5A inhibitors, the resistance-associated substitutions testing at baseline should be considered for treatment-experienced patients and cirrhotic individuals, irrespective of treatment history, for whom SOF/VEL is being considered. The identification of the Y93H substitution indicates the need for RBV addition or an alternative regimen administration[78,79].
The second potent pangenotypic option is a combination of GLE and PIB, which was approved for the treatment of patients without or with liver cirrhosis irrespective of previous therapy. As protease inhibitors containing regimens carry a risk of decompensation during antiviral treatment, GLE/PIB is not recommended for decompensated cirrhotic patients[88]. This regimen provides the opportunity for shortening therapy to 8 wk in the majority of patients[89]. Based on findings from the ENDURANCE-3, SURVEYOR-II, and EXPEDITION-8 clinical trials, an 8-wk regimen has been registered for all previously untreated patients, including those with compensated liver cirrhosis, whereas treatment-experienced GT3-infected individuals should be treated for 16 wk regardless of liver fibrosis[90-94]. RWE studies reported effectiveness for an 8-wk GLE/PIB regimen, which exceeded 96% in treatment-naïve patients without liver cirrhosis[95-99]. Since the shortening of therapy in previously untreated cirrhotic patients infected with GT3 has been approved very recently, the available RWE data are very limited and only include a small number of patients[100,101]. Therefore, further studies are needed to determine the treatment outcome in this subpopulation. Although the implementation of SOF/VEL and GLE/PIB regimens has resulted in a high efficacy rate among GT3-infected individuals, there is still room for improvement, especially in those who did not achieve SVR, particularly following NS5A containing regimens. For such patients, a 12-wk salvage therapy with a singletablet combination of SOF/VEL and next-generation NS3/4A protease inhibitor voxilaprevir (VOX) is recommended[102]. Safety and efficacy of SOF/VEL/VOX in GT3-infected patients without and with liver cirrhosis, both treatment-naïve and treatmentexperienced were demonstrated in the POLARIS studies (Table 1)[102,103].
The other option to address failed DAA treatment in GT3-infected patients is a combination of GLE/PIB plus SOF and RBV, as investigated in the MAGELLAN-3 study. This demonstrated a 100% SVR rate, however, the small number of patients enrolled may have limited the broad applicability of these findings[104].
SOMETHING WENT WRONG
On the way to developing highly effective pangenotypic regimens against GT3, there were multiple paths that appeared to be dead ends. Some of them were not investigated despite showing encouraging initial results, due to disappointing treatment outcomes in selected subpopulations of GT3 patients. One good example is an open-label study of 12-wk treatment with an NS5A inhibitor—ledipasvir and SOF, plus RBV, which demonstrated a 100% SVR rate among treatment-naïve GT3 infected individuals. Unfortunately, due to limited efficacy in treatment-experienced patients, especially those with liver cirrhosis, as well as low antiviral potency without RBV against GT3, that direction of search has proved a blind alley[105]. Alisporivir, a cyclophilin inhibitor, applied alone or with RBV in treatment-naïve non-cirrhotic patients has resulted in SVR rates of 76% and 93%, respectively, however, research involving other subgroups of patients was suspended due to a safety issue[106]. Efficacy was observed to be below expectations with the combination of the NS3/4A inhibitor grazoprevir (GZR) and the NS5A inhibitor elbasvir (EBR) with RBV, as well as with a regimen consisting of the NS5A inhibitor ruzasvir and the NS5B inhibitor uprifosbuvir[107,108]. The unsatisfactory outcome of the treatment with NS35A inhibitor ombitasvir, and NS3/4A inhibitor paritaprevir boosted by ritonavir with or without RBV, was subsequently improved by the addition of SOF, but ultimately these regimens were not further evaluated, because there were new potent pangenotypic options on the horizon[109,110]. For this same reason, investigations into a regimen of GZR/EBR combined with uprifosbuvir or SOF were discontinued, despite the high effectiveness demonstrated in phase 2 clinical trials. However, GZR/EBR + SOF is currently recommended by AASLD/IDSA as an alternative option for the specific subpopulation of pegIFNα+RBV-experienced patients with compensated liver cirrhosis[79,111-113].
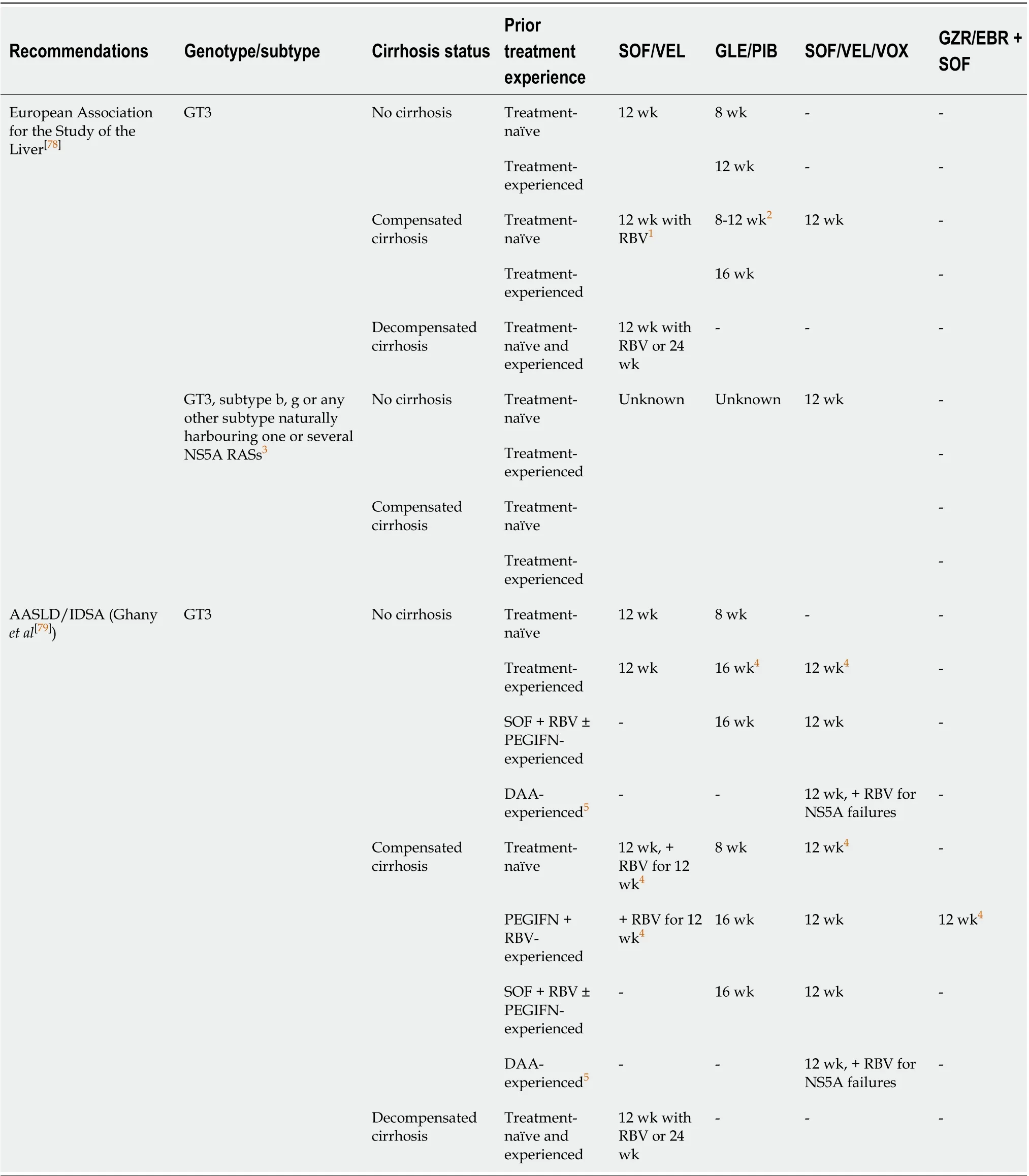
Table 2 European Association for the Study of the Liver and American Association for the Study of Liver Diseases/Infectious Diseases Society of America current recommendations on the treatment of genotype 3-infected patients
CONCLUSION
Despite the high efficacy and safety of pangenotypic therapies, that may sooner or later cure all or at least almost all identified HCV infections, including GT3, there will still be many infections that go unrecognized and are therefore impossible to cure with even the best drug. The major problem that remains to be solved worldwide is screening people who are unaware of the risk of liver disease progression from a virus in their body. It is a shame for national governments that, despite having access to the perfect tool to eliminate a dangerous virus and rule out one of the most difficult-totreat cancers, are not doing enough.
 World Journal of Gastroenterology2021年11期
World Journal of Gastroenterology2021年11期
- World Journal of Gastroenterology的其它文章
- Chronic renal dysfunction in cirrhosis: A new frontier in hepatology
- How to manage inflammatory bowel disease during the COVID-19 pandemic: A guide for the practicing clinician
- Efficacy and safety of endoscopic submucosal dissection for gastric tube cancer: A multicenter retrospective study
- Study on the characteristics of intestinal motility of constipation in patients with Parkinson's disease
- Apolipoprotein E polymorphism influences orthotopic liver transplantation outcomes in patients with hepatitis C virus-induced liver cirrhosis
- Prospective single-blinded single-center randomized controlled trial of Prep Kit-C and Moviprep: Does underlying inflammatory bowel disease impact tolerability and efficacy?
