Chronic renal dysfunction in cirrhosis: A new frontier in hepatology
Ramesh Kumar, Rajeev Nayan Priyadarshi, Utpal Anand
Abstract Chronic kidney disease (CKD) in patients with liver cirrhosis has become a new frontier in hepatology. In recent years, a sharp increase in the diagnosis of CKD has been observed among patients with cirrhosis. The rising prevalence of risk factors, such as diabetes, hypertension and nonalcoholic fatty liver disease, appears to have contributed significantly to the high prevalence of CKD. Moreover, the diagnosis of CKD in cirrhosis is now based on a reduction in the estimated glomerular filtration rate of < 60 mL/min over more than 3 mo. This definition has resulted in a better differentiation of CKD from acute kidney injury (AKI), leading to its greater recognition. It has also been noted that a significant proportion of AKI transforms into CKD in patients with decompensated cirrhosis. CKD in cirrhosis can be structural CKD due to kidney injury or functional CKD secondary to circulatory and neurohormonal imbalances. The available literature on combined cirrhosis-CKD is extremely limited, as most attempts to assess renal dysfunction in cirrhosis have so far concentrated on AKI. Due to problems related to glomerular filtration rate estimation in cirrhosis, the absence of reliable biomarkers of CKD and technical difficulties in performing renal biopsy in advanced cirrhosis, CKD in cirrhosis can present many challenges for clinicians. With combined hepatorenal dysfunctions, fluid mobilization becomes problematic, and there may be difficulties with drug tolerance, hemodialysis and decision-making regarding the need for liver vs simultaneous liver and kidney transplantation. This paper offers a thorough overview of the increasingly known CKD in patients with cirrhosis, with clinical consequences and difficulties occurring in the diagnosis and treatment of such patients.
Key Words: Acute kidney injury; Cirrhosis; Chronic kidney disease; Renal failure; Hepatorenal syndrome; Renal function
INTRODUCTION
Most attempts to assess renal impairment in cirrhosis have so far concentrated on acute kidney injury (AKI), and as a result, detailed knowledge of AKI in cirrhosis is now available[1]. However, there is still scarce evidence on the prevalence, clinical impact and treatment of chronic kidney disease (CKD) in cirrhosis. A sharp rise in the diagnosis of CKD among patients with cirrhosis has been observed in recent years. The prevalence of CKD in hospitalized patients with cirrhosis, which used to be approximately 1% in 2005, has now risen to as high as 46.8% in 2019[2,3]. The growing prevalence of CKD in patients with cirrhosis may represent the convergence of several important epidemiological patterns: the continuing increase in the prevalence of metabolic risk factors such as obesity, hypertension and Medicine degree (DM); the increasing prevalence of nonalcoholic fatty liver disease (NAFLD) as a major contributor to the burden of cirrhosis; and the aging cohort of cirrhosis[4-6]. Moreover, some emerging evidence indicates that the risk of developing de novo CKD remains high for AKI survivors[7]. Liver cirrhosis patients are susceptible to developing AKI due to circulatory abnormalities, neurohormonal changes and the involvement of risk factors such as bacterial infection, gastrointestinal bleeding, medication and paracentesis[1,8]. Depending on the severity, length and frequency, AKI increases the risk of developing incident CKD due to decreases in renal mass and nephron number, vascular insufficiency, and maladaptive repair mechanisms[9]. Therefore, rather than separate entities, AKI and CKD may represent a continuum. The term CKD now encompasses both structural CKD due to structural damage to the kidney and functional CKD due to circulatory and neurohormonal imbalances in cirrhosis. The differentiation between various forms of renal dysfunction in cirrhosis is crucial, as each requires a different treatment plan.
DEFINITION AND CLASSIFICATION
The definition of CKD in cirrhosis was originally based on a serum creatinine level of > 1.5 mg/dL until 2011, when an updated definition was introduced by a working group composed of experts from various disciplines[10]. The definition endorsed by kidney disease: Improving global outcomes was largely adopted by this group, and CKD was defined as an estimated glomerular filtration rate (eGFR) of < 60 mL/min for more than 3 mo, measured using the Modication of Diet in Renal Disease-6 (MDRD-6) equation. While the group further agreed that the MDRD-6 equation was not perfect for estimating GFR in patients with cirrhosis, it may still be adopted until better alternatives become available. Currently, the diagnosis of CKD does not require corroborating evidence of kidney damage, such as proteinuria, hematuria, abnormal renal imaging or pathology.
Kidney Disease: Improving Global Outcomes has classified CKD into structural and functional CKD on the basis of the presence or absence of kidney injury. The old entity, type 2 hepatorenal syndrome (HRS), now referred to as HRS-CKD, is essentially a functional CKD[11]. While functional CKD is considered potentially reversible, since biomarkers of renal tubular damage have been found in patients with HRS, this may not exactly be the case[12-14]. Patients with cirrhosis may have several risk factors for developing structural CKD per se, such as DM, NAFLD, and atherosclerosis[4-6]. In addition, persistent renal vasoconstriction in functional CKD can lead to structural changes, transforming it into structural CKD.
THE GROWING PREVALENCE OF CHRONIC KIDNEY DISEASE IN CIRRHOSIS
In recent years, not only has the prevalence of CKD increased significantly in the general population, but an increasing rise in the prevalence of CKD has also been reported in patients with cirrhosis (Table 1)[3,15-17]. The prevalence rates of CKD in cirrhosis, however, vary significantly across studies due to variations in parameters used to describe CKD and differences in the severity of patients with chronic liver disease (CLD).
In a study by Rustgi et al[18], although the prevalence of CKD among 94431 patients with cirrhosis collected from the insurance claim database was 3.37%, the proportion of patients with decompensated cirrhosis was higher in the combined CLD-CKD group (27.2% vs 11.8%), suggesting that the prevalence of CKD increases with the severity of CLD. In a recent retrospective analysis of a large cohort of patients with cirrhosis (n = 78640) awaiting liver transplantation (LT), while the prevalence of CKD was 7.8% in 2002, it increased to 14.6% in 2017. This is a documented increase in the CKD prevalence rate of 187% in just 15 years. Moreover, among 39719 LT recipients, 6269 (16%) patients met the CKD criteria at the time of last transplant[4]. Another study evaluating the prospectively managed database of the North American Consortium for the End-Stage Liver Disease Study reported a 46.8% prevalence of CKD among 2346 admitted patients with cirrhosis[3]. In an Indian study, the occurrence of CKD was observed in 32.8% of a large prospective cohort (n = 818) of patients with cirrhosis[16]. Functional CKD is considered to be relatively uncommon and accounts for only approximately 3.9% to 15.8% of renal impairments among hospitalized cirrhotic patients; however, new data need to be developed in light of the updated concept of CKD in cirrhosis[2,19].
DETERMINANTS OF INCREASING CHRONIC KIDNEY DISEASE IN CIRRHOSIS
Apart from the aging and the high incidence of AKI in cirrhosis, a rising recognition of this condition and a rising trend in the prevalence of DM, hypertension and NAFLD seem to be the key factors behind the increased prevalence of CKD in cirrhosis (Figure 1).
AKI to CKD transition
AKI is an independent risk factor for developing CKD in the general population. AKI to CKD transition appears to reflect a continuum. In a meta-analysis of 13 studies, the pooled adjusted hazard ratio for developing CKD among patients with AKI was 8.8 (95% confidence interval: 3.1-25.5)[20]. AKI occurs very frequently in patients with decompensated cirrhosis because of pre-existing circulatory abnormalities, neurohormonal changes and the involvement of risk factors such as DM, bacterial infection, gastrointestinal bleeding, medications and therapeutic paracentesis[1,7,10,11]. Emerging data suggest that in patients with decompensated cirrhosis, a large proportion of AKI progresses to CKD. In a recent study, 25% of patients with decompensated cirrhosis with AKI who survived for at least 3 mo developed CKD, compared with only 1% of those without AKI[7]. Moreover, the odds of developing CKD in patients with decompensated cirrhosis with AKI were 31, suggesting that they are more prone to CKD development than general AKI patients. A higher transition from AKI to CKD was seen when the severity of AKI was higher and when it developed after hospitalization. In another study published in India, 32.8% of 818 patients with cirrhosis developed CKD, approximately 80% of patients with CKD hadat least one episode of AKI, and one-third of patients with AKI had progression to CKD[16]. The mechanisms underlying AKI-CKD progression are still poorly understood. In general, it is believed to be a result of maladaptive repair in the interstitial, vascular and tubular structures of the kidney[9]. However, it is not clear whether the same mechanisms contribute to the development of CKD in cirrhosis. Patients with higher baseline levels of serum creatinine are more likely to develop AKI and less likely to recover from such AKI episodes[21]. Because DM and hypertension patients are more likely to have intrinsic renal disease, such as diabetic nephropathy or hypertensive nephrosclerosis, they are not only at higher risk of developing AKI episodes but also less likely to recover from these episodes due to a lower renal reserve.
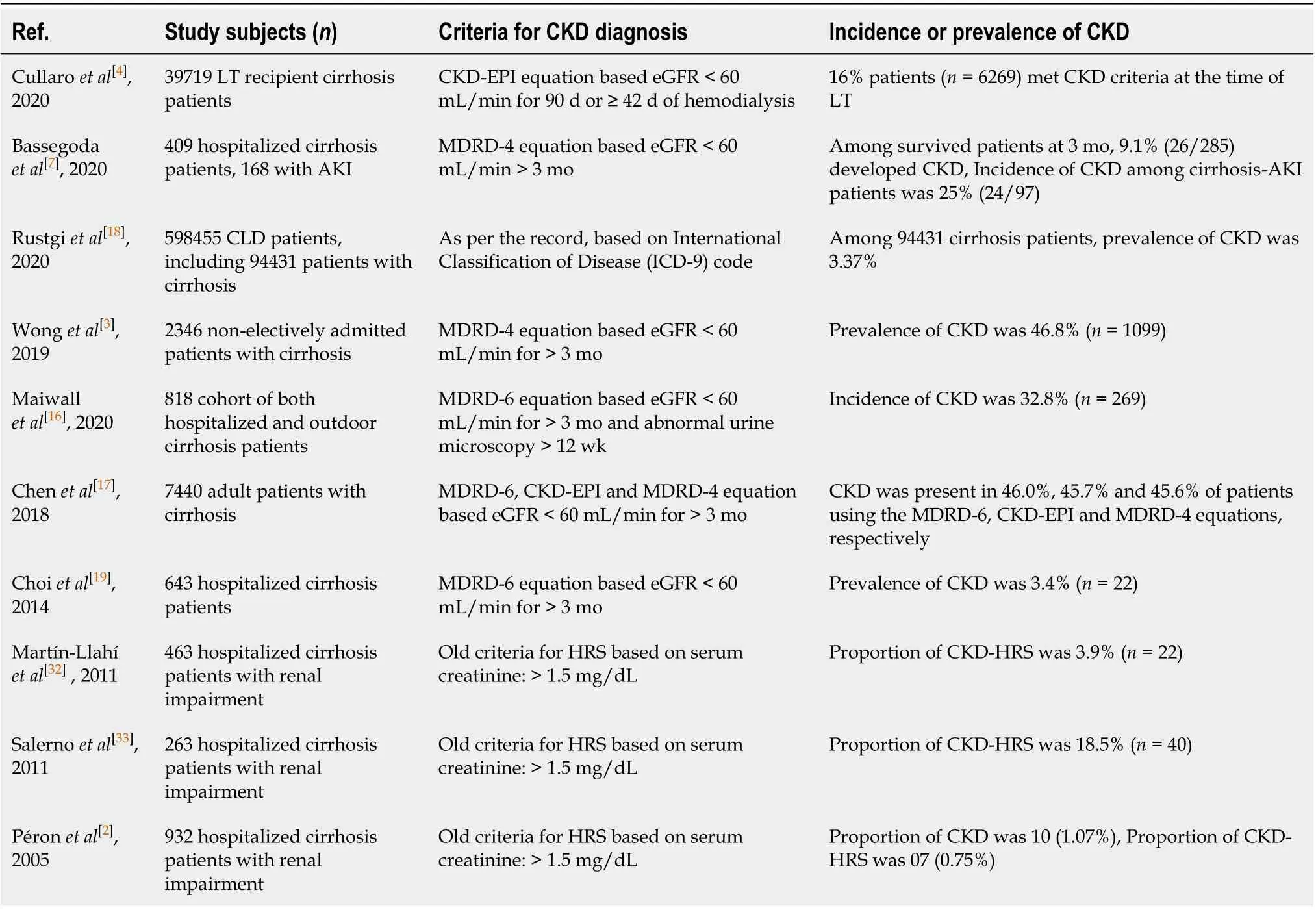
Table 1 Incidence and prevalence of chronic kidney disease in cirrhosis patients
Increase in the multiple risk factors
There has been a substantial increase in the multiple shared risk factors for cirrhosis and CKD over the years. NAFLD is independently and significantly associated with an increased incidence and prevalence of CKD[22,23]. In a recent meta-analysis that included nearly 64000 subjects, NAFLD was associated with an approximately 2-fold increased risk of both prevalent and incident CKD[23]. Multiple factors, such as the proinflammatory environment, insulin resistance, oxidative stress, and the activated renin-angiotensin system, may account for the accelerated development and progression of CKD in NAFLD subjects, apart from the common occurrence of DM and hypertension[24]. In addition, NAFLD has been strongly associated with atherosclerosis, as shown by increased intima media thickness or atherosclerotic plaques in the carotid arteries, and atherosclerosis has been associated with glomerulosclerosis, a process that can lead to CKD[25]. In one study, approximately 20%-25% of LT candidates were found to have severe coronary artery disease, suggesting that atherosclerosis in cirrhosis is not uncommon[26]. Over the last 2 decades, the prevalence of NAFLD and NAFLD-related cirrhosis has also increased considerably[27]. In a study, NAFLD accounted for a substantial rise in simultaneous liver and kidney transplantation (SLKT), from 8.2% in 2002 to 22% in 2011[28]. In cirrhosis, DM is highly prevalent, with recorded prevalence rates ranging from 35% to 71%, which is far higher than in the general population[29]. Glomerulopathy, which can progress to CKD, may be associated with certain specific causes of cirrhosis, such as hepatitis B virus (HBV) or hepatitis C virus (HCV). Glomerular involvement in patients with viral hepatitis occurs via an immune pathogenic mechanism. Circulating immune complexes containing viral antigens have been found in the kidney[30].
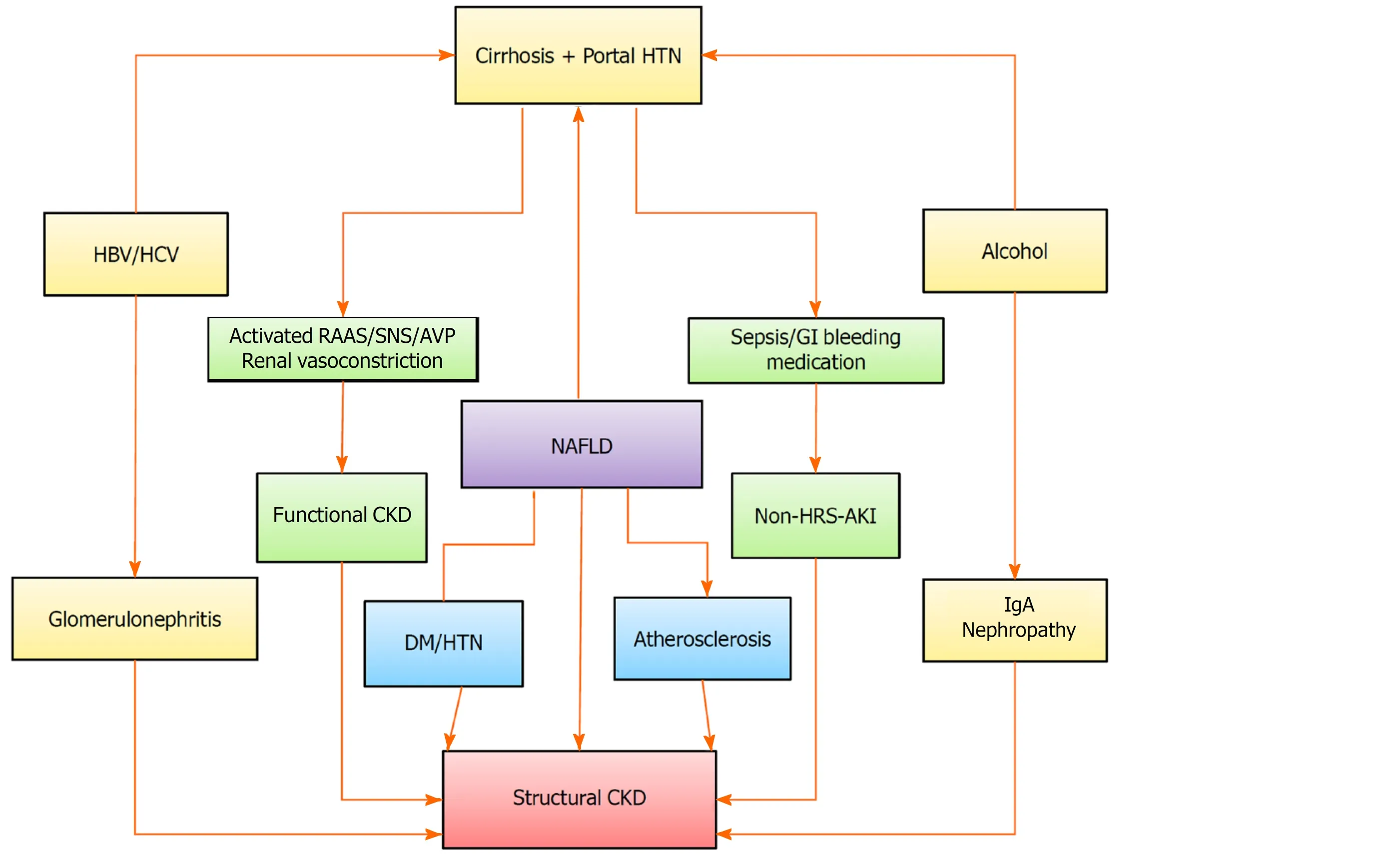
Figure 1 Risk factors associated with chronic kidney disease in patients with liver cirrhosis. A rising trend in the prevalence of medicine degree, hypertension and non-alcoholic fatty liver disease seem to be the key factors behind the increased prevalence of chronic kidney disease in cirrhosis. The risk of developing de-novo chronic kidney disease remains high for acute kidney injury survivors. CKD: Chronic kidney disease; HTN: Hypertension; HBV: Hepatis B virus; HCV: Hepatitis C virus; RAAS: Renin-angiotensin-aldosterone system; SNS: Sympathetic nervous system; AVP: Arginine vasopressin; GI: Gastrointestinal; NAFLD: Non-alcoholic fatty liver disease; HRS: Hepatorenal syndrome; AKI: Acute kidney injury.
CLINICAL IMPLICATIONS OF CHRONIC KIDNEY DISEASE IN CIRRHOSIS
In several ways, CKD can affect the clinical manifestations, complications, therapeutic decisions, and outcomes of patients with cirrhosis.
Impact on clinical manifestations
Anorexia, anemia, ascites, bleeding tendency and encephalopathy can be independently due to both hepatic and renal diseases, so the contributions from individual diseases are often difficult to determine in patients with cirrhosis with CKD. This may create uncertainty about optimal therapeutic choices, such as requirements of renal replacement therapy. In patients with cirrhosis, CKD may contribute to ascites and edema in various ways, such as nephrogenic ascites, chronic fluid overload, hypoproteinemia, and cardiomyopathy[31]. Refractory ascites is almost universal in patients with functional CKD[32,33]. Due to multiple and complex hemostasis abnormalities, patients with concurrent hepatorenal dysfunction may have a higher tendency to bleed. On the other hand, even thrombotic complications are not unusual in such patients[34]. CKD is an independent cardiovascular mortality risk factor, and it can worsen anemia due to cirrhosis[35,36]. Both CKD and cirrhosis can cause immunodepression, leading to an increased risk of infection[37]. Patients with cirrhosis and CKD appear to have an increased risk of developing malignancy[27,38]. In recent years, NAFLD, which is significantly linked to CKD, has also emerged as one of the leading causes of hepatocellular carcinoma (HCC)[27]. Alcohol, HBV infection and HCV infection are other shared risk factors that can be associated with both HCC and CKD. HCC has been found to be associated with a higher prevalence of CKD than any other cancer[39]. After adjustment for many possible confounders, a lower GFR has been shown to be independently associated with a higher risk of incident renal cell and urothelial cancer[40]. CKD has been shown to be associated with increased mortality from liver, kidney, and urothelial cancers[41].
Impact on complications and outcomes
CKD in cirrhosis is associated with poor outcomes and an increased frequency of complications[3,7]. Wong et al[3]found that patients with cirrhosis with CKD had higher rates of superimposed AKI (68% vs 21%), need for dialysis (11% vs 2%) and 30-d mortality rates (16% vs 7%) than patients with cirrhosis without CKD. A 10 mL/min decrease in eGFR was found to be associated with a 13% increase in 30-d mortality in patients with cirrhosis. In a study by Bassegoda et al[7], patients with cirrhosis with CKD had a higher frequency of AKI (75% vs 45%), refractory ascites (25% vs 7%), bacterial infections (58% vs 34%) and LT requirement (25% vs 10%) compared with those without CKD[7]. In addition, the involvement of cirrhosis is independently related to a poor outcome in patients with CKD[42]. CKD impacts not only waitlist mortality but also worsens post-LT survival. Cullaro et al[4]reported that the one-year post-LT mortality rate in patients with CKD was 12%, compared with 9% in those without CKD. In addition, posttransplant renal outcomes may also be affected by the presence of CKD[13].
Impact on health care utilization
Wong et al[3]found that cirrhosis patients with CKD had higher rates of hospitalization during the preceding 6 mo (70% vs 63%) than those without CKD. Similarly, Bassegoda et al[7]reported a higher 3-mo readmission rate (67% vs 37%) in cirrhosis-CKD patients compared to cirrhosis alone. A recent study analyzed the usage of health care services and the cost burden associated with CKD in patients with CLD (n = 9869) compared to patients with CLD alone (n = 588586) by using real-world insurance claims data. In a propensity-matched cohort analysis, patients with combined CLDCKD were found to have substantially greater annual per-person all-cause health care costs than patients with CLD alone[18].
DIAGNOSTIC EVALUATION
The diagnosis of CKD in cirrhosis is based on GFR. Abnormal urine analysis and/or abnormal findings on renal ultrasonography are usually found in advanced CKD and hence are not required for diagnosis. Cirrhosis can be diagnosed in patients with CKD by histopathology or hepatic ultrasound, as well as by clinical manifestations of portal hypertension and/or hepatic decompensation.
Since CKD diagnosis in cirrhosis requires a decrease in GFR to < 60 mL/min for 12 wk, a reliable and reproducible method is required to estimate GFR. The direct iothalamate clearance test is the gold standard for GFR measurement; however, the cumbersome technique and lack of widespread availability limit its usage in clinical practice[43]. Several indirect methods are available to calculate eGFR in clinical practice.
Creatinine-based eGFR
For the assessment of renal function, an eGFR based on the serum creatinine level is commonly used in clinical practice. In patients with cirrhosis, the most commonly used creatinine-based equation is the MDRD. However, serum creatinine levels in patients with cirrhosis may be unreliable due to hepatic dysfunction causing decreased production of creatine, reduced skeletal muscle mass causing decreased creatine-tocreatinine conversion, increased tubular secretion of creatinine, and underestimation of the serum creatinine level by hyperbilirubinemia[44-46]. Therefore, GFR in cirrhosis is typically overestimated by the creatinine-based equation, where a normal serum creatinine level cannot rule out renal dysfunction. In a meta-analysis, the formula based on creatinine was found to overestimate GFR by 18 mL/min[47]. However, despite limitations and until better substitutes become available, the latest creatininebased MDRD equation (MDRD-6) has been recommended by expert panels to be used in patients with cirrhosis[4,44]. The MDRD-6 equation includes 6 variables: Age, sex, race, serum creatinine, serum albumin, and blood urea nitrogen.
Cystatin-based eGFR
Cystatin C is a protein produced by all nucleated cells in the body that is exclusively removed by glomerular filtration. Hepatic function, muscle mass, sex, hyperbilirubinemia and tubular secretion do not affect the level of cystatin C. Therefore, cystatin C-based eGFR may be a better alternative to the serum creatinine-based equation for patients with cirrhosis[48-50]. However, the level of cystatin C is affected by hypoalbuminemia, elevated C-reactive protein and leukocytosis, which may limit its role in estimating GFR in cirrhosis[50,51]. Additionally, in patients with cirrhosis, the diagnostic performance of all cystatin C-based GFR equations has been found to be lower than in those without cirrhosis[44]. Combining serum creatinine and cystatin C in an equation appears to predict GFR more accurately than either alone[52]. However, eGFR measurement based on cystatin C has not yet been approved for routine use in patients with cirrhosis.
Biomarkers of kidney damage
The role of conventional urinary markers such as albuminuria is very limited in patients with cirrhosis, which may be because of hypoalbuminemia and relatively increased capillary permeability[44]. In addition, a normal proteinuria or urine examination may not exclude parenchymal changes in the kidney. The recent identification of many urinary biomarkers of renal tubular injury, such as urinary neutrophil gelatinase-associated lipocalin (uNGAL), interleukin-18, liver-type fatty acid-binding protein and kidney injury molecule-1, has revolutionized research into organic renal dysfunction[44,53-55]. These biomarkers have, however, been studied primarily in the context of AKI, and their role in the assessment of CKD is not yet clear. The most extensively assessed biomarker in cirrhotic patients has been uNGAL, an inflammatory biomarker produced by damaged renal tubular cells. The uNGAL levels can help distinguish organic from functional AKI; however, cutoff values for such discrimination lack specificity. In addition, the existence of concomitant infections or prolonged renal vasoconstriction in patients with HRS may significantly increase uNGAL levels, thereby limiting its discriminatory function in patients with cirrhosis[14,53]. In general, uNGAL has a positive correlation with the severity of renal dysfunction in patients with CKD, which indicates its prognostic significance for CKD[53]. However, the prognostic value of uNGAL in patients with cirrhosis with CKD is not known.
The profiles of urinary microRNAs may be an attractive noninvasive tool for future kidney damage assessment[54]. Other biomarkers, such as osteopontin and metalloproteinase-1 tissue inhibitor, are usually elevated in patients with CKD, but their clinical significance has not yet been established[55].
Role of duplex Doppler ultrasonography
Renal duplex Doppler ultrasound is a simple, noninvasive and efficient method that can be used in patients with cirrhosis to study intrarenal hemodynamics (Figure 2). It is a test to assess renal vascular resistance as a vasoconstriction marker, and the renal resistive index (RRI) can be used to detect early renal dysfunction in patients with cirrhosis[56]. In general, there is a progressive increase in RRI as cirrhosis patients move from without ascites to with ascites and then to HRS[57]. Since severe renal vasoconstriction is a feature of HRS, duplex ultrasonography can play a potential role in the assessment of functional CKD in patients with cirrhosis. Furthermore, the RRI can predict CKD progression as it correlates with renal histopathological changes such as glomerular sclerosis, interstitial fibrosis, and arteriolosclerosis[58].
Differentiation between functional and structural CKD
It is important to determine what proportion of CKD in cirrhosis is functional due to HRS and what proportion is associated with structural renal damage. Such differentiation has important therapeutic and prognostic implications. This would assist the clinician in deciding on the use of diuretics, vasoconstrictor treatment and the recommendation of LT vs SLKT. Structural CKD patients are more likely to be indolent and have higher survival rates than functional CKD patients[32]. However, in the absence of a renal biopsy, it is often difficult to differentiate a functional CKD from a structural CKD. Abnormal urine analysis (proteinuria > 500 mg/d or hematuria > 50/high power field) and/or abnormal findings on renal ultrasonography (reduced cortical thickness, increased cortical echogenicity and scarring) are features of advanced structural CKD (Figure 2). Currently, no accurate biomarkers are available that can diagnose subclinical renal parenchymal injury or differentiate between reversible and permanent renal injury. Importantly, prolonged renal vasoconstriction in patients with functional CKD may lead to irreversible structural changes in the kidney[32,33]. Studies on the outcome of LT in patients with type 2 HRS have found that 50%-60% of patients develop stage 3 CKD during the posttransplant period, even when HRS reverses[13,59]. Therefore, essentially a long-standing functional CKD can be regarded as structural CKD. In the absence of abnormal early imaging features and reliable biomarkers of CKD, renal biopsy remains the only choice to diagnose and further characterize CKD. However, due to coagulopathy, thrombocytopenia, and the presence of large ascites in cirrhosis, as well as scarred kidneys due to CKD, percutaneous renal biopsy may be technically challenging in patients with decompensated cirrhosis.
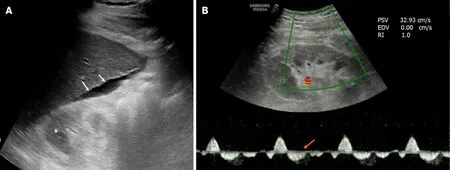
Figure 2 Ultrasonographic image of a 65-year-old diabetic patient with liver cirrhosis and chronic kidney disease. A: The liver outline is irregular (white arrows) and there is ascites around it. The right kidney is small and the parenchymal echogenicity is increased with loss of corticomedullary differentiation (asterisk), suggesting chronic kidney disease; B: Doppler sonogram of the same kidney showed reversal of diastolic flow (orange arrow) with absent end-diastolic velocity, indicating very high resistance vessels.
Pitfalls in the diagnosis of CKD
A dramatic rise in the diagnosis of CKD in patients with cirrhosis raises some concerns as to whether, in the absence of any corroborating evidence of renal injury, the dependence solely on eGFR leads to overdiagnosis of CKD[60]. An arbitrary single threshold of eGFR < 60 mL/min might have a high propensity to cause overestimation of CKD in elderly subjects. There is a natural steady decrease in GFR with increasing age, and eGFR levels between 50 and 60 mL/min can be insignificant for older individuals, with very little propensity to progress to symptomatic kidney disease[61]. The risks of overdiagnosis of CKD may be significant in patients with cirrhosis since many of them belong to the old age group. In addition, a varying degree of deterioration in GFR may occur in patients with decompensated cirrhosis due to neurohormonal alterations and circulatory dysfunction well before the detection of overt renal disease, which may lead to overdiagnosis of CKD. Therefore, on the basis of a single eGFR threshold and in the absence of any corroborating evidence of kidney damage, caution before labeling CKD in elderly patients with cirrhosis may be needed. Since creatinine-based equations tend to overestimate the GFR, they can underestimate CKD and thus may provide clinicians with false reassurance. Studies need to be performed to determine whether the diagnosis of CKD in patients with decompensated cirrhosis requires a different GFR cutoff.
MANAGEMENT IMPLICATIONS
The presence of CKD in patients with cirrhosis presents many challenges to clinicians with regard to medical care. In particular, fluid mobilization to control ascites and edema becomes a real challenge. The use of diuretic therapy has several limitations. As ascites often reaccumulates rapidly, patients require repeated large volume paracentesis. This puts them at risk of multiple complications, such as worsening circulatory dysfunction, infection and bleeding, in addition to causing discomfort to the patients.
Diuretic therapy
Diuretic therapy is often not prescribed in patients with functional CKD because of the concern that it may further worsen renal failure by causing intravascular volume loss and may precipitate electrolyte imbalance[33]. In patients with structural CKD, the use of diuretics seems appropriate to manage ascites and edema. However, a varying degree of diuretic resistance is usually present in patients with CKD. This occurs primarily because of decreased renal blood flow, hyperuricemia and organic anion accumulation[62]. The organic anions, uric acid and hypoalbuminemia interfere with the function of loop diuretics. A higher dose of diuretics is therefore required to overcome diuretic resistance in the presence of CKD.
There is a lack of evidence to guide clinicians as to which single or combination diuretic agent is most appropriate for these patients. Furosemide is primarily eliminated by the kidney, while torsemide has predominant hepatic clearance[63]. Therefore, if kidney dysfunction is a predominant issue, torsemide might be preferred over furosemide, while in the case of severe hepatic dysfunction, furosemide may be preferred over torsemide. A recent meta-analysis found that coadministration of albumin with furosemide had a modest effect on overcoming diuretic resistance in hypoalbuminemic patients[64]. Correction of metabolic acidosis and hyperuricemia, adequate restriction of fluid and salt intake, and avoidance of medications that interfere with peritubular diuretic uptake, such as nonsteroidal anti-inflammatory drugs and beta-lactam antibiotics, may be other measures to enhance the diuretic response[62]. Spironolactone should be better avoided in advanced CKD to prevent hyperkalemia.
Vaptans
Vaptan, an antagonist of vasopressin 2 receptor, may be considered in patients with CKD with cirrhosis who are intolerant to or poorly responsive to diuretics. Tolvaptan has been found to be potentially safe with an efficacy rate of 77% for the treatment of refractory ascites in decompensated cirrhosis patients with coexisting type 2 HRS[65]. Tolvaptan significantly increases urine volume in patients with CKD with liver cirrhosis without worsening renal dysfunction[66]. However, its diuretic response gradually diminishes with progression of the CKD stage[67]. Due to the possible risks of hepatocellular damage identified during a clinical trial involving patients with autosomal dominant polycystic kidney disease, the United States Food and Drug Administration issued a warning for tolvaptan use in 2013. However, very high doses of tolvaptan had been used for a long period of time in this study (120 mg/d for 3 years), and no such adverse effects have been reported from the study on patients with cirrhosis where the recommended dose was much lower.
Vasoconstrictor therapy
Midodrine is an orally available α1-agonist that serves as a vasoconstrictor and has been found to have an effect on the systemic hemodynamics of cirrhotic patients. However, midodrine trials in patients with cirrhosis have shown contradictory results. There is insufficient evidence about its use in patients with CKD. In patients with type 2 HRS, midodrine has only a slight beneficial effect on systemic hemodynamics, with no effect on renal hemodynamics[68]. Additionally, treatment with terlipressin or noradrenaline along with albumin appears to have a limited role in patients with CKD. While there are several cases of reversal of type 2 HRS, recurrence after withdrawal of therapy is very common. In addition, evidence on the impact of this treatment on the outcomes of patients is controversial. Few studies have assessed the efficacy of terlipressin in a limited number of patients with type 2 HRS, and the findings have been equivocal[69,70]. In a recent study, 46% of treated patients demonstrated reversal of type 2 HRS; however, nearly half of responders experienced relapse[71]. Furthermore, reversal of type 2 HRS before LT does not appear to provide a major benefit over patients who are untreated or who have failed treatment before LT[13]. Therefore, most of the current guidelines do not recommend vasoconstrictor treatment in functional CKD.
Transjugular intrahepatic portosystemic shunt
Transjugular intrahepatic portosystemic shunt (TIPS) decreases portal pressure, improves kidney function and relieves ascites. While it is being used increasingly to treat patients with refractory ascites and functional renal failure, there is limited evidence on its use in patients with advanced CKD. In a study on TIPS in 17 patients with cirrhosis with CKD, Lakhoo et al[72]found that ascites control occurred in 83% of patients but at the expense of a high incidence (47%) of new or worsening hepatic encephalopathy (HE). Michl et al[73]reported improvement in renal function and a decrease in the frequency of paracentesis following TIPS in 10 patients with cirrhosis, including three with structural kidney disease. A recent systematic review and metaanalysis found a potential survival benefit of TIPS in patients with HRS but with a high (49%) incidence of HE. In type 2 HRS, the pooled short-term and 1-year survival rates after TIPS were 86% and 64%, respectively. Moreover, 83% of patients with HRS experienced improvement in renal function after TIPS[74]. In summary, TIPS appears to be very effective in patients with functional CKD, and limited data indicate its effectiveness in structural CKD as well. However, TIPS may increase the incidence of HE, so it should be avoided in patients with encephalopathy, cardiopulmonary disease, and significant hepatic dysfunction.
Cell-free and concentrated ascites reinfusion therapy
Cell-free and concentrated ascites reinfusion therapy (CART) is an apheresis therapy in which ascitic fluid is filtered to remove unwanted cells, sterilely concentrated, and then intravenously reinfused[75]. It was introduced in Japan as a novel treatment for refractory ascites in patients with cirrhosis. The potential advantages of CART include its ability to maintain nutritional status, control ascites, and improve quality of life. Unlike large volume paracentesis, CART is not associated with the risk of hypoproteinemia, hemodynamic instability, renal dysfunction or fatigue. It has been used for both cirrhotic and malignant ascites; however, its safety and efficacy need to be assessed in CLD-CKD patients with refractory ascites. CART has been found to be equally as effective as large volume paracentesis plus albumin infusion[76]. However, its routine use can be limited by the high cost of the CART apparatus[77].
Concerns related to medications
Since the majority of drugs are metabolized and/or excreted by the hepatorenal system, it is a challenging task to prescribe medicines in patients with advanced cirrhosis with renal impairment[78]. There are no evidence-based guidelines for the use of medicines in such patients. Drugs with significant hepatotoxic or nephrotoxic potential or both need to be avoided in such patients. In addition, the dosage of several antibiotics needs to be modified in accordance with GFR. Nonselective β-blockers can increase mortality in patients with advanced cirrhosis with renal dysfunction due to their adverse impact on cardiac compensation[79]. Treatment with hepatitis B nucleos(t)ide analogues can also raise the risk of lactic acidosis if renal dysfunction is present[80].
Treatment modifications according to the etiology of cirrhosis
There are no large controlled studies available to direct appropriate antiviral therapy for patients with CKD with HBV cirrhosis. The use of tenofovir disoproxil fumarate has been associated with a mild risk of CKD progression, but a recent meta-analysis has shown that such a decrease in renal function is inappreciable compared with entecavir[81,82]. Nevertheless, entecavir tends to be the most preferred drug for these patients. However, entecavir therapy may not be as effective in patients with lamivudine resistance, so tenofovir alafenamide, an orally bioavailable tenofovir prodrug with a lower risk of renal toxicity, may be considered in such patients. In patients with HCV-cirrhosis and CKD, high sustained virologic response rates with glecaprevir/pibrentasvir combination in all genotypes and with elbasvir/grazoprevir in genotypes 1 and 4 can be achieved. However, these drugs are largely metabolized in the liver and are therefore not safe in advanced cirrhosis[83]. Patients with advanced decompensated cirrhosis and renal dysfunction are a difficult-to-treat category for which there are no guidelines for treatment; therefore, a treatment decision should be made on a case-by-case basis. While a treatment based on sofosbuvir is not recommended for patients with severe renal impairment, it may be used in patients with mild renal impairment[84]. In patients with nonalcoholic steatohepatitis-CKD, statins have been shown to decrease cardiovascular disease mortality, and sodiumglucose cotransporter-2 inhibitors have been found to slow the progression of CKD and minimize all-cause mortality[85]. However, it is important to determine the role of these drugs in nonalcoholic steatohepatitis-cirrhosis patients with CKD.
Renal replacement therapy
The shared symptoms between the two diseases and overestimation of eGFR make it difficult for patients with cirrhosis with CKD to determine the ideal time for commencing renal replacement therapy. The hemodynamic alterations of cirrhosis pose a challenge to maintaining hemodynamic stability during dialysis, where a sudden decrease in intravascular volume due to ultrafiltration may cause hypotension. A sharp change in blood osmolarity and electrolyte levels increases the risk of developing HE. In addition, thrombocytopenia, platelet dysfunction, and coagulopathy due to combined CKD-cirrhosis may increase the risk of bleeding complications. In these patients, peritoneal dialysis may be a better choice because it will not only resolve many problems associated with intermittent HD but will also allow ascitic fluid to be regularly evacuated[86,87]. Studies evaluating the survival of cirrhotic patients on peritoneal dialysis have reported a modest survival rate of 8 to 66 mo[88].
LT vs SLKT
Increased post-LT mortality is associated with any form of renal dysfunction. However, most studies evaluating the impact of renal function on post-LT survival do not differentiate between CKD and AKI[4,89,90]. Few studies have reported the progression of CKD, including the development of end-stage renal disease and increased post-LT mortality in cirrhotic patients with CKD receiving LT alone[90-92]. In a recent study, the presence of CKD at the time of LT increased the risk of post-LT mortality by 16%[4]. Thus, there has been a drive to perform more SLKT in patients with combined cirrhosis-CKD. However, in patients with cirrhosis, predicting renal recovery post-LT is difficult, and the degree or severity of CKD that warrants SLKT vs LT remains undefined. The 2012 SLKT summit guidelines indicate that SLKT should be considered for cirrhosis-CKD patients with an eGFR of ≤ 40 mL/min measured by the MDRD-6 equation[93]. Once again, however, the use of SLKT is highly variable, and the role of kidney transplantation in nondialysis CKD is controversial. Singh et al[94]recently reported the outcome of LT only in nine CKD-cirrhosis patients who had persistently low eGFR < 40 mL/min for ≥ 12 wk but relatively normal kidney biopsy findings. Post-LT, eGFR increased in all nine patients within a week and remained stable afterwards; one patient progressed to ESRD 9 years post-LT, and another patient expired 7 years after LT. While no definite conclusions can be drawn from this small study, there is an indication that, in the absence of other indicators of renal injury, low eGFR alone below an arbitrary cutoff value does not constitute an absolute requirement for SLKT in patients with liver cirrhosis. Functional CKD is potentially reversible after LT[13,52]. However, prolonged renal ischemia can cause permanent tubular or glomerular damage that may not recover with LT, leading to post-LT CKD progression. Because the assessment of renal function may be difficult in patients with advanced cirrhosis, a renal biopsy should be considered whenever possible for identifying parenchymal changes and to decide between LT and SLKT. In patients with low eGFR and kidney biopsy showing > 30% glomerulosclerosis and/or interstitial fibrosis, SLKT should be considered[93]. Future prediction models to assist in decision-making between SLKT and LT should consider integrating kidney injury markers, including new CKD biomarkers.
CONCLUSION
In conclusion, the incidence of CKD in patients with cirrhosis has increased significantly as a result of a rise in risk factors and a change in the diagnostic criteria from a fixed level of serum creatine to a dynamic change in GFR. The available data on this condition are extremely limited. Future studies on this subject are required to explain several contentious issues. Taking into account the problems related to the calculation of GFR in patients with cirrhosis, the main issue to be addressed would be the refining of diagnostic criteria. The other areas that require future research are the identification of reliable biomarkers of chronic kidney damage, the formulation of management strategies based on phenotypic features of CKD in cirrhosis, and the development of prediction models to assist in decision-making between SLKT and LT.
 World Journal of Gastroenterology2021年11期
World Journal of Gastroenterology2021年11期
- World Journal of Gastroenterology的其它文章
- Genotype 3-hepatitis C virus' last line of defense
- How to manage inflammatory bowel disease during the COVID-19 pandemic: A guide for the practicing clinician
- Efficacy and safety of endoscopic submucosal dissection for gastric tube cancer: A multicenter retrospective study
- Study on the characteristics of intestinal motility of constipation in patients with Parkinson's disease
- Apolipoprotein E polymorphism influences orthotopic liver transplantation outcomes in patients with hepatitis C virus-induced liver cirrhosis
- Prospective single-blinded single-center randomized controlled trial of Prep Kit-C and Moviprep: Does underlying inflammatory bowel disease impact tolerability and efficacy?
