Health-related quality of life in patients that have undergone liver resection:A systematic review and meta-analysis
Tomohiro Ishinuki,Shigenori Ota,Kohei Harada,Hiroomi Tatsumi,Keisuke Harada,Koji Miyanishi,Minoru Nagayama,Ichiro Takemasa,Toshio Ohyanagi,Thomas T Hui,Toru Mizuguchi
Tomohiro Ishinuki,Toru Mizuguchi,Department of Nursing,Surgical Sciences,Sapporo Medical University,Sapporo 060-8556,Japan
Shigenori Ota,Minoru Nagayama,Ichiro Takemasa,Departments of Surgery,Surgical Science and Oncology,Sapporo Medical University,Sapporo 060-8543,Japan
Kohei Harada,Division of Radiology,Sapporo Medical University,Sapporo 060-8543,Japan
Hiroomi Tatsumi,Department of Intensive Care Medicine,Sapporo Medical University Hospital,Sapporo 060-8543,Japan
Keisuke Harada,Department of Emergency Medicine,Sapporo Medical University Hospital,Sapporo 060-8543,Japan
Koji Miyanishi,Department of Medical Oncology,Sapporo Medical University Hospital,Sapporo 060-8543,Japan
Toshio Ohyanagi,Department of Liberal Arts and Sciences,Center for Medical Education,Sapporo Medical University,Sapporo 060-8556,Japan
Thomas T Hui,Department of Surgery,Division of Pediatric Surgery,Stanford University School of Medicine,Stanford,CA 94598,United States
Abstract BACKGROUND Mortality after hepatectomy has decreased,and the quality of various surgical approaches to hepatectomy have been evaluated.Various assessments of quality of life (QOL) after hepatectomy have been developed and investigated in different clinical settings.AIM To conduct a systematic review and meta-analysis to examine two clinical topics:Laparoscopic hepatectomy vs open hepatectomy,and preoperative QOL status vs postoperative QOL status.METHODS A systematic literature search was performed using PubMed and MEDLINE,including the Cochrane Library Central.The following inclusion criteria were set for inclusion in this meta-analysis:(1) Studies comparing preoperative QOL and postoperative QOL;and (2) Studies comparing QOL between laparoscopic hepatectomy and open hepatectomy.RESULTS A total of 8 articles were included in this meta-analysis.QOL was better after laparoscopic hepatectomy than after open hepatectomy.CONCLUSION The outcomes of evaluations of QOL after hepatectomy can depend on the type of questionnaire used,the timing of the assessment,and the etiology of the hepatic disease.
Key Words:Quality of life;Hepatectomy;Laparoscopy;Transarterial chemoembolization;Functional Assessment of Cancer Therapy-Hepatobiliary;36-Item Short-Form Health Survey;European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire
INTRODUCTION
Recently,hepatectomy has become safe,and the mortality rate of the procedure is now less than 1%[1,2].Besides surgery,various other approaches have been developed for managing liver tumors,such as ablation,chemotherapy,molecular targeted therapy,and immunotherapy[3,4].Furthermore,various surgical approaches have been developed,such as laparoscopic hepatectomy,robot-assisted hepatectomy,hybrid methods,hand-assisted methods,and classic open hepatectomy[5-9].Therefore,selecting the optimal approach is essential for ensuring patients receive high-quality treatment.
Patient-reported outcomes (PRO) are considered to be gold-standard methods for evaluating quality of life (QOL) and comparing different management strategies[10].Various PRO,such as the Functional Assessment of Cancer Therapy–Hepatobiliary(FACT-Hep)[11],the 36-Item Short-Form Health Survey (SF-36)[12],the European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire(QLQ-C30)[13],the EuroQol 5-dimension,5-level questionnaire[14],and others,have been investigated in patients who underwent hepatectomy.The FACT-Hep consists of 5 subscales,physical well-being,social well-being,emotional well-being,functional well-being,and the hepatobiliary cancer subscale[15].The sum of the scores for the five subscales gives a total score ranging from 0 to 180.A higher score indicates better QOL.The SF-36 consists of eight subscales,which are used to produce a physical component score and a mental component score[16].The EORTC developed the QLQC30.The QLQ-C30 consists of three subscales:global health status,functional scales,and symptom scales[17].Each subscale gives a score ranging from 0 to 100.Higher scores in the global health status and functional scales represent better QOL.
Although many studies have investigated PRO-QOL after hepatectomy,even the best QOL questionnaires are imprecise.Also,the timing of the evaluation is usually unknown.We attempted to examine QOL in patients who had undergone hepatectomy.The first clinical question we investigated was whether postoperative QOL was better among patients who underwent hepatectomy or transarterial chemoembolization (TACE).The second question was whether QOL was better among patients who underwent laparoscopic hepatectomy or classic open hepatectomy.Finally,we compared the changes in QOL scores seen after hepatectomy.This systematic review and meta-analysis examined the current status of QOL studies of patients who underwent hepatectomy.In addition,it revealed a future clinical question and provided an idea for a future clinical study.
MATERIALS AND METHODS
Literature search
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines were followed when obtaining and reporting the meta-analysis data[18].The PICOS scheme was followed when reporting the inclusion criteria.A systematic literature search was performed independently by two authors (Ishinuki T and Ota S)using PubMed and MEDLINE,including the Cochrane Library.The search was limited to human studies whose findings were reported in English.No restriction was set for the type of publication,the publication date,or publication status.Patients of any age or sex who underwent liver resection for any type of hepatic lesion were considered as outlined in the PICOS scheme.The search strategy was based on different combinations of words for each database.For the PubMed database the following combination was used:("qol"[All Fields] AND ("liver"[MeSH Terms] OR"liver"[All Fields] OR "livers"[All Fields] OR "liver s"[All Fields]) AND("surgery"[MeSH Subheading] OR "surgery"[All Fields] OR "surgical procedures,operative"[MeSH Terms] OR ("surgical"[All Fields] AND "procedures"[All Fields]AND "operative"[All Fields]) OR "operative surgical procedures"[All Fields] OR"general surgery"[MeSH Terms] OR ("general"[All Fields] AND "surgery"[All Fields])OR "general surgery"[All Fields] OR "surgery s"[All Fields] OR "surgerys"[All Fields]OR "surgeries"[All Fields]).For the Medline database,the following combination was used:(QOL and Liver and Surgery).
Study selection
The two independent authors screened the titles and abstracts of the primary studies identified in the database search.Duplicate studies were excluded.The following inclusion criteria were set for inclusion in the meta-analysis:(1) Studies comparing preoperative QOL and postoperative QOL in patients who underwent liver resection for any type of hepatic lesion;(2) Studies comparing QOL between laparoscopic hepatectomy and open hepatectomy in patients who underwent liver resection for any type of hepatic lesion;(3) Studies reporting at least one QOL outcome;and (4) If the same institute reported more than one study,only the most recent or the highest level study was included.
The following exclusion criteria were set:(1) Original studies assessing the outcomes of liver transplantation;(2) Review articles,letters,comments,and case reports;and (3) Studies for which it was impossible to retrieve or calculate the data of interest.The Cohen kappa statistic was used to quantify the agreement between the investigators.
The protocol was registered with PROSPERO (#CRD42021225970).
Data extraction
The same two authors extracted the following primary data:(1) The questionnaires used for each QOL evaluation;(2) The first author,year of publication,and type of study;(3) The etiology of the disease and the number of times each intervention was performed;and (4) The timing of the evaluations.The reasons why studies were excluded from the full-text evaluation are shown in Supplementary Tables 1-3.All excluded references are listed in the supplemental references.
Risk of bias assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included studies,as they included observational studies (http://www.ohri.ca/).The NOS consists of three domains,patient selection,comparability of study groups,and outcome assessment.The minimum risk of bias gain 9 points.We considered studies that scored ≥ 7,4-6,and < 4 to be high quality,moderate quality,and low quality,respectively[19].
Statistical analyses
All analyses were performed using the RevMan software (version 5.3.;The Cochrane Collaboration).The mean differences (MD) between groups were calculated for continuous variables.The interquartile ranges of the data were transformed by dividing them by 1.35 to produce alternative standard deviation values[20].Multiple means and standard deviations were combined using the StatsToDo online web program (https://www.statstodo.com/index.php).
Theχ2test was used to evaluate heterogeneity,and the CochranQandI2statistics were reported.TheI2value describes the percentage variation between studies in degrees of freedom.Low,moderate,and high heterogeneity were defined based on cut-off values of 25%,50%,and 75%,respectively,using the obtainedI2test values[21].
All results were considered significant atPvalues of < 0.05.
RESULTS
Study selection
The literature search yielded 248 articles,and the abstracts were reviewed by two independent researchers (Figure 1).Of these,30 articles were selected for full-text review.Two articles were excluded due to different comparison.Nine articles were excluded due to no data description being provided.Eleven articles were excluded as they did not involve appropriate timepoints.Detailed information about the excluded articles is shown in Supplementary Tables 1-3.Finally,a total of 8 articles were included in this meta-analysis (Table 1).Two studies used the FACT-Hep[22,23],four studies used the SF-36[24-27],and two studies used the QLQ-C30[28,29].None of them were randomized controlled studies.
FACT-Hep
The FACT-Hep was used to compare QOL before and after hepatectomy.None of the FACT-Hep domains differed significantly from their preoperative levels at 3 mo(Figure 2A) or 12 mo (Figure 2B),although several domains at 3 mo after hepatectomy tends to be better than those at 12 mo.
SF-36
The SF-36 was used to compare QOL between laparoscopic hepatectomy and open hepatectomy at 3-6 mo after treatment (Figure 3A).Although the physical component score did not differ significantly between the groups (P= 0.08),the mental component score for the laparoscopic hepatectomy group was significantly more favorable than that for the open hepatectomy group (P= 0.001).On the other hand,the physical component scores and mental component scores seen at 3 mo after hepatectomy were significantly more favorable than those observed before hepatectomy (Figure 3B;P=0.04 andP= 0.02,respectively).
QLQ-C30
The QLQ-C30 was used to evaluate QOL at 6 mo and 12 mo after hepatectomy(Figure 4).No significant differences in global health,emotional function,or social function were observed between the preoperative assessment and 6 mo or 12 mo after hepatectomy.However,the patients’ preoperative physical function scores were better than those seen at 6 mo or 12 mo after hepatectomy (P= 0.0004 andP= 0.04,respectively).Although role function and cognitive function differed significantly between the preoperative assessment and 6 mo after hepatectomy (P= 0.01 andP=0.02,respectively),they did not differ significantly between the preoperative assessment and 12 mo after hepatectomy.

Table 1 Data extracted from the included studies

Figure 1 Flow diagram of systematic reviews and meta-analyses.
Quality assessment
The quality assessment was conducted using the NOS score (Supplementary Table 4).Three studies were of moderate quality,and seven studies were of high quality.
DISCUSSION
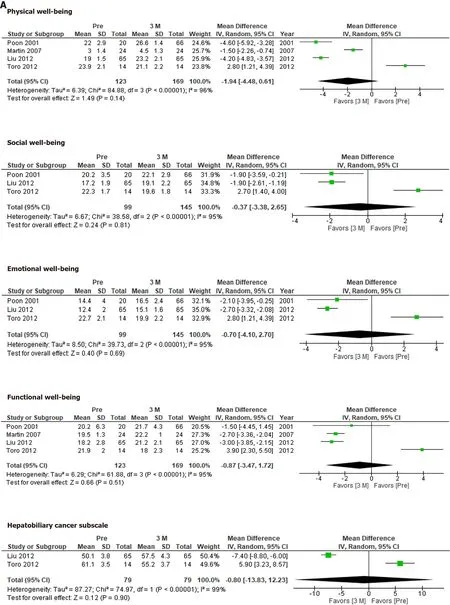
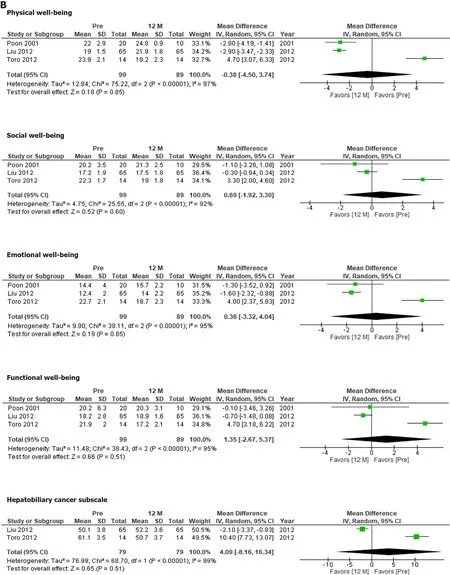
Figure 2 Functional Assessment of Cancer Therapy–Hepatobiliary scores before and at 3 mo,12 mo after hepatectomy.A:Before and at 3 mo after hepatectomy;B:Before and at 12 mo after hepatectomy.
Liver resection has become a safe surgical procedure for liver tumors and is now used for living transplantation[2,3,5,13].The clinical outcomes of hepatectomy have been reported based on quality assessments since 2000,and evidence has accumulated rapidly within the last decade[30].We evaluated two crucial clinical questions in this study.The first was whether hepatectomy or TACE resulted in better QOL.The second was whether laparoscopic or open hepatectomy resulted in better QOL.Furthermore,we examined the changes in QOL seen at 3 mo,6 mo,and 12 mo after hepatectomy.
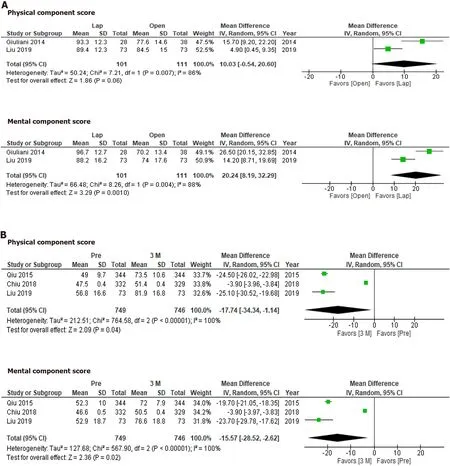
Figure 3 The 36-Item Short-Form Health Survey scores at 3 mo and 6 mo after laparoscopic or open hepatectomy or at 3 mo after hepatectomy.
The findings of postoperative QOL assessments can vary according to the type of questionnaire used,the surgical approach,the etiology of the disease,and the timing of the evaluations[30].
Most of the studies examined in the present review were conducted using a paperbased approach or face-to-face interviews.A more mobile approach would allow the comprehensive collection of a greater variety of data[31].However,some questionnaires are not suitable for extensive prospective surveys due to cost issues.In addition,language translation is also an obstacle to international comparisons among questionnaires.Therefore,the statistical power of the studies was limited.
The FACT-Hep did not identify any significant changes in QOL after hepatectomy.Our results indicate that HCC patients’ QOL recovered within 12 mo after hepatectomy.Although the QOL scores for each subdomain at 3 mo did not differ significantly from those observed before hepatectomy,the integrated mean tended to be more favorable at 3 mo after hepatectomy than before hepatectomy.This would depend on the condition of the patients who were eligible for the studies.Therefore,the QOL scores for these patients would have improved after hepatectomy.


Figure 4 The European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire scores at 6 mo and 12 mo after hepatectomy.
According to the SF-36,QOL was significantly better at 3 mo after hepatectomy than before hepatectomy.On the other hand,different results might have been obtained if the studies had involved asymptomatic patients[26].Another concern is the sensitivity of each questionnaire.It is possible that the SF-36 is more sensitive than the FACT-Hep in these circumstances.
Laparoscopic hepatectomy has become the standard approach for liver resection[5,7,9].It is considered that the reduced invasiveness associated with the minimal wound length of the laparoscopic approach allows patients to recover faster than is possible with the open approach[5].As we demonstrated in this study,QOL could be better after laparoscopic hepatectomy than after open hepatectomy.In addition,the mental component scores of the patients that underwent laparoscopic hepatectomy were significantly better than their physical component scores.The reduced invasiveness of laparoscopic hepatectomy is considered to improve physical outcomes.However,this was not proven,presumably due to the long period of time between the surgery and the assessments.The degree to which mental QOL was preserved is a unique feature of laparoscopic hepatectomy.
The QLQ-C30 produced different results from the other QOL questionnaires.Physical function and role function had deteriorated significantly at 6 mo after hepatectomy,but had recovered at 12 mo after hepatectomy.The changes in the QLQC30 seen after hepatectomy seem to be more reasonable.They indicate that surgery itself temporarily reduces patients’ QOL.In addition,the examined studies included asymptomatic patients who had metastatic liver tumors from colorectal cancer[28,29],which could also explain why QOL deteriorated after surgery.
CONCLUSION
Laparoscopic hepatectomy resulted in better QOL than open hepatectomy.The results of QOL evaluations performed after hepatectomy could depend on the type of questionnaire used,the timing of the assessment,and the etiology of the hepatic disease.
ARTICLE HIGHLIGHTS
Research background
The quality of life (QOL) assessment after hepatectomy has never been summarized.Therefore,comprehensive systematic review and meta-analysis would have great scientific value.
Research motivation
Lack of randomized controlled trial motivate us to plan prospective study.However,sample size calculation is difficult due to lack of the QOL value.This analysis would be helpful to conduct future trials.
Research objectives
Research objectives were to elucidate QOL after hepatectomy.
Research methods
Systematic review and met a-analysis was conducted according to PROSPERO guidelines with Preferred Reporting Items for Systematic Reviews and Meta-Analyses check lists.
Research results
A total of 8 articles were included in this meta-analysis.QOL was better after laparoscopic hepatectomy than after open hepatectomy.Physical and mental component score of the 36-Item Short-Form Health Survey at 3 mo was significantly better than before hepatectomy.
Research conclusions
The outcomes of evaluations of QOL after hepatectomy can depend on the type of questionnaire used,the timing of the assessment,and the etiology of the hepatic disease.
Research perspectives
The values from this study could be useful to plan future randomized control trial.
ACKNOWLEDGEMENTS
We thank Tan S and Nara M for their help in preparing this manuscript and valuable discussions.
 World Journal of Meta-Analysis2021年1期
World Journal of Meta-Analysis2021年1期
- World Journal of Meta-Analysis的其它文章
- Should we use full analgesic dose of opioids for organ procurement in brainstem dead?
- Magic and forensic psychiatry:A case study and review of the literature
- Efficacy and safety outcomes with remdesivir in COVID-19 patients:A meta-analysis
- Mortality of critical care interventions in the COVID-19:A systematic review
- Risk factors,manifestations,diagnosis and treatment of cholelithiasis in children
- Non-invasive diagnosis of Crohn’s disease:All that glitters is not gold
