Exosomes:A new frontier under the spotlight for diagnosis and treatment of gastrointestinal diseases
Maliha Naseer,Syeda Hadi,Ali Syed,Amer Safdari,Veysel Tahan
Maliha Naseer,Division of Gastroenterology and Hepatology,Department of Internal Medicine,East Carolina University,Greenville,NC 27834,United States
Syeda Hadi,Rawalpindi Medical University,School of Medicine,Rawalpindi 46000,Punjab,Pakistan
Ali Syed,Veysel Tahan,Division of Gastroenterology and Hepatology,Department of Internal Medicine,University of Missouri,Columbia,MO 65212,United States
Amer Safdari,Illinois College of Medicine,School of Medicine,Chicago,IL 60612,United States
Abstract Exosomes are small plasma membrane-bound multivesicular bodies ranging in size from 20-100 nm.Exosomes are degraded fragments of mRNA,microRNA,and enriched in proteins,lipids,and nucleic acid.They are produced in the endosomes of most eukaryotic cells and once secreted,exosomes are involved in cell to cell communication and remodeling of the matrix in the extracellular compartment.Exosome biogenesis plays a crucial role in cellular development,inflammation,immunity,hemostasis,carcinogenesis,and degeneration.Due to their unique biochemical and biophysical properties,exosomes serve a variety of functions including biomarkers of diagnostic and prognostic significance.Besides,there is an increasing level of evidence to expand our understanding of the exosomes as novel therapeutic agents.Inflammatory bowel disease (IBD) such as Crohn's disease and ulcerative colitis,hepatic fibrosis,and gastrointestinal malignancies such as colorectal cancer are the potential avenues where exosomes can be applied as cell therapy and immunotherapy and have shown promising results in several in-vitro and animal models.The purpose of this review article is to highlight the emerging role of exosomes as the diagnostic and therapeutic tool in various diseases involving the gastrointestinal tract like IBD,hepatocellular carcinoma,and colon cancer.A thorough literature search was performed on databases such as PubMed,Ovid Medline,and EMBASE to achieve the objectives of this review article.
Key Words:Exosomes;Gastrointestinal diseases;Inflammatory bowel disease;Colorectal cancer;Hepatocellular carcinoma
INTRODUCTION
Most eukaryotic cells can internalize portions of their intracellular components to create endosomes.These endosomes then serve as a recycling plant for the cells[1].However,some endosomes can internalize parts of their membranes to create multivesicular bodies.Vesicles found within the endosomes are called intraluminal endosomal vesicles.If these multivesicular bodies fuse with the plasma membrane releasing their intraluminal content extracellularly,then those vesicles are referred to as exosomes[2,3].The size of the parent multivesicular bodies ultimately determine the size of the exosomes,which can range between 20-100 nm[4].Although poorly understood at the time,exosomes first made their appearance in literature as early as the 1940s[5].In the 1970s these extracellular “membrane fragments” were considered a universal feature of viable cells.In the early 1980s exosomes were considered nothing more than cellular debris that budded directly from the plasma membrane[6].In 1987 exosomes were given their name by Rose Johnstone who able to isolate endosomederived vesicles proving their intracellular origin[7].The next major advancement in the history of exosomes was the discovery of mRNA,microRNA (miR),and proteins found within them[8,9].Exosome protein composition can be divided into two categories.The first category depends on the endosome from which they originated and contains components from the Golgi apparatus,endoplasmic reticulum,nucleus,and mitochondria.The second category consists of evolutionarily conserved protein molecules that have the potential to serve as a biomarker (Figure 1).Exosomes also contain mRNA or miR which have been shown to alter gene expression in target cells[8].This has led to increased research in the field and exosomes have now been shown to patriciate in biological processes including signaling,inflammation,waste management,immune response,coagulation,cell death,angiogenesis,neurodegenerative diseases,and cancer[10,11].Exosomes also play a role in altering the microenvironment of tumor cells to help facilitate angiogenesis and metastasis to a more favorable environment in the setting of hypoxia[12].Multiple studies have demonstrated the potential for exosomes to be used as novel biomarkers and therapeutic tools in combating cancer.Current research is focused on bringing exosomes to the clinical arena as said biomarkers and therapeutic vectors[13].The purpose of this review article is to highlight the emerging role in the diagnostic and therapeutic utility of exosomes in various cancerous and non-cancerous diseases involving the gastrointestinal tract.
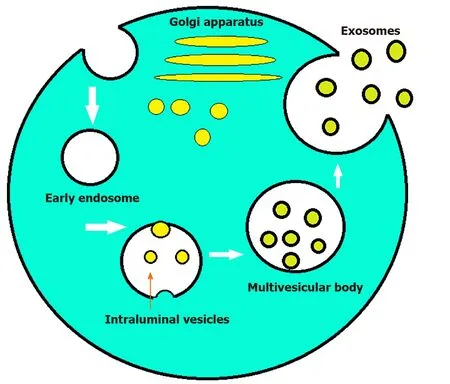
Figure 1 Formation of exosomes.
ROLE OF EXOSOME IN NON-CANCEROUS GASTROINTESTINAL DISEASES
Exosomes in inflammatory bowel disease
Intestinal epithelial cells interact daily with numerous dietary-derived antigens and bacteria without eliciting immune reactions.Yet,when enteric pathogens are introduced,the immune system activates to fight the novel insult.Homeostasis depends on the ability of the immune system to distinguish normal intraluminal content from invading pathogens.The failure of this process results in inflammatory bowel diseases[14-16].Although the exact etiology of the disease remains debated,a combination of factors including genetic,immunoregulatory,and environmental likely play a major role[17].Inflammatory bowel disease (IBD) is a systemic,chronic,noninfectious,and relapsing inflammatory disorder of the gastrointestinal tract consisting primarily of Crohn’s disease and ulcerative colitis.The symptoms of the disease commonly include abdominal pain,bloody diarrhea,weight loss,and fever[18,19].Current therapy for the disease focuses primarily on immunosuppression with agents such as anti-tumor necrosis factor (TNF) monoclonal antibodies,immunomodulators,anti-inflammatory drugs,and thiopurine agents[20].The incidence of IBD is increasing in developed countries and the United States alone one to 1.5 million people estimated to be affected by the disease[21-23].Patients with the disease experience a reduction in their overall quality of life and increased mortality primarily due to an increased risk for colitis associated colorectal cancer (CRC)[24-26].Current therapies for IBD have their limitations and many patients are unable to achieve remission.As such,novel therapies ranging from the use of herbal extracts to the introduction of harmless parasites have been proposed[27-29].One potential avenue worth exploring in the treatment of IBD is the use of exosomes as novel biomarkers and therapeutic vectors.
Currently,the gold standard for diagnosing IBD is ileocolonoscopy.Endoscopic visualization is necessary due to the discrepancy between clinical symptoms and the extent of the disease.The endoscopic assessment provides critical information about the severity of the disease and aids in decision making regarding medical management.However,colonoscopies are associated with increased cost and patient discomfort.As such development of less invasive tools for diagnosing IBD are necessary.
Exosomes show promise as novel biomarkers for diagnosing IBD and assessing its progression (Table 1).One such biomarker is proteasomes subunit alpha type 7(PSMA7) which is markedly upregulated in the salivary exosomes of a patient with IBD when compared to healthy individuals[30].The exact relationship between thedevelopment of IBD and salivary exosomal PSMA7 has yet to be elucidated,but the ease with which they can be collected may represent a better diagnostic approach towards IBD[30].Another potential biomarker for diagnosing IBD is annexin-1 containing exosomes.These exosomes arise from the intestinal epithelial cell and are overexpressed during inflammatory responses.Annexin-1 has been shown to play a role in the healing process that occurs in intestinal epithelial cells following mucosal damage.Patients with IBD have shown have increased levels of annexin-1 containing exosomes making them a potential new biomarker for diagnosing IBD[31].
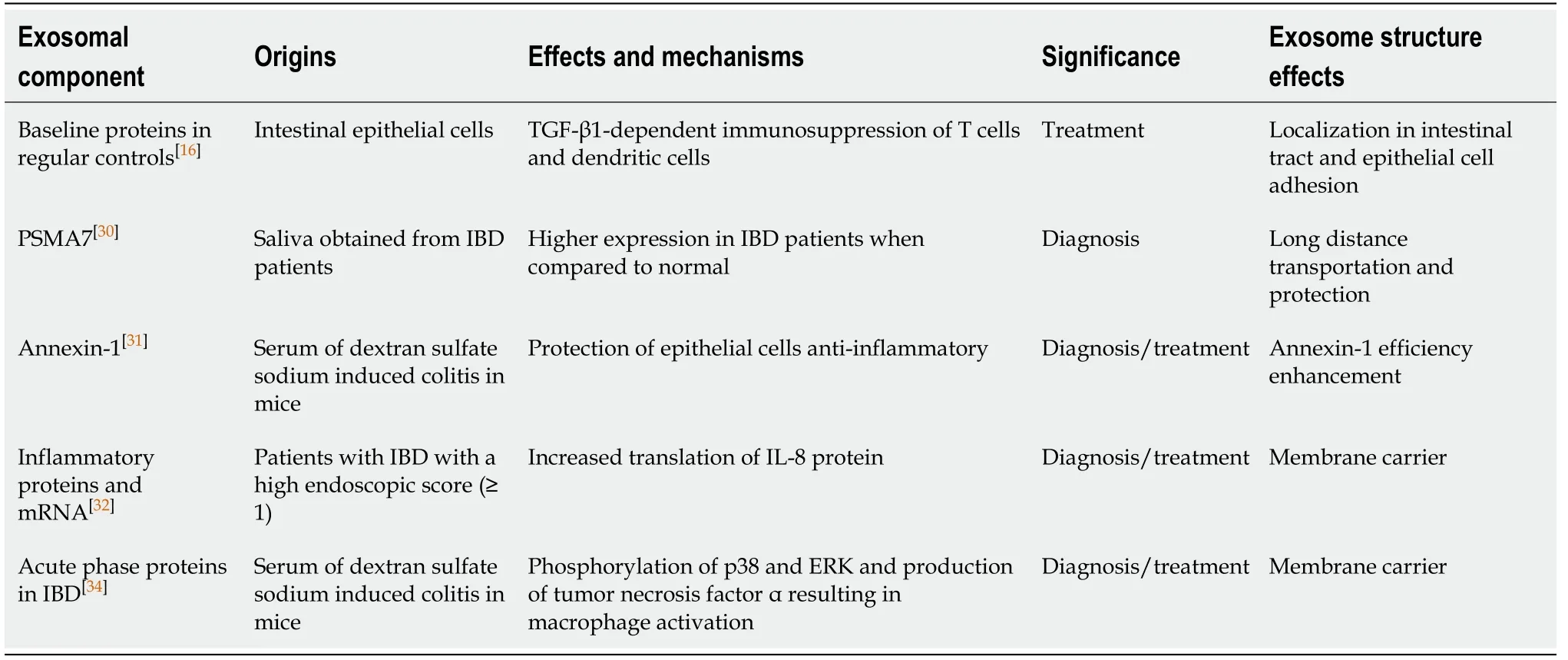
Table 1 Exosomes as promising novel biomarkers for diagnosis of inflammatory bowel disease and its progression
The pathophysiology of IBD involves a variety of factors including environmental factors,genetic factors,microbial dysbiosis,and immune system dysregulation.Current treatment for IBD centers on maintaining immuno-tolerance to obtain remission.Current research suggests that immune tolerance may be established through the regulation of activated T cells.Exosomes have been shown to have an immunosuppressive activity that can down-regulate T cells resulting in decreased severity of the disease[16].Although the mechanism is not fully understood,the transfer of intestinal exosomes from healthy mice into those with IBD has been shown to decrease the severity of IBD[16].On the other hand,the transfer of exosomes from IBD patients into human colonocytes cell line DLD-1 has been shown to increase the expression of inflammatory markers such as interleukin (IL)-8 within the cell line[32].Another way that immune tolerance may be established is through the suppression of macrophages.The activation of macrophages has been suggested to play a critical role in the pathogenesis of IBD[33].Exosomes have been shown to play a role in intercellular communication and can thus modulate the macrophage-mediated immune response[34].Inducing acute colitis in mice results in the exosomal expression of 56 proteins to change when compared to healthy controls[35].Most of the proteins that are expressed are acute phase immunoglobulins and proteins which participate in the coagulation cascade and the complement pathway[36].As such exosomes represent excellent targets for blocking the activation of macrophages.
Exosomes in hepatic fibrosis and cirrhosis
Chronic liver injury and inflammation result in the production of insoluble collagen fibers and extracellular matrix components resulting in fibrosis and eventually cirrhosis.Exosomes have been shown to participate in this process and thus can serve as potential biomarkers and therapeutic vectors[37].The gold standard for diagnosis of liver fibrosis and cirrhosis is a biopsy.This is an invasive and expensive procedure the carries the risk of infection,internal bleeding,and injury to the biliary tract.Early research in exosomes shows promise in detecting fibrosis and cirrhosis.Exosomes collected from the serum of a patient with chronic hepatitis C (HCV) have been associated with inflammation and severity of fibrosis when enriched with CD81[38].Human patients with F3-4 fibrosis were also noticed to have decreased levels of miR-34c,miR-151-3p,miR-483-5p,or miR-532-5p in their serum exosomes.These same miRNAs were also decreased in the serum exosomes of mice with carbon tetrachloride-induced liver fibrosis[39].Although promising,more research is needed before exosomes can be used in a clinical setting.However,the potential of exosomes and the ease in which they can be collected has the promise of reducing invasive procedures in the future for diagnosing fibrosis or cirrhosis.
Exosomes in acute and chronic pancreatitis
Emerging research shows that exosomes play a role in pancreatic diseases including acute pancreatitis,chronic pancreatitis,and pancreatic cancer.In acute pancreatitis exosomes have been shown to participate in pulmonary dysfunction by penetrating the alveolar endothelial barrier and activating macrophages by changing their phenotype from M2 to M1 resulting in injury to the lungs[40].In pancreatic cancer exosomes derived from pancreatic ductal adenocarcinoma are internalized by Kupffer cells and cause secretion of transforming growth factor (TGF)-β.This results in increased fibronectin production by hepatic stellate cells resulting in activation of fibrotic pathways and proinflammatory milieu to facilitate tumor cell metastasis[41].Exosomes have been shown to participate in complex signal pathways and such have a potential to serve as novel biomarkers when it comes to pancreatic diseases.In the case of pancreatic cancer,the first presentation are the clinical symptoms and the workup to confirm the diagnosis is costly and invasive in the form to computed tomography scan,endoscopic ultrasound,and biopsy.The current biomarker for pancreatic cancer is carbohydrate antigen 19-9 (CA19-9) which has a sensitivity and specificity of 79%-81% and 82%-90% respectively[42].However,this is only the case in which patients are suspected to have symptoms of pancreatic cancer.When it comes to its function as a screening maker it has a low positive predictive value (0.5%-0.9%)[42].As a result of this,better biomarker is necessary,and exosomes have the potential to fill this role.
Most of the research concerning the use of exosomes as biomarkers is in its early stages and more rigorous clinical studies are needed before they play a role in the clinical arena.However,two candidates merit further attention.One is the combination of proteoglycan glypican-1 and circulating exosomes.These exosomal markers are found to be significantly elevated in patients with pancreatic ductal adenocarcinoma when compared to healthy control[43].Furthermore,these markers could discriminate between patients with each stage of pancreatic cancer (carcinomain situ,stage I as well as stages II-IV) from patients with benign pancreatic disease with a 100% sensitivity and specificity[43].Second is a recent study which compared exosomal proteoglycan glypican-1 and CA19-9 to high exosomal levels of miR-10b,miR-21,miR-30c,and miR-181a and low miR-let7a.This study found the exosomal miRNAs mentioned could not only differentiate pancreatic ductal adenocarcinoma patients from healthy controls,but also those with chronic pancreatitis.Furthermore,these exosomal levels decreased to normal values 24 h following resection whereas the levels of proteoglycan glypican-1 remained elevated[44].Exosomes can serve as a frontier for much needed novel biomarkers in the arena of pancreatic diseases and the early research have been promising.With further studies these markers can soon have a role to play in the clinical setting as well.
Exosomes in gastritis
Chronic gastritis occurs when the stomach lining becomes inflamed.This can be due to various factors including helicobacter pylori,ethanol use,autoimmune diseases,chronic stress,or certain medication.Currently this disease is diagnosed clinically or with the aid of endoscopy.Untreated chronic atrophic gastritis has the potential for evolving into gastric cancer.Exosomes have been suggested as novel biomarkers for the detection of chronic gastritis.Currently more research is needed in this field,but early studies suggest that exosomes play a role in the development of chronic gastritis.In one study,exomes from chronic gastritis patients withHelicobacter pylori(H.pylori)infection were shown to up regulate the expression of a proinflammatory cytokine (IL-1α)viaIL-6 trans-signaling[45].The proinflammatory effect that results due to the influence of IL-1α is a known initiator of inflammation seen in human diseases[46].In another study,the exosomal miRNA of patients with chronic atrophic gastritis was compared to those with chronic non-atrophic gastritis.The results suggested that hsamiR-122-5p is significantly upregulated compared to other components and has the potential to serve as a biomarker for chronic atrophic gastritis[47].Exosomes also have been shown to play a role in the protective effect seen withH.pyloriinfection in inflammatory bowel disease.Exosomes derived fromH.pyloriinfected patient were shown to promote NLRP12 expressionin vitrohuman intestinal epithelial cells.NLRP12 was then shown to inhibit the Notch signaling pathway resulting in a decrease in chemokine MCP-1 and MIP-1α.These exosomes where then injected into dextran sulfate sodium induced colitis mice which had an improvement in colitis symptoms associated with an increase in NLRP12[48].Currently the studies looking at the relationship between exosomes and chronic gastritis are ongoing.Early data shows that exosomes do play a role a role in gastritis,but further studies are needed to figure out the nature of this interaction.
Exosomes in viral hepatitis
Exosomes have been known to participate in complex cellular communication.One way in which they accomplish this is by transfer genetic material between cells.This normal process,however,can also be hijacked by viruses to promote proliferation and avoid immune response.In a study by Linet al[49]the exosomes found within the serum of patients with chronic hepatitis B (HBV) infection contained HBV proteins and nucleic acids.Furthermore,these components were transferred to hepatocytes in an active manner.Natural killer cells from healthy donors and those with chronic HBV infection were shown to interact with and uptake exosomes containing HBV nucleic acids[49].These exosomes then impaired natural killer cells resulting in dysfunction[49].Similarly,patients infected with HCV were also found to have HCV viral RNA and proteins within their exosomes[50].Exosomes that contained HCV were shown to increase the infectivity of HCV compared to exosomes free of HCV[51].Also,exosomes helped HCV escape immune response up upregulating Gal-9 which is associated with T-cell inhibition[52].Due to the role exosomes play in propagating infection by HBV and HCV,they also have the potential to serve as novel therapeutic targets.More research is needed to further understand the nature of exosome use by viruses and development of therapies.
ROLE OF EXOSOME IN CANCEROUS GASTROINTESTINAL DISEASES
Exosomes in CRC
Emerging evidence is beginning to show the potential for exosomes in the disease process of CRC.Due to its resistance to treatment and metastatic properties,CRC is the third leading cause of cancer mortality worldwide[53].CRC has a high prevalence in both men and women and carries a low 5-year survival,especially when it has progressed to stage IV[54].Of those newly diagnosed with CRC,approximately 25%have distant metastases at the time of presentation[55,56].Furthermore,an additional 25% will develop distant metastases two years after the initial diagnosis.The prevalence of metastasis observed in CRC serves as a major challenge in its treatment.CRC is known to preferentially metastasize to the liver and a lesser extent the brain,bones,and lungs[57,58].Exosomes from cancer cells have been shown to preferentially fuse at predicted sites of their metastasis[59].The unique integrin expression pattern is thought to be the cause of this observed effect[59].As exosomes likely play a role in the metastasis of CRC;they have the potential to serve as targets for therapeutic interventions.Exosomes also have the potential to serve as novel biomarkers for detecting and monitoring the progression of the disease.
Cancers of different types are known to be associated with their specific exosomal membrane and cytosolic proteins.These proteins can serve as potential biomarkers for detecting CRC in patients.In the exosomes of a patient with CRC different proteins are upregulated when compared to healthy controls[60].An interaction between FN1 and matrix metalloproteinase 9,both of which are upregulated in patients with CRC,has been shown to cause changes in tumor microenvironment favoring progression and metastasis by integrin signaling and cytoskeletal reorganization[60].Another potential biomarker for the diagnosis of CRC is a heat shock protein 60 (Hsp60).This protein is known to play a pivotal role in tumorigenesis and is upregulated in exosomes of patients with CRC.Hsp60 can also be used to monitor response to treatment as its level return to normal after resection of CRC tumor[61].
Exosomes also carry miRNAs which are known to play an important role in the progression of CRC.The expression of these molecules differs in CRC when compared to healthy controls.As such,miRNAs can serve as reliable biomarkers for the diagnosis and staging of CRC.Seven of these molecules (let-7a,miR-21,miR-23a,miR-150,miR-223,miR-1229,and miR-1246) are known to be upregulated even in the early stages of CRC.These markers can also be used to monitor treatment response,as their levels in the serum drop after tumor resection[62].Another potential biomarker that can help in the staging of CRC is miR-193a.Higher levels of circulating miR-193a are associated with advanced stages of CRC,whereas lower levels are seen in the early stages and healthy controls[63].In addition,miRNAs can be used to monitor for the recurrence of CRC as higher levels of miR-17-92a after therapy correlates with recurrence.The difference in expression of miRNA can also be used as a prognostic tool,as a higher expression of miRNA-19a is associated with a poorer prognosis when compared to those with low expression[64].
Through their role in altering the tumor microenvironment,exosomes have been shown to impact tumor initiation,progression,chemoresistance,and even metastasis.The precise mechanism by which they accomplish this has not been delineated.As such,more research is needed before exosomes can play a central role as novel biomarkers.Although the use of exosomes as biomarker show promise in research done on cell lines,animal models,and human body fluids;large randomized clinical trials are still needed for validation.Moreover,further studies comparing the existing modalities and exosomes are still needed to show if exosomal biomarkers allow for earlier detection of CRC.
Research focusing on strengthening host immunity to combat CRCviaimmunotherapy shows promise.Dendritic cells (DCs) are potent antigen-presenting cells that play an important role in regulating both the adaptive and innate immune responses[65].Dendritic cell-derived exosomes (Dex) are exosomes that are secreted by DCs and have been shown to elicit a strong immune response against cancer cells[50].This response is due to an abundance of immunostimulatory membrane proteins being present on the surface of Dex (integrins,CD81,CD63,MHC class I and II)[66,67].In mouse models,Dex loaded with tumor peptides can invoke a stronger immune response in cytotoxic T cells than even tumor peptide loaded DCs themselves[68].Exosomes produced by cancer cells inhibit the proliferation and cytotoxic effects of natural killer cells[69].Furthermore,they carry the Fas ligand on their surface to induce T cell apoptosis[70].In contrast,exosomes produced by DCs activate anti-tumor immunity and cause tumor cell lysis[71,72].Dex loaded with tumor antigens have already been tested in clinical trials and were found to be safe and effective in activating antitumor immunity in the cases of melanoma and non-small cell lung cancer[73,74].While the use of Dex in the treatment of CRC remains to be further studied,phase I clinical trials have already begun.In the cases of advanced CRC,the combination of ascites-derived exosomes and granulocyte-macrophage colony-stimulating factor has been shown to induce the activation of antitumor cytotoxic T cells.Treatment with this immunotherapy was not only safe but also prolonged the survival of patients with CRC in the metastatic stage of the disease[75].While the use of Dex in the treatment of CRC shows great promise,its success has only been observed in a few small trials.Large randomized clinical trials are still needed before Dex can play a role in the clinical arena.Furthermore,research is still needed to modify Dex to elicit a stronger immune response from innate host immunity.
Exosomes play an important role in the progression of CRC and thus serve as excellent targets for therapeutic interventions.They have the potential to serve as delivery vectors for chemotherapeutic agents.Since exosomes have a slower rate of clearance from the bloodstream,they are superior to existing modalities such as liposomes.They also boast the advantage of a greater degree of specificity for CRC cells.Exosomes can also serve to enhance host immunity against cancer cells as shown by dendritic cell-derived exosomes.They could also be used to enhance chemosensitivity in cancer cells through the use of miRNA,increasing the efficacy of chemotherapeutic agents.While large clinical trials and translational studies are still needed to validate the use of exosomes in a clinical setting;they represent promising therapeutic opportunities in the fight against CRC.
Exosomes in hepatocellular carcinoma
The incidence of hepatocellular carcinoma (HCC),one of the most frequently diagnosed cancer,is increasing worldwide[76-78].Increased technological development and the availability of novel screening methods are likely contributing factors;but despite advancements in screening and prevention mortality rates continue to increase[79,80].Current screening and diagnostic tools are limited to abdominal ultrasound,computed tomography scans,magnetic resonance imagines,liver biopsies,and surveillance by alpha-fetoprotein (AFP) levels[81].Although AFP levels > 400 ng/mL are highly correlated with HCC,only 30% of patients with HCC present with elevated AFP levels[81].As a result,early-stage tumors are often missed causing patients to present with advanced stages of the diseases;less than 30% of these cases are amenable to surgical intervention[82-85].As such,more sensitive methods are needed to detect these tumors.Exosomes have the potential to not only offer new tools for detection and surveillance but also open new avenues in the treatment of HCC.
The intercellular communicationviaexosomes has been linked with helping cancer cells create a microenvironment that serves to induce angiogenesis,avoid host cell immune response,interact with the extracellular matrix,create a pre-metastatic niche,and even develop resistance to chemotherapeutic agents[86-91].As such,the information contained within exosomes reflects the real-time status of cancer cells.Analysis of this data can help in cancer detection,determining the stage of cancer,its recurrence,and response to chemotherapy.This information presents itself in the form of miR or protein biomarkers that have been concentrated within the exosomes which would otherwise go undetected in serum[92].
miRNA expression varies based on conditions and diseases affecting hepatocytes.Patients with HCC have higher exosomal levels of miR-18a,miR-221,miR-222,and miR224 when compared with those who have chronic hepatitis B or cirrhosis without HCC[75].A strong correlation was also noticed between miR-21 and patients with chronic HBV[92].A subsequent study confirmed that high levels of miR-21 were correlated with the advanced tumor stage.Patients with HCC had higher levels of miR-21 when compared to those with chronic HBV or healthy volunteers[93].Certain miRs (939,595,and 519d) have also been shown to be superior to AFP as a diagnostic biomarker based on receiver operating characteristic curve analysis[94].
In a separate study comparing patients with chronic HCVvsthose with HCC secondary to HCV,patients with HCC had significantly lower miR-16 Levels.Moreover,miR-16 could be used to discriminate HCC from chronic HCV with a sensitivity of 57.5% and specificity of 70%.When miR-16 was combined with AFP,the sensitivity of the test improved to 85%,and the diagnostic accuracy improved to 87.5%.Additional associations were also noticed between miR-16 and the number of HCC tumors and their size[95].In rat models,hepatocytes were converted into HCC cellsviadiethylnitrosamine.Variation in miR-10b,miR-21,miR-122,and miR-200a expression gradually began to appear with the development of liver disease and achieved the greatest change as cells transitioned to cirrhosis and HCC.In comparison,AFP levels remained unchanged until the early stages of HCC[96].
These studies demonstrate that exosomes directed research continues to progress in the area of primary liver disease.Current trends indicated that exosomes may have a role to play in the surveillance and diagnosis of HCC in the future clinical setting.However,further studies are needed to find reliable exosomal biomarkers and more work is needed in developing technologies to extract and accurately test the samples obtained.
Although the mechanism of resistance is not yet fully understood,HCC is known to be highly resistant to chemotherapy.Mounting evidence is showing that exosomes facilitate intercellular communications between HCC cells allowing them to develop resistance to chemotherapeutic agents.One study demonstrated that treatment with TGF-β and sorafenib caused increased expression of long-noncoding RNA within exosomes.Long intergenic non-protein coding RNA,regulator of reprogramming was then shown to enhance the expression of CD133+ tumor-initiating cells resulting in chemoresistance[97].Another study demonstrated exosomal lincRNA-VLDLR not only promoted cell cycle progression in cancer cells,but also helped to develop resistance by expression of ABCG2 transporter which actively exported chemotherapeutic agents such as sorafenib,camptothecin,and doxorubicin out of the cell[98].Anin vitromodel demonstrated that exosomes derived from patients with HCC activated the HGF/c-Met/Akt signaling pathway inducing resistance to sorafenibviainhibition of sorafenib-induced apoptosis[99].
Exosomes present a novel avenue to explore in the treatment of HCC,as they serve an integral part in cancer cell communication.Vps4A is a protein that weakens cell response to exosomes and thus shows potential as a therapeutic agent for blocking said communication.This molecule is frequently downregulated in HCC and its expression deactivates the PI3K/AKT pathway resulting in decreased growth,migration,and invasion by HCC cellsin vitro[100].Exosomes naturally accumulate in the liver after intravenous delivery.This makes them an excellent vehicle for drug delivery;they are also stable,biocompatible,and resistant to proteases or RNases[101].MiR-355 is a molecule that has shown to have both anti-fibrotic and anti-neoplastic capabilities.In one study,the hepatic stellate cell was used to generate and deliver exosomes containing miR-335 to HCC cells.When this treatment was administered to HCC cells in vitro,inhibition of cell proliferation,and invasion was noted.When tumor-bearing mice were treated,tumor shrinkage was observed[102].
A potential therapeutic option for HCC might lie in strengthening host immunity.Exosomes are efficiently taken up by dendritic cells and can participate in antigen presentation to tumor-specific cytotoxic T cells.In mice exosomes from HCC cells have been used to confirm cross-protection against various tumors with shared antigens independent of the MHC molecules of the donors[103].In a separate study,a tumorsuppressive effect was noticed when exosomes derived from HCC cells expressed CD9 and CD63 surface markers.These exosome markers are known to induce the cytotoxic effects of dendritic and NK cells.The activation of this cell-mediated immunity was likely the cause of the observed effect[102].In a separate study,a significant decrease in tumor growth and prolonged survival rates were observed;when mice with HCC were exposed to exosomes obtained from AFP-expressing dendritic cells.These exosomes stimulated CD8+ T cells,activated interferon gamma,IL-2,IL-10,TGF-β,and inhibited CD25+,Foxp3+,regulatory T cells resulting in a strong antigen-specific immune response.The same response was observed with exosomes obtained from AFP-expressing bone marrow stromal cells[104].
Lastly,exosomes may also have the potential to increase chemosensitivity in cancer cells resulting in increased efficacy of chemotherapeutic agents.MiR-122 has been shown to promote chemosensitivity in HCC cells by down-regulating IGF1-R,metalloprotease 10,and cyclin G1[105].When HCC cells were treated with miR-122 containing exosomes,they displayed increased sensitivity to sorafenib and 5 fluorouracil[106].Despite the potential of exosomes,more research is needed before they can play a role in a clinical setting.One significant flaw of exosomes lies in the ability to produce exosomes that contain therapeutic siRNA and active drugs.Preliminary research in the field shows promise,but more work is still needed.Mesenchymal stem cells derived exosomes have been used to pack and deliver therapeutic siRNA and active drugs such as paclitaxel and deserve further investigation[107].
Exosomes in gastric cancer
Gastric cancer (GC) is associated with poor prognosis despite all the recent advancements in chemotherapy,surgical techniques,and radiotherapy[108].This in part is due to GC presenting in later stages of the disease due to its relatively asymptomatic nature early in its course.Exosomes have been shown to participate in the progression of GC and as such have the potential to serve as novel biomarkers resulting in early diagnosis and increased long-term survival of patients.Examples include exosomes derived from GC cells which have been shown to promote proliferation in an autocrine manner by activation of PI3K/Akt and MAPK/ERK pathways[93].GC cell exosomes have also been shown to increase proliferation and invasion in an exosomedependent manner by activation of the MAPK signaling pathwayviaCD 97[109,110].
Potential GC biomarker targets include the BARHL2 gene found within the exosomes in gastric juice.By looking at the methylation level of the gene,GC patients can be discriminated from non-GC control with a sensitivity of 90% and a specificity of 100%.However,this study only contained 20 GC patients and 10 non-GC control and further studies with a larger sample are needed[96].Other promising biomarkers include exosomal proteins such as GKN1,TGF-β1,and TRIM3.GNK1 Levels are lower in healthy controls when compared to a patient with GC[111].Increased exosomal TGFβ1 Levels are associated with the advanced stage of GC and increased TRIM3 Levels are associated with GC when compared to healthy controls[112-115].Other potential biomarkers include exosomal miRNA which has also been shown to not only have diagnostic,but prognostic value as well.Elevated miR-19b-3p and miR-106a-5p levels in serum exosomes are more sensitive and specific for GC than AFP and CA-199[116].High levels of miR-423-5p in serum exosomes have been correlated with lymph node metastasis and poor prognosis[117].Post-surgery higher miR-451 Levels predict a significantly poorer prognosis than lower level[118].Lastly,exosomal lncRNA could also serve as promising new biomarkers.Exosomes found in the plasma of stage I GC patients have significantly upregulated lncUEGC1 when compared to healthy controls.Plasma exosomal lncUEGC1 had a better diagnostic value than CEA in distinguishing stage I GC patients from healthy controls[49].Overall exosomes show great potential as diagnostic and prognostic biomarkers for GC.However,further research is still needed to test these biomarkers in larger clinical trials to validate preliminary observations.
Exosomes in pancreatic cancer
Globally,pancreatic cancer remained one of the most aggressive malignancy with 5-year relative survival rate as low as 40% in patients with localized disease and 3% in patients with distant metastasis[119].The poor prognosis is attributed to factors such as lack of early diagnosis,high risk of lymphovascular invasion leading to early distant metastasis,and sub-optimal response to traditional therapies[107].Exosomes have been identified to play a key role in cellular communication,signaling,and transportation.Due to these important properties,exosomes can serve as potential biomarkers for a better understanding of the tumor behavior and potential for metastasis.A recent study published by Queet al[120]highlights the use of pancreatic adenocarcinoma-relate miRNAs (miR-21,miR-155,miR-17-5p,and miR-196a) as potential biomarkers in 49 patients.Compared to controls,patients with advanced or unresectable pancreatic adenocarcinoma were found to have to higher expression of miR-17-5p and miR-21[108].However,for localized pancreatic canceri.e.stages I and IIA plasma microsomal exosome miR-196a is found to be elevated in pancreatic ductal adenocarcinoma,and miR-1246 is significantly elevated in patients with intraductal papillary mucinous neoplasms[121].Certain exosomal proteins are also found to be elevated in a patient with pancreatic cancer.A study published by Meloet al[122]indicated that exosomes in a patient with pancreatic cancer are enriched in glypical-1 and the levels were significantly elevated in the cancer group than in controls with sensitivity and specificity of 100%.Other exosomes that were found to be expressed by the metastatic pancreatic cell to liver and lung are macrophage migration inhibitory factor and exosomal integrins such as α6β4 and α6β1[41,43].In addition,exosomes have a role in the management of pancreatic cancer due to their unique biochemical structure and protein arrangement which enable them to act either as potential therapeutic targets or as nanoparticle carriers to transport proteins and drugs across cell membranes[123].
Exosomes in the cholangiocarcinoma
Cholangiocarcinoma (CCA) is a rare but aggressive malignancy involving the biliary tree.Globally,CCA accounts for 10%-25% of the primary liver cancers which constitute second most common cancer after HCC[124].Though CCA is more prevalent in Asian countries but in recent years the incidence is even rising in Western world.CCA arises from biliary epithelium and classified as intra-hepatic and extra-hepatic(peri-hilar and distal) subtypes based on location[125].Chronic inflammatory diseases such as primary sclerosing cholangitis,chronic cholestasis and infections are the common risk factors for CCA.However,there are significant number of cases arising in the absence of known risk factors.The cancer carries poor prognosis and so far,surgical resection is the only curative treatment available with chemoradiation therapy for locally advanced and distantly metastatic cancers[126].
There is substantial evidence that exosomes play important role in the progression of the CCA through regulating tumor microenvironment[127].CCA microenvironment consists of dense stroma enriched with immune cells and extracellular matrix.Exosomes derived from CCA modulates tumor microenvironment and promotes formation of the tumor stroma by regulating the expression of α-smooth muscle actin mRNA,differentiation of fibroblasts particularly cancer-associated fibroblasts (CAFs)and interleukin-6 by interacting with mesenchymal stem cells[128].CAFs are known to promote tumorigenesis by producing and releasing tumor growth factors like fibroblast growth factors and vascular endothelial growth factor.There is increasing evidence that CCA-derived exosomes are involved in cell-cell communication and transfer of the oncogenic proteins.Duttaet al[129]studied the protein profiles of CCAderived exosomes and their potential roles in CCA progression.In this study,CCA derived exosomes named KKU-M213 and KKU-100 were incubated with normal human cholangiocyte cellsi.e.H69 and proteomic analysis was performed.These isolated exosomes were found to be internalized into H69 cells,leading to migration and invasions of these cells by increasing the expression β-catenin and reducing the expression of E-cadherin.In addition,they are involved in metastasis by inhibiting the function of tumor suppressing genes such as p53 and chemoresistance.Furthermore,by inhibiting cytokine-induced killer (CIK) cells exosomes enables tumor cells for immune escape[130].Like the proteins in exosomes,non-coding RNA such as miRs and circular RNAs are found to be involved in the progression of CCA.Particularly,miR-205-5p and circ-0000284 Levels were associated with invasion and migration of CCA cells in several studies[131,132].
Therapeutic role of exosomes in the management of CCA has been studied well in human and animal models both as a primary target to inhibit tumor progression and drug delivery.Chenet al[133]explored the impact of tumor derived exosomes on the antitumor activities of CIK cells.The results of the study showed that by down regulating the population of CD3+,CD8+,NK (CD56+),CD3+CD56+cells and decreasing secretion of TNF-α and perforin,RBA derived exosomes inhibit the antitumor activity of CIK cells.In another study conducted by Wang and coauthors,exosomal circ-0000284 found to be a therapeutic target for CCA[134].
CONCLUSION
Exosomal composition changes in response to the state of the cells from which they are released.They provide a view of the physio-pathological process occurring within cells in real-time.Current research indicates that exosomes play a role in modulating gene expression and cell function.They are also readily available in blood,urine,and saliva marking them as promising candidates as a novel biomarker in the detection of IBD and cancer.An increasing number of studies are expanding our understanding of the potential of exosomes in diagnostic testing.However,a standard method to isolate,analyze,and track exosomes has yet to be developed limiting their application in a clinical setting.Tumorigenesis is a complex process and while certain molecules show promise,further research is still needed to identify biomarkers unique to cancerderived exosomes.These new biomarkers then need to be tested against current detection and surveillance techniques in large randomized clinical trials.Exosomes have also been shown to play a role in managing the tumor microenvironment,helping cancer cells grow,metastasize,and avoid host immune response.They have also been shown to play a role in the development of cancers such as HCC and CRC.Given the key role they play in the propagation of cancer,they have the potential to serve as novel therapeutic vectors.The biocompatibility of exosomes makes them excellent biocarriers.Many different cell types have been used to engineer exosomes for delivering therapeutic cargos as a means for targeted therapy.The clinical application of current models,however,is limited by inefficiency.As research in the field expands,more efficient methods for generating exosomes will emerge,allowing their transition into the clinical arena.Exosomes carry miRNA and proteins that have been shown to have both a negative and positive effect on cancer cells.These molecules have been shown to limit cancer growth,increase sensitivity to chemotherapy,and activate the immune response resulting in tumor lysis.On the other hand,miRNA and exosomal proteins have also been linked to cancer growth,propagation,and metastasis.Different tumors have different characteristics and likewise,the effect of exosomal cargo on them is equally varied.Further research is needed to characterize these effects so appropriate therapeutic vectors can be selected.Cancer is an adaptive process and more innovation is needed before exosomes can be used for cancer therapy.However,early studies show considerable promise and with further research,exosomes may yet play an increasing role in a clinical setting.
 World Journal of Meta-Analysis2021年1期
World Journal of Meta-Analysis2021年1期
- World Journal of Meta-Analysis的其它文章
- Inflammatory pseudotumor-like follicular dendritic cell sarcoma:Literature review of 67 cases
- Biofat grafts as an orthobiologic tool in osteoarthritis:An update and classification proposal
- Non-invasive diagnosis of Crohn’s disease:All that glitters is not gold
- Risk factors,manifestations,diagnosis and treatment of cholelithiasis in children
- Mortality of critical care interventions in the COVID-19:A systematic review
- Efficacy and safety outcomes with remdesivir in COVID-19 patients:A meta-analysis
