Association between estradiol levels and clinical outcomes of IVF cycles with single blastocyst embryo transfer
Arie A.Polim,Nining Handayani,Tri Aprilliana,Roza Silvia,Batara Sirait,5,Arief Boediono,6,Ivan Sini
1Morula IVF Jakarta Clinic,Jakarta,Indonesia
2IRSI Research and Training Centre,Jakarta,Indonesia
3Department of Obstetrics and Gynecology,School of Medicine and Health Sciences,Atmajaya Catholic University of Indonesia,Jakarta,Indonesia
4Morula IVF Padang Clinic,Padang,Indonesia
5Department of Obstetrics and Gynecology,Faculty of Medicine,Universitas Kristen Indonesia,Jakarta,Indonesia
6Department of Anatomy,Physiology and Pharmacology,IPB University,Bogor,Indonesia
ABSTRACT
KEYWORDS:In-vitro fertilization; Estradiol; IVF outcomes;Single blastocyst transfer
1.Introduction
In the ovary,estradiol 17-β(E)is a predominant natural potent form of estrogen,which is synthesized through the aromatization of androstenedione upon stimulation by a follicle-stimulating hormone in the granulosa cells.The prominent function of estradiol is to regulate the secretion of gonadotropin through a feedback mechanism.It also plays a pivotal role in folliculogenesis,primarily during the post antral follicular stage[1].In the proliferative and secretory phase of endometrium,both estradiol and progesterone act as essential regulators of endometrial receptivity[2].Estrogen induces the proliferation of functional epithelial layers and the expression of progesterone receptors.Through these receptors,progesterone then initiates the conformational and biochemical changes of the epithelium to begin opening the window of implantation during the endometrium mid-secretory phase[3,4].
In an in-vitro fertilization(IVF)cycle,a controlled ovarian stimulation treatment stimulates the simultaneous development of multiple follicles in the ovary causing an unavoidable elevation of estradiol.Compared to a natural cycle,the estradiol level during controlled ovarian stimulation might be increased by more than ten-fold[5],which resulted in supra-physiologic conditions.Such conditions have raised concern about the plausible effects that elevated estradiol levels have on endometrial receptivity and failed embryo implantation[6].
In attempt to gain a comprehensive understanding of this issue,several molecular investigations and observational studies of IVF practice data have long been conducted.Recently,an analysis of protein profile using Ishikawa cells as an ideal proxy for the invitro endometrial study revealed that a dose-dependent increase of estradiol-induced the expression of different proteins[7].However,despite the advancements in the molecular understandings of the deleterious effects of high estradiol concentration,the relationship between elevated estradiol levels and IVF clinical outcomes produces mixed findings[8-12].Inconsistent findings across several studies have been a matter of debate for over two decades yet a majority of the clinical evidence has relatively shown that the elevation of serum estradiol did not impact the clinical outcomes of IVF[8-10,13,14].
However,as well-discussed by the latest systematic review and meta-analysis conducted by Karatasiou et al in 2020[15],one of the main limitations of examining the correlation between levels of estradiol and clinical pregnancy is the discrepancy in the number[8,16]and developmental stage[14,17]of the transferred embryo.This study aimed to clarify the correlation between the different serum estradiol levels and IVF outcomes.Only IVF patients who underwent a fresh single embryo transfer at the blastocyst stage were recruited for this study to ensure an equal probability of pregnancy throughout the different estradiol level study groups.
2.Materials and methods
2.1.Study population
This study was a retrospective observational design that employed couples who underwent an IVF program with antagonist protocol in Morula IVF Jakarta clinic from January 2016 to December 2018.To reduce confounding factors and guarantee a comparable probability of achieving pregnancy,the inclusion criterion for this study was women who underwent a single fresh embryo transfer of top-quality blastocyst.Patients with uterine pathology(such as myomas,polyp,and adenomyosis),freeze-all cycle,mini-stimulation,and natural cycle were excluded.A total of 542 subjects had met the eligible criteria.The median age of women included in this study was 33 years old,ranging from 22 to 45 years old.The median BMI was 22.80 kg/mwith a range of 14.88 kg/mto 41.75 kg/m.Of all subjects,86.7%(470/542)were diagnosed with primary infertility.The primary causes of infertility were associated with male factors(31.0%)followed by unexplained factors(30.6%),and tubal factors(22.9%).Top-quality blastocysts were assessed according to Gardner Criteria(degree of blastocoel expansion:3-5,the combination of inner cell mass and trophoblast score:AA,AB,BA)[18].To investigate the effects of estradiol dynamic levels on ovulation trigger day,the patients were sorted into 4 groups,i.e.group 1 [E:<2 000 pg/mL,(n=190)],group 2 [E:2 000-2 999 pg/mL,(n=209]],group 3 [E:3 000-3 999 pg/mL,(n=94)],and group 4 [E:≥ 4 000 pg/mL,(n=49)].The primary objective of this study was to evaluate the effects of different estradiol levels on clinical pregnancy and miscarriage rates.The secondary objective was to assess the IVF laboratory outcomes in association with the serum estradiol levels which included the number of retrieved,mature,and fertilized oocytes,number of obtained embryos and number of top-quality embryos at cleavage and blastocyst stage.Complete indexes of subject baseline(age,BMI,type of infertility,infertility causes,infertility duration)and clinical characteristics(hormonal basal levels,hormonal levels on the trigger day,and ovarian stimulation indexes)of each group were presented in Table 1 to provide a comprehensive summary about the studied subject and cycle characteristics.
项目组对调查河段(图1)进行了4次采样,分别为2014年10月(秋季)、2015年1月(冬季)、2015年5月(春季)和2015年7月(夏季)。具体采样站位信息如表1所示。
2.2.Ovarian stimulation
All research participants were subjected to controlled ovarian stimulation using antagonist protocol and different forms of gonadotropin products.Administration of gonadotropin [Gonal-F(Merck,Serono),Pergoveris(Merck,Serono),or Menophur(Ferring)]started on day 2/3 of the menstrual cycle.The starting dose was individualized based on the patient's characteristics [age,antral follicle count,anti-Müllerian hormone(AMH)level,and body mass index(BMI)]ranging from 150 IU to 375 IU.Ovarian stimulation response was evaluated after four daily primings of gonadotropin.Subsequent dose alterations were determined by estradiol assays and follicular growth assessments through ultrasonography.A dose of 0.25 mg of Cetrotide(Merck KGaA,Darmstadt,Germany)was primed on day 6/7 of the menstrual cycle.The ovulation trigger,using 6 500 IU of Ovidrel(Merck,Serono),was given when at least three follicles had reached a diameter of≥18 mm.On that day,serum estradiol and progesterone level were measured.Luteal support [vaginal progesterone:200 mg Utrogestan(Besins Healthcare,Belgium)/12 hours and 90 mg Crinone(Merck,Serono)/day]was administered after ovum pick-up until the human chorionic gonadotropin(hCG)test.In a case of biochemical pregnancy detection(hCG > 50 mIU/mL),patients continued to receive the supporting drugs until the 8th week of pregnancy.
2.3.Oocyte retrieval,sperm insemination,and embryo culture
Oocyte retrieval was performed 36 h after follicular maturation trigger injection utilizing transvaginal ultrasound guidance while patients were under sedation.Intracytoplasmic sperm injection or intracytoplasmic morphologically-selected sperm injection was performed after three hours of cumulus-oocytes complex incubation,followed by embryo culture in a triple gas incubator(37 ℃,5% O,6% CO)for 5/6 days.A single top-quality blastocyst was transferred into the uterus through trans-abdominal ultrasound guidance.
2.4.Study parameters
Clinical pregnancy was confirmed via ultrasonography or clinical documentation of at least one fetus with a discernible heartbeat.The clinical pregnancy rate was calculated as the number of successful clinical pregnancies divided by the number of subjects performing embryo transfer.Miscarriage was defined as the loss of pregnancy before 20 weeks of gestation.The miscarriage rate was calculated as the number of miscarriage events divided by the number of clinically pregnant subjects[19].
The number of oocytes retrieved was calculated as the median number of total oocytes collected per ovum pick-up procedure.The number of mature oocytes was calculated as the median number of metaphase Ⅱ oocytes that were viable for intracytoplasmic sperm injection or intracytoplasmic morphologically-selected sperm injection.The number of fertilized oocytes was calculated as the median number of fertilized zygotes after insemination,manifested by two pronuclear.The total number of cleavage(day 3)and blastocyst(day 5/6)stage embryos were calculated as the median number of acquired embryos at the respective developmental stages,regardless of the quality.The number of top-quality cleavage and blastocyst were calculated as the median number of good graded day-3-embryos at the cleavage stage and day-5/6-embryos at the blastocyst stage.
2.5.Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences version 25.0(SPSS,Inc.,Chicago,IL,USA).A minimum of 44 subjects per group was needed to have a statistical power of 90%.A non-parametric test was utilized due to the nonnormal data distribution.Multiple analyses using logistic regression were conducted to determine the modification effects of baseline and clinical characteristics variables on the clinical pregnancy and miscarriage outcomes.Confounders were then adjusted to compare the clinical pregnancy rates among the varying estradiol level groups.Beta coefficient adjusted odds ratio,95% confidence interval of the adjusted odds ratio and P-value were calculated.Statistical significance was considered when P-value was < 0.05.
2.6.Ethics statement
The study protocol was evaluated and approved by the Ethics Committee of the Faculty of Medicine University of Indonesia(Ethical approval number:KET-1324/UN2.F1/ETIK/PPM.00.02/2019).
3.Results
3.1.Baseline and clinical characteristics
Differences in baseline and clinical characteristics among the study groups that showed statistical significance were median age,basal follicle-stimulating hormone(FSH),basal luteinizing hormone(LH),progesterone level on the trigger day,AMH,antral follicle count,the starting dose of gonadotropin,stimulation duration,total gonadotropin usage,and suppression duration(Table 1).The highest median age was found in group 1(E<2 000 pg/mL),whereas the lowest age in group 3(E:3 000-3 999 pg/mL).Consistent with the highest median age,the highest median total of gonadotropin usage was also observed in group 1.
Almost three-fourths of the patients had a trigger day estradiol level of less than 3 000 pg/mL,which was relatively equally distributed in groups 1 and 2.Group 1 had the lowest median antral follicle count.In this study,antral follicle count was shown to have a positive correlation with the estradiol level.The lowest median basal LH level,trigger day progesterone level,AMH,and the highest basal FSH level were also observed in group 1.Other characteristics including BMI,infertility duration,types of infertility,infertility etiology,basal estradiol,basal progesterone,and endometrial thickness were comparable among the studied groups(Table 1).Overall mild ovarian hyper-stimulation syndrome rate of the study participants was 2.58%(14/542).
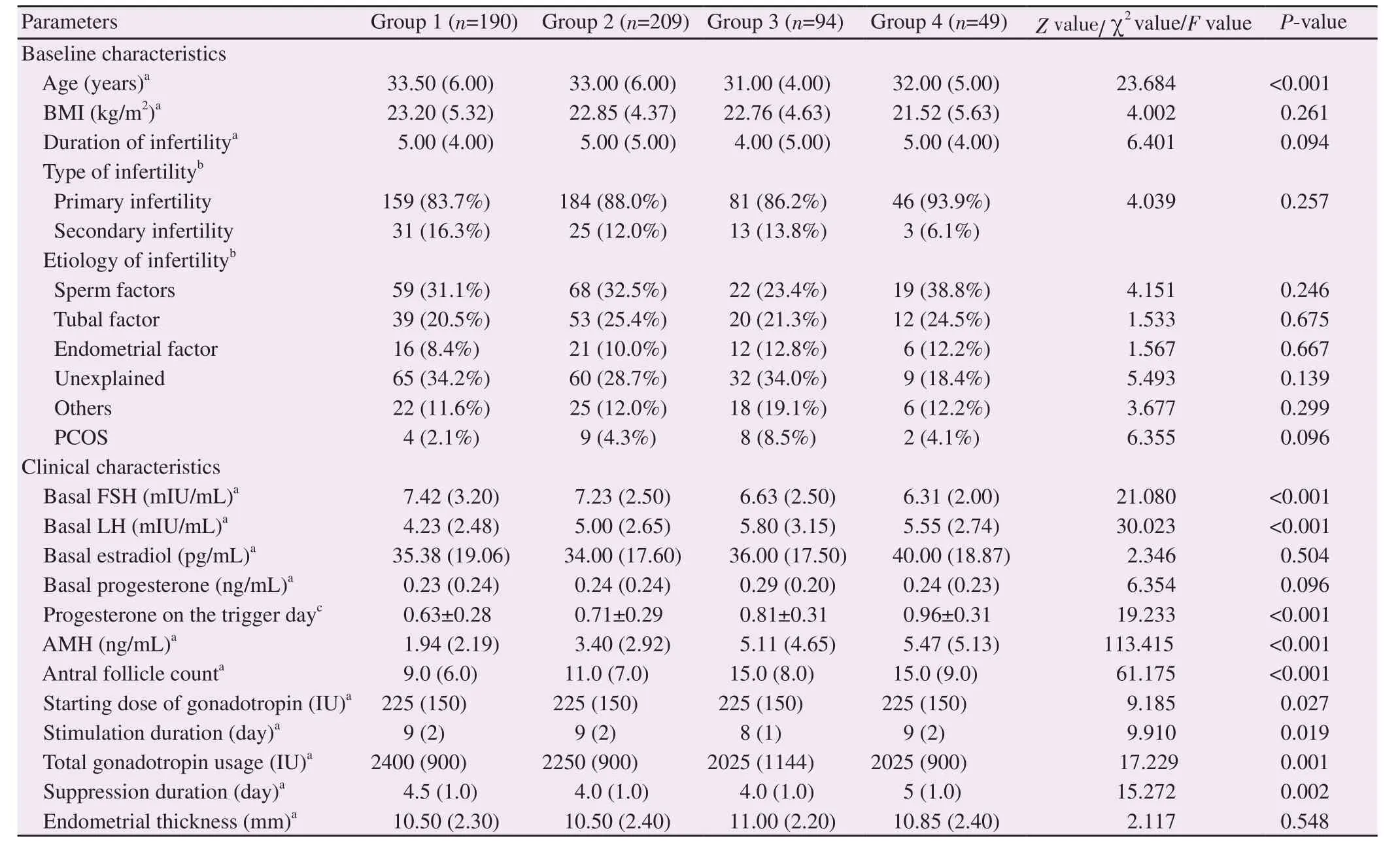
Table 1.Baseline and clinical characteristics of the study population.
3.2.Primary and secondary outcomes

Table 2.Clinical outcomes of different estradiol level groups [n(%)].
Irrespective of the estradiol levels,the clinical pregnancy rate in the overall studied population was 42.43%(230/542).Evaluation of the receiver operating characteristic curve(ROC curve)was performed to better apprehend the correlation between estradiol levels and pregnancy events.The analysis implied that the estradiol level on trigger day was less adequate to become a predictor of a pregnancy event [area under the curve(AUC)0.594,95% CI 0.55 - 0.64](Figure 1).
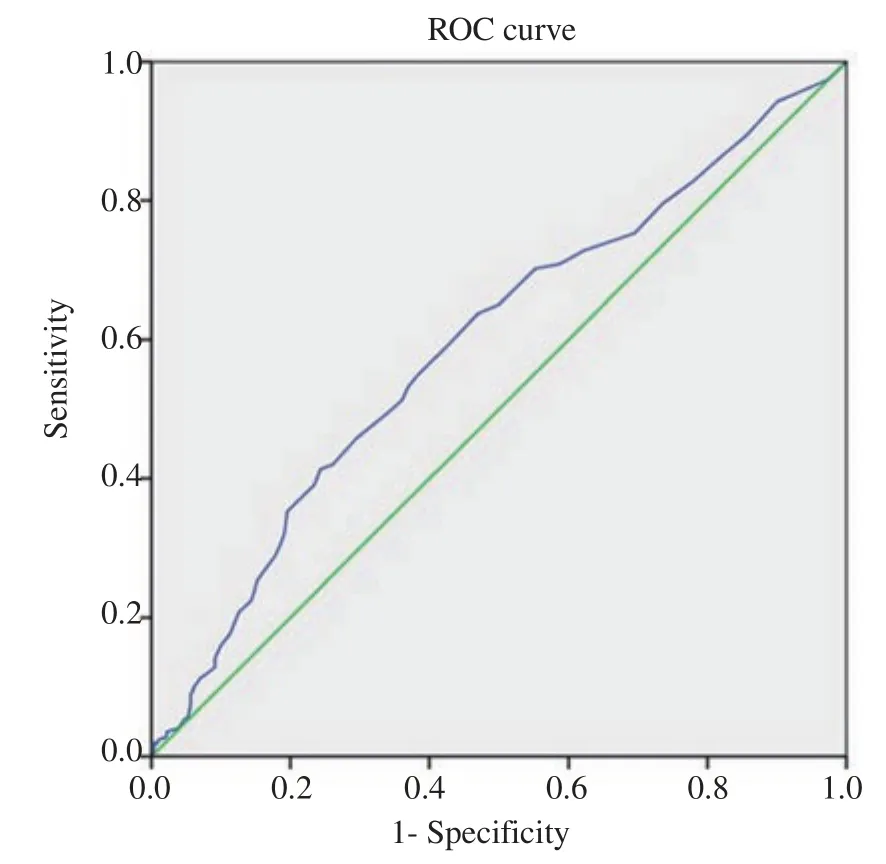
Figure 1.Receiver operating characteristic(ROC)curve obtained from analyzing the correlation between estradiol level and pregnancy event.Diagonal segments are produced by ties.
On the contrary,laboratory outcomes including the number of retrieved oocytes,mature oocytes,fertilized oocytes,total and topquality cleavage-,and blastocyst-stage embryos were shown to positively correlate with the series of estradiol levels(P<0.001)(Table 3).Increased serum estradiol concentration on the day of hCG injection was associated with improved yields and achievements in the overall laboratory outcomes.The positive correlation could be explained by the reason of the multiple follicle growth during stimulation,leading to the higher production of estradiol.
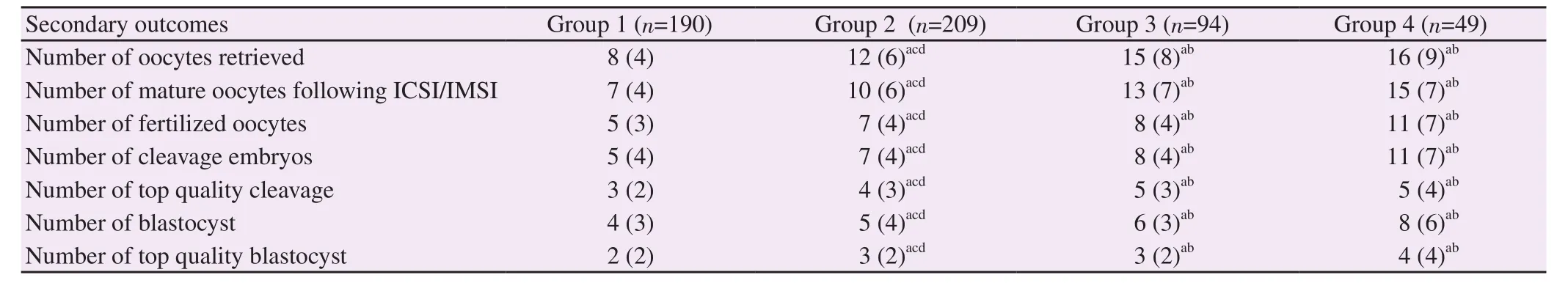
Table 3.Embryology laboratory outcomes among the study groups.
4.Discussion
The existing practical data in this study have demonstrated no statistically significant correlation between the estradiol levels on trigger day of follicular maturation and the rate of clinical pregnancy and miscarriage,even after ensuring an equal number of transferred embryo in the study subjects.Our results indicated that estradiol level of <3 000 pg/mL was the optimal range for embryo implantation and pregnancy.This finding was similar to the previous studies conducted by Siddharta et al[13]and Taskin et al[9].Both studies emphasized the lack of statistical differences in the pregnancy rate or implantation rate among responders exhibiting low and elevated estradiol levels.Moreover,Taskin et al[9]have demonstrated a weak correlation between estradiol levels and clinical pregnancy rate through evaluation of area under the curve(AUC 0.532,95% CI 0.471- 0.593)[9].The result of AUC in our study,likewise,presented a comparatively similar outcome(AUC 0.594,95% CI 0.55 - 0.64).A large retrospective study of 3 393 fresh IVF cycles in a Chinese population by Wang et al[8]has shown that patients who exhibited an elevated estradiol level(≥3 757 pg/mL)on trigger day seemed to achieve a better clinical pregnancy rate than those with a lower estradiol level(<3 757 pg/mL).Moreover,the elevated estradiol level did not increase the risk of adverse perinatal outcomes including preterm birth,low birth weight,and fetal malformation.
Contrary to our findings,in a prospective controlled in-vitro study,Valbuena et al[12]highlighted the deleterious effects of increasing estradiol levels on both endometrial and embryo development.The study concluded that high estradiol concentration could impair embryo's adhesion phase,causing a lower implantation rate.Supporting those findings,a randomized controlled trial performed by Shapiro et al[20]discovered that the clinical pregnancy rate per frozen embryo transfer was significantly higher compared to the fresh embryo transfer.The authors suggested that the use of exogenous gonadotropins during controlled ovarian stimulation might have contributed to the impaired endometrial receptivity leading to the decreased chance of embryo implantation and pregnancy.Supra-physiologic conditions or elevated estradiol levels due to controlled ovarian stimulation during IVF were also indicated to negatively affect the process of trophoblast invasion and placentation,thus,increasing the risk of pregnancy complications[21].Several animal model and in-vitro studies have also demonstrated the possible negative effect of elevated estradiol levels on the endometrial receptivity for embryo implantation.A 2003 study on mice has demonstrated that the expressions of implantation-related genes such as Lif,Hegfl,and Ptsg2 as well as the expressions of genes associated with the preparatory uterine receptive phase(Ptsg1,Areg)were compromised in response to a high dose estradiol injection[22].Following that,Zhang and colleagues proposed a novel mechanism about the negative effects of supra-physiological concentration of estradiol in mice,which has led to the over-expression of waterchannel genes(Aqp5/8)thus giving rise to excessive intra-uterine fluid accumulation.Such a condition could perturb the apposition of an embryo during implantation to the endometrium[23].A proteomic analysis was also investigated by Ullah and colleagues in 2017.Protein quantification through mass spectrometry utilizing iTRAQ isobaric tags coupled with 2D Nano LC-MS/MS managed to evaluate the physiological impacts of estradiol concentrations on endometrial cells by screening the comparative proteomic profiles of Ishikawa cells pre-treated with different estradiol levels(10M and 10M).When the cells were treated with 10M estradiol,the expression of 45 proteins were altered in which 43 were up-regulated while 2 proteins were down-regulated[7].Despite the deleterious effects of high estradiol concentration at a molecular level,our study failed to find such a negative correlation between increased estradiol levels and IVF outcomes.
Our study,particularly,showed a positive correlation between the elevation of estradiol level and the overall laboratory outcomes.In this study,increasing estradiol levels corresponded to the multiple follicular growths induced by gonadotropin stimulation.Therefore,elevated estradiol level is commonly affiliated with an increased number of retrieved oocytes which consequently lead to a higher number of mature oocytes and hence potentially higher derivation of embryos.The previous studies could justify our findings[16].Through a retrospective review of 128 IVF cycles,positive correlation of the elevated estradiol level was found with the number of retrieved oocytes,and the mean number of viable embryos.While Fatemeh et al[16]also indicated a positive correlation between the estradiol level and the clinical pregnancy rate,this study did not.The discrepancy identified was the different number of transferred embryo among subjects.This study confirmed that by limiting the confounding variables through the recruitment of subjects who proceeded with an equal number of transferred embryo,the positive correlation between estradiol level and pregnancy rate became inconspicuous.
This study also implied that the superior laboratory outcomes which corresponded to the high estradiol level did not improve the clinical pregnancy rate.These results agree with a study by Sunkara et al[24]to some extent,in which a non-linear association was identified between the number of retrieved oocytes and the livebirth rate following fresh IVF cycles.In the study,an IVF cycle with around 15 retrieved oocytes culminated in a high live-birth rate.The increased number of retrieved oocytes(>15),however,did not improve the live-birth rate and even exhibited a decline when ≥20 oocytes were derived.Hence,the theoretical beneficial expectation that a high number of retrieved oocytes has in yielding more goodquality embryos and subsequently improving the pregnancy rate has yet been proven.
Finally,analyzing the clinical outcomes of IVF based upon data of single embryo transfer cycles of the top-quality blastocyst has failed to correlate the estradiol level with the clinical outcomes in the terms of the clinical pregnancy and miscarriage rates.Other aspects such as the human endometrial response are to be taken into consideration as factors responsible for the adverse outcomes in relation to the high estradiol levels on the trigger day or the association to ovarian hyperstimulation syndrome.Further studies are still required to investigate the effect of elevated estradiol levels on the endometrium.
Some limitations have pertained to our study.Firstly,although multiple analysis was conducted to the outcomes of interest,other confounders such as recurrent implantation failure were not calculated.Secondly,this study also lacked to provide prominent indexes of IVF such as pregnancy complications and live-birth outcomes due to the limited availability of such data.
In conclusion,although the overall laboratory outcomes were shown to positively correlate with the estradiol levels,this study did not identify a statistically significant correlation between the different serum estradiol levels on trigger day and the clinical outcomes of IVF.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors' contributions
Arie Adrianus Polim conceived and designed the study.Nining Handayani and Tri Aprilliana performed data collection and analysis.Arie Adrianus Polim,Nining Handayani,and Tri Aprilliana interpreted the results.Arie Adrianus Polim,Nining Handayani,Roza Silvia,Batara Sirait,Arief Boediono,and Ivan Sini conducted study literature and drafted the manuscript.All authors have read and approved the final version of the publication.
 Asian Pacific Journal of Reproduction2021年2期
Asian Pacific Journal of Reproduction2021年2期
- Asian Pacific Journal of Reproduction的其它文章
- Evaluation of pig oocyte in vitro maturation and fertilization using three gonadotropin-based hormonal compounds
- Semen characteristics of the three genetic types of boars reared in Benin
- Aphrodisiac potential of Polyalthia bullata(Tongkat Ali)in fowl
- Combined effects of Gymnema sylvestre and Pergularia daemia on letrozole-induced polycystic ovarian syndrome in rats
- Antifertility effects of 60-day oral gavage of ethanol extract of Spondias mombin leaves in guinea pigs:A biochemical,reproductive and histological study
