Strain and interfacial engineering to accelerate hydrogen evolution reaction of two-dimensional phosphorus carbide∗
Tao Huang(黄韬), Yuan Si(思源), Hong-Yu Wu(吴宏宇), Li-Xin Xia(夏立新),Yu Lan(蓝郁), Wei-Qing Huang(黄维清),†, Wang-Yu Hu(胡望宇), and Gui-Fang Huang(黄桂芳),‡
1Department of Applied Physics,School of Physics and Electronics,Hunan University,Changsha 410082,China
2Department of Physics,Kashgar University,Kashgar 844006,China
3College of Physics and Electronic Engineering,Hengyang Normal University,Hengyang 421002,China
4School of Materials Science and Engineering,Hunan University,Changsha 410082,China
Keywords: phosphorus carbide,hydrogen evolution reaction(HER),coordination configuration,electrocatalysis,first principle calculations
1. Introduction
Excessive consumption of fossil fuel makes it urgent to explore alternative sources of energy to substitute fossil fuel or reduce its dependency.[1]Therefore, hydrogen, which is sustainable and environment-friendly, attracts plenty of interests in recent decades.[2]Considered as an effective route to generate hydrogen,the electrocatalytic hydrogen evolution reaction(HER)is the cathodic half-reaction of electrochemical water splitting, where the electrocatalyst is the key to affect the efficiency.[3–6]At present,the noble-metal platinum(Pt)is the most efficient electrocatalyst for HER,[7]which is located near the peak of volcano plots,but the rareness and high cost of Pt hinder the widespread utilization for electrocatalysis.[8,9]Thus, great effort has been made on seeking the nonprecious and highly efficient catalysts to replace the Pt-based materials, such as transition metal phosphides, carbides, borides,nitrides, sulfides and carbon materials, and metal–organic complexes.[10–15]

Most recently,considerable attention has been focused on the HER catalysts based on two-dimensional (2D) materials because of the unique planar structure with atomic thickness and the tunable electronic properties,which can give some obvious advantages for HER catalytic performance.[16,17]For instance, the atomic-thickness structure provides more exposed active sites to facilitate the contact between the reactants and active sites, further permits 2D materials as the ideal platform to facilely interact with other materials or substrates,and makes them easily activated and optimized to obtain higher HER catalytic activity by introducing strain, defects or heteroatoms.


Appendix A:Supporting information
In order to better understand and explain the descriptions in the main text,the following figures are given below for references.

Fig.A1. Optimized atomic configuration of (a) P–P stacking vdW bilayer P–C structure and (b) P–C stacking bilayer PC structure. The blue rhomb represents the redefined cell. The P and C atoms are denoted as pink and gray spheres, respectively. The equilibrium distance between two layers is represented by d.
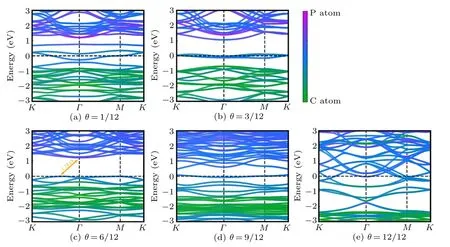
Fig.A2. (a)–(e)Band structures of bilayer PC under different H coverages. The Fermi level is set to zero.
——记4808工厂威海修船厂
- Chinese Physics B的其它文章
- Statistical potentials for 3D structure evaluation:From proteins to RNAs∗
- Identification of denatured and normal biological tissues based on compressed sensing and refined composite multi-scale fuzzy entropy during high intensity focused ultrasound treatment∗
- Folding nucleus and unfolding dynamics of protein 2GB1∗
- Quantitative coherence analysis of dual phase grating x-ray interferometry with source grating∗
- An electromagnetic view of relay time in propagation of neural signals∗
- Negative photoconductivity in low-dimensional materials∗

