Human Serum-derived Extracellular Vesicles Protect A549 from PM2.5-induced Cell Apoptosis*
ZHOU Qiu Lian,BAI Yu Zheng,GAO Juan,DUAN Yi,LYU Yi Cheng,GUAN Long Fei,ELKIN Kenneth, XIE Yu Ling, JIAO Zheng, and WANG Hong Yun,#
1.Cardiac Regeneration and Ageing Lab,Institute of Cardiovascular Sciences,School of Life Science,Shanghai University,Shanghai 200444,China; 2.Shanghai Applied Radiation Institute,School of Environmental and Chemical Engineering,Shanghai University,Shanghai 200444,China; 3.School of Medicine,Shanghai University,Shanghai 200444,China; 4.China-America Institute of Neuroscience,Beijing Luhe Hospital,Capital Medical University,Beijing 101149,China; 5.Department of Neurosurgery,Wayne State University School of Medicine,Detroit,MI 48201,USA
Abstract Objective Epidemiological studies reveal that exposure to fine particulate matter (aerodynamic diameter ≤ 2.5 μm,PM2.5) increases the morbidity and mortality of respiratory diseases.Emerging evidence suggests that human circulating extracellular vesicles (EVs) may offer protective effects against injury caused by particulate matter.Currently,however,whether EVs attenuate PM2.5-induced A549 cell apoptosis is unknown.Methods EVs were isolated from the serum of healthy subjects,quantified via nanoparticle tracking analysis,and qualified by the marker protein CD63.PM2.5-exposed (50 μg/mL) A549 cells were pretreated with 10 μg/mL EVs for 24 h.Cell viability,cell apoptosis,and AKT activation were assessed via Cell Counting Kit-8,flow cytometry,and Western blot,respectively.A rescue experiment was also performed using MK2206,an AKT inhibitor.Results PM2.5 exposure caused a 100% increase in cell apoptosis.EVs treatment reduced cell apoptosis by 10%,promoted cell survival,and inhibited the PM2.5-induced upregulation of Bax/Bcl2 and cleaved caspase 3/caspase 3 in PM2.5-exposed A549 cells.Moreover,EVs treatment reversed PM2.5-induced reductions in p-AKTThr308 and p-AKTSer473.AKT inhibition attenuated the anti-apoptotic effect of EVs treatment on PM2.5-exposed A549 cells.Conclusions EVs treatment promotes cell survival and attenuates PM2.5-induced cell apoptosis via AKT phosphorylation.Human serum-derived EVs may be an efficacious novel therapeutic strategy in PM2.5-induced lung injury.
Key words:Cell apoptosis; PM2.5; Extracellular vesicles; Therapy; AKT
INTRODUCTION
The effect of fine particulate matter(aerodynamic diameter ≤ 2.5 μm,PM2.5) on human health is an increasing global concern.PM2.5refers to tiny particles that are generated from volatile organic compounds during coal burning,vehicle exhaust,and other similar sources[1].Epidemiological studies reveal that PM2.5contributes to lung injury[1]and increases the morbidity and mortality of respiratory diseases[2-4],including asthma[5,6],chronic obstructive pulmonary disease (COPD)[7], and lung cancer[8,9]. The mechanism of PM2.5toxicity is mainly related to oxidative stress[10,11]and inflammation[12],which trigger cell apoptosis in bronchial and alveolar epithelial cells[13,14].Even low doses of PM2.5have been demonstrated to induce acute oxidative stress,inflammation,and pulmonary impairment in healthy mice[15,16].PM2.5-induced lung injury results from not only the augmented production of reactive oxygen species (ROS) but also DNA damage,nitric oxide production,lipid peroxidation,and cell death[17-20].Thus,the development of effective strategies to prevent or mitigate PM2.5-induced cell apoptosis and lung injury is an urgent undertaking.
Extracellular vesicles (EVs) are particles released by cells that exist in various biofluids,including blood,urine,and bronchoalveolar lavage fluid[21].EVs contain exosomes or small EVs (40-200 nm),microvesicles (200-1,000 nm),and apoptotic bodies(1-2 μm)[22,23].An increasing number of studies demonstrate that EVs are involved in certain pathological processes of lung diseases[24],such as asthma[25]and COPD[26-29].Compared with healthy human controls,acute COPD patients exhibit an increase in exosomes[24].EV release significantly increases in response to particulate matter-induced lung injury[30].EV cargo offers protective effects against several pathologic processes.Exosomes derived from human plasma play a protective role in the myocardium during ischemia-reperfusion (I/R)injury[31-33].Serum-derived EVs are reported to promote the proliferation of rat myoblast H9C2 cells[34-36]. Mechanistically, the pro-proliferation effect of EVs is associated with miR-17-3p upregulation,which further inhibits tissue inhibitor of metalloproteinase 3 expression.Serum EVs protect H9C2 against hydrogen peroxide-induced apoptosis via activation of the ERK1/2 signaling pathway.
Bax,a member of the Bcl-2 family,is a proapoptotic protein that can translocate to the mitochondria and induce cytochrome C release.An elevated Bax/Bcl-2 ratio is considered a marker of cell apoptosis[37].Increasing evidence indicates that activation of the phosphatidylinositol 3 kinaseprotein kinase B (PI3K-AKT) signaling pathway can block the translocation of cytoplasmic Bax to the mitochondria[38].Although serum-derived EVs may protect cardiomyocytes against I/R injury,their role in PM2.5-induced cell apoptosis remains unknown.As serum-derived EVs may be a potential therapeutic target for lung injury,we hypothesize that human serum-derived EVs may attenuate PM2.5-induced cell apoptosis by activating AKT in human alveolar epithelial A549 cells.Therefore,in this work,we isolate EVs from human serum and investigate their role in PM2.5-induced A549 apoptosis.
METHODS
Human Serum Collection and Extracellular Vesicle(EV) Isolation
All human-related experimental protocols were given consent by the Institutional Review Board,approved by the appropriate institutional review committees (ECSHU2020-004),and performed in accordance with the Helsinki Declaration as revised in 2013.Three pooled serum samples (n=3 samples/pool) were collected from healthy males aged 50-60 years.Serum pools were obtained as follows.Blood without anticoagulant was incubated at room temperature for 1 h and centrifuged at 2,500 ×g for 15 min at 4 °C.The supernatant was then collected.Three samples were collected into one pool.EV isolation was conducted using ExoQuickTMExosome Precipitation Solution (EXOQ5A-1,System Biosciences,USA) according to the manufacturer’s instructions.In brief,250 μL of the pooled serum samples was added to the appropriate volume of ExoQuick precipitation solution.The solution was mixed well,refrigerated for 30 min at 4 °C,and then centrifuged at 1,500 ×g for 30 min. After centrifugation,the EV pellets at the bottom of the vessel were collected and resuspended in 100 μL of sterile phosphate buffer solution (PBS).The EVs were used immediately or stored at -80 °C for further study.A total of 10 μL of the isolated EVs was dropped onto an ultra-thin carbon film for transmission electron microscopy (TEM) imaging.The liquid was removed using filter paper,and the film was washed thrice with distilled water.The carbon film was dried naturally at room temperature.TEM images were obtained using an LVEM5 transmission electron microscope (Delong Instruments) operated at 5 kV.
Nanoparticle Tracking Analysis
The EV pellets were resuspended and diluted with the appropriate volume of PBS.Next,the concentration and size distribution of EVs were determined by nanoparticle tracking analysis (NTA,Version 3.1 Build 3.1.54,Malvern,UK) as previously described[39].The EV experiments were adjusted to the MISEV2018 guideline[40].
Cell Culture and Treatment
Human alveolar epithelial A549 cells were acquired from the Cell Bank (Chinese Academy of Sciences) and cultured with Dulbecco’s modified Eagle’s medium (DMEM,Corning,USA) plus 1%penicillin/streptomycin and 10% fetal bovine serum(BioInd,Israel) at 37 °C and 5% CO2.PM2.5was purchased from NIST (SRM 1650b, USA) and dissolved in an appropriate volume of dimethyl sulfoxide (DMSO).Prior to treatment,the A549 cells were seeded in 12-well plates at a density of 2 ×105cells/mL and allowed to attach for 12 h.The cells were then exposed to 50 μg/mL PM2.5with or without 10 μg/mL EV pre-treatment for 24 h.An equal volume of DMSO in DMEM (DMSO < 0.1%) was used as the negative control,and an equal volume of sterile 1× PBS was used as the EV control.The concentration of PM2.5utilized in the experiments was based on a previous study[41].According to the Stochastic Human Exposure and Dose Simulation(SHEDS-PM) model[42],the exposure dose of cells used in this work is parallel to that of real-life exposure.
Cell Viability and Apoptosis Tests
Cells were incubated in 96-or 6-well plates and treated with 10 μg/mL EVs or PBS for 24 h.Next,the cells were exposed to PM2.5for 24 h and subjected to cell viability and apoptosis analyses.An enhanced Cell Counting Kit-8 (CCK-8; Beyotime,China) was used for the cell viability test.Briefly,10 μL of enhanced CCK-8 solution was added to each well,and the plate was incubated for 2 h according to the manufacturer’s instructions.Cells were subjected to flow cytometric analysis with an Annexin V-FITC/PI kit (Dojindo, #AD10, Japan) according to the manufacturer’s instructions to determine cell apoptosis.A549 cells in 1× binding buffer were incubated in the dark with FITC Annexin V and PI for 15 min at room temperature and then immediately analyzed by flow cytometry.
Western Blot
The supernatants (without EVs) and EV pellets were lysed with EV-specific lysis buffer (Umibio,Shanghai,China).A549 cells were lysed with RIPA(KeyGen Biotech,China) containing 1% PMSF and phosphatase inhibitor cocktail (1 tablet per 10 mL of lysis buffer, 4906837001-PhosSTOP™, Roche).Western blot analysis was performed as previously described[39],and β-actin was used as an internal control.Primary antibodies against β-actin were purchased from Bioworld Technology,Inc.(USA).Primary antibodies against Bax,Bcl2,caspase 3,CD63,CD9,and Tsg101 were purchased from ABclonal (China).Primary antibodies against p-AKTThr308and p-AKTSer473were purchased from Cell Signaling Technology (USA). Primary antibodies against AKT were purchased from ProteinTech(China).All of the primary antibodies used in this study were diluted to 1:1,000 with 5% BSA,and all of the secondary antibodies were diluted to 1:10,000 with 5% defatted milk.The corresponding Research Resource Identifiers are reported in Supplementary Table S1,available in www.besjournal.com.
Statistical Analysis
All experiments were performed independently at least three times.All data are presented as mean± SD and analyzed using SPSS (version 20).One-way ANOVA followed by Bonferroni’s post hoc test was performed for multiple group comparisons.A Pvalue less than 0.05 was considered statistically significant.
RESULTS
Human Serum-derived Particle Isolation and Characterization
According to the MISEV2018 guideline,EVs less than 200 nm in size are called small EVs or exosomes.Here we performed precipitation to collect EV pellets (Figure 1A).Previous reports indicated that at least two markers are needed to identify EVs[40]. Therefore, Western blot was performed to analyze marker protein expression,including those of CD63,CD9,and Tsg101,to evaluate EV recovery.The results indicated that few EVs exist in the supernatant,whereas rich signals could be found in the EV pellets (Figure 1B).This finding indicates a high recovery of EVs from serum.The EVs were quantified by NTA,and results showed an EV concentration of approximately 17.04 ×108particles/mL (Figure 1C).The morphology of the EVs was determined by TEM,as shown in Figure 1D.
Serum EVs attenuate PM2.5-induced Cell Apoptosis and Promote Cell Survival
A549 cells were treated with EVs (10 μg/mL) and then exposed to PM2.5for 24 h to investigate the protective role of serum EVs in PM2.5-induced cell apoptosis.Cell apoptosis in A549 was subsequently tested by flow cytometry.PM2.5caused a 15%increase in cell apoptosis,whereas EVs treatment reduced cell apoptosis by approximately 10%-42%(Figure 2A,Supplementary Figure S1,available in www.besjournal.com). Analysis of apoptosis by Western blot indicated that the ratios of protein Bax/Bcl2 and cleaved caspase 3/caspase 3 expression increased in PM2.5-treated cells when compared with those of the control group.However,EV pretreatment significantly elevated Bcl2 levels and inhibited Bax and the ratio of cleaved caspase 3/caspase 3 expression (Figure 2B).
Serum EVs Promote AKT Phosphorylation in PM2.5-exposed Cells
Previous studies revealed that AKT is involved in PM2.5-induced pulmonary injury[43]and lung cancer[44],which may be activated by EVs.Threonine-308 (Thr308) and serine-473 (Ser473) of AKT were analyzed in EV-treated cells by Western blot to investigate whether EVs influence AKT phosphorylation (p-AKT). PM2.5exposure could inhibit p-AKT by reducing the expression of p-AKTThr308and p-AKTSer473.However,pre-treatment with EVs reversed PM2.5-induced AKT inhibition and promoted the expression of p-AKTThr308and p-AKTser473(Figure 3).These findings indicate that human serum-derived EVs could promote p-AKT and protect cells from PM2.5injury.
Inhibition of AKT Reversed the Protective Role of EVs in PM2.5-exposed A549 Cells
AKT activation is recognized as an anti-apoptotic signal in pathological diseases by phosphorylating and inhibiting a forkhead transcription factor,caspase 9[45].MK2206 is an effective allosteric inhibitor of AKT that inhibits AKT activation[46].Thus,MK2206 was used to determine whether AKT activation mediates the anti-apoptotic effect of serum EVSon PM2.5-exposed A549 cells.
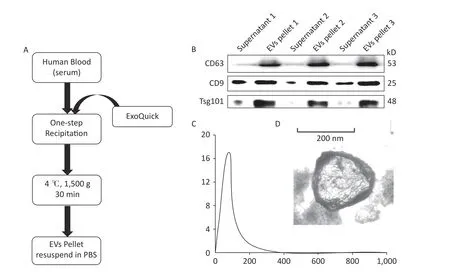
Figure 1.Extraction and characterization of human serum-derived extracellular vesicles (EVs).(A) Method and workflow of EV isolation from human serum.The EV pellets were resuspended in PBS.(B) Western blot for EV marker proteins (i.e.,CD63,CD9,and Tsg101) in the supernatant and EV pellets (n=3).(C) The concentration and size distribution of EV were determined via nanoparticle tracking analysis.(D) The morphology of serum-derived EVs was determined by TEM.Scale bar=50 nm.
MK2206 could attenuate the protective role of EV in PM2.5-exposed cells and lead to a 10% increase in apoptosis compared with that in the non-MK2206 group (Figure 4A-B).Cell viability was assessed using a CCK-8 kit,and results showed that MK2206 causes significant decreases in cell viability (Figure 4C).Western blot analysis demonstrated that MK2206 significantly inhibits Bcl2 expression and augments Bax/Bcl2 and cleaved caspase 3,thus supporting the observed increase in apoptosis (Figure 4D).Overall,human serum-derived EVs could protect cells against PM2.5-induced cell apoptosis and viability loss via AKT activation.
DISCUSSION
In this work,A549 cells were treated with EVs purified from healthy human blood and then exposed to PM2.5for 24 h to explore the role of human serum-derived EVs in PM2.5-induced cell apoptosis.This study presents two new major findings.First,human serum-derived EVs could attenuate PM2.5-induced cell apoptosis and promote cell survival.EV treatment inhibited the protein expression of Bax/Bcl2 and cleaved caspase 3/caspase 3.Second,EVs mediated cell protection via AKT activation,which promotes the expression of p-AKTThr308and p-AKTSer473.Inhibition of AKT by MK2206 counteracted the protective role of EVs in PM2.5-exposed A549 cells.
PM2.5is a major component of air pollution that affects the whole body and systemic homeostasis.PM2.5is acknowledged as a significant threat to the respiratory system[47-49]and human health[50].According to an assessment report from the World Health Organization, approximately 4.2 million people died from PM2.5exposure in 2016[51].Thus,PM2.5has merited broad attention and concern over the last few years.Interestingly,the toxicity of PM2.5varies according to its source,time,and space.To determine the specific effect of stable particulate matter,we purchased a standard,well-characterized PM2.5from the NIST (SRM 1650b).According to the Standard Reference Material (https://wwws.nist.gov/srmors/certificates/1650B.pdf),SRM 1650b is prepared from the same bulk diesel particulate material that was issued in 1985 as SRM 1650 and in 2000 as SRM 1650a.The composition of SRM 1650b has been well analyzed and includes polycyclic aromatic hydrocarbons,reactive metals,and organic and elemental carbon[52,53].SRM 1650b induces DNA damage in A549 cells[54]as well as pulmonary injury[55]. Mechanistically, 1) PM2.5exacerbates secondary injury to organs via oxidative stress and inflammation.PM2.5can carry toxic substances,such as pathogenic bacteria,to the alveoli and interact with macrophages and alveolar epithelial cells,thereby causing an increase in ROS production and pro-inflammatory response.PM2.5-induced oxidative stress and inflammation exacerbate secondary injury and promote the development of respiratory diseases, including asthma,COPD,and lung cancer.2) PM2.5exposure directly triggers systematic injury.PM2.5can pass through respiratory membranes and the blood-brain barrier to enter into the systemic circulation and cause cell death, systemic inflammation, and cardiovascular and cerebrovascular diseases.3) PM2.5exposure disturbs metabolic homeostasis.PM2.5exposure has been demonstrated to lead to insulin tolerance and energy metabolism dysfunction via inhibition of AMP-activated protein kinase activity.Previous studies reported that exposure to PM2.5could induce cell death, inflammation, and fibrosis[13,56-58]by promoting the secretion of the inflammatory factors TNFα,IL-6,and TGF-β[59-61]and the expression of apoptotic proteins.In the present study,cell apoptosis and cell viability analyses indicated that PM2.5causes significant upregulations in Bax/Bcl2 and cleaved caspase 3/caspase 3(Figure 2),thus indicating extensive apoptosis after PM2.5exposure.
EVs are released from cells (40-2,000 nm) and may be divided into two subgroups according to the MISEV2018 guideline:EVs (40-200 nm) and medium or large EVs (> 200 nm).EVs with a diameter of approximately 40-200 nm have a goblet shape and double-layer membrane; they exist in blood,urine,and cell culture medium.EVs have sparked great research interest on account of their potential applications in therapeutics, particularly those related to drug delivery.EVs have been used to carry uracil phosphoribosyltransferase for tumor therapy and have successfully inhibited schwannoma tumor growth in vivo[62].Studies reveal that EVs from human blood could protect cells and tissue against injury, especially myocardial infarction[31,63].However,no investigation has yet studied the role of EVs in PM2.5-induced injury.In this work,EVs were extracted from human serum and utilized therapeutically in PM2.5-exposed A549 cells to investigate the clinical prevention of PM2.5-induced injury via EV therapy.Western blot and flow cytometric analyses indicated that EV pre-treatment could attenuate PM2.5toxicity by promoting AKT activation at Thr308 and Ser473 (Figure 4).
EVs have potential therapeutic applications on account of their innate biocompatibility and low immunogenicity and toxicity.Many investigations have suggested that EVs could be used for drug delivery and disease treatment[64].However,our study was performed in vitro,and several constraints continue to limit the utilization of EVs in mammalian studies and clinical trials.For example,the optimal dose of EVs to use under different conditions should be determined in pre-clinical trials.A previous study recommended 50 μg of EVs from plasma exosomes as an effective dose to protect the heart against I/R injury[30].While the study[30]rightly addresses the concern that low production of EVs limits their clinical use,we believe that this limitation could be solved with further scientific development.
EVs can carry micro RNA (miRNA),proteins,and lipids and transfer them to target cells,thereby regulating physiological and pathological processes.The primary goal of our study is to reveal the EV components responsible for the observed effects.Human plasma-derived exosomal RNAs have been characterized by deep sequencing[65].For example,the blood EV contents of patients exposed to particulate matter have been studied.Pergoli et al.investigated the effects of PM10exposure on exosomal miRNA in plasma from overweight individuals and found that short-term exposure to PM10is associated with increased EV release.In particular,nine EV-miRNAs (i.e.,let-7c-5p,miR-106a-5p,miR-143-3p,miR-185-5p,miR-218-5p,miR-331-3p, miR-642-5p, miR-652-3p, miR-99b-5p) are downregulated after exposure to PM10[30].
AKT is a downstream target of phosphatidylinositol 3-kinase, the activation of which promotes cell survival by targeting caspase 9 and cytochrome C[66].An increasing number of studies indicate that PM2.5exposure regulates the AKT pathway and cell apoptosis.Our previous investigation demonstrated that PM2.5exposure inhibits AKT activation in A549 cells[67],while another study found that PM2.5exposure may significantly inhibit p-AKT[31],which is consistent with the present findings (Figure 3). Specifically, EV treatment reversed PM2.5-induced AKT dephosphorylation but failed to modify the mRNA level of TNFα and IL8 in PM2.5-exposed A549 cells (data not shown).Abundant miRNAs in EVs may play important roles in p-AKT.Huang et al.systemically characterized extracellular RNA species from human plasmaderived exosomes and found that many miRNAs(e.g., miR-99a-5p, miR-99b-5p, miR-486) are enriched in these EVs[65].Li et al.revealed that an miR-486 mimic can protect A549 cells against PM2.5-induced AKT dephosphorylation[41].Therefore,we propose that exosomal miR-486 in human EVs may be related to the activation of AKT.However,further investigation of the component(s) of EVs responsible for the observed effects is necessary.
CONCLUSIONS
In conclusion,our findings indicate that human serum-isolated EVs exert protective roles in PM2.5-induced A549 cell apoptosis.EVs target AKT and induce p-AKT at Thr308 and Ser473, thereby rendering cells resistant to stimuli induced by PM2.5.The potential therapeutic relevance of EVs is underscored by previous studies indicating that human blood-derived EVs improve cardiac function[30].These previous studies,however,neither establish the relationship between human blood-derived EVs and pulmonary function nor demonstrate that EVs protect cells against PM2.5-induced cell apoptosis.Our investigation revealed that:1) EVs derived from 50-60-year-old healthy males have great potential to protect cells against PM2.5-induced cell apoptosis; 2)human blood-derived EVs can activate AKT signaling and promote cell survival,thereby providing new insights into the protective mechanism of EVs; and 3)EVs may be used as novel therapeutic strategy for PM2.5-related respiratory diseases.Future studies investigating the clinical application of human bloodderived EVs as functional medicine would be of considerable interest.
ACKNOWLEDGMENTS
We thank Dr.HU Xi (Quantum Design China &Delong Instruments) for supporting the TEM image acquisition procedures.
AUTHOR CONTRIBUTIONS
Conception and design:WANG HY and JIAO Z.Administrative support:GAO J,WANG HY,and JIAO Z.Provision of study materials and patients:ZHOU QL and GUAN LF.Collection and assembly of data:ZHOU QL,DUAN Y,LYU YC,and GUAN LF.Data analysis and interpretation:ZHOU QL,BAI YZ,GAO J,DUAN Y,LYU YC,XIE YL,and ELKIN K.Manuscript writing:All authors.Final approval of manuscript:All authors.
DECLARATION OF COMPETING INTEREST
The authors declare no potential conflicts of interest concerning the research,authorship,and/or publication of this article.
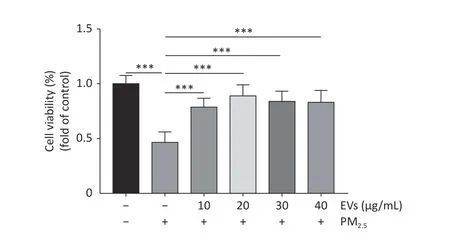
Supplementary Figure S1.Dose response experiments of EVs in PM2.5-exposed A549 cells.Cell viability of PM2.5-exposed cells was determined by the CCK-8.A549 cells were treated with small EVs (0,10,20,30,40 μg/mL) for 24h followed by PM2.5 exposure (50 μg/mL for 24 h).The data was presented as Means ±SD and analyzed using SPSS (Version 20).All experiments were performed independently at least three times,n=8.***P < 0.001.
 Biomedical and Environmental Sciences2021年1期
Biomedical and Environmental Sciences2021年1期
- Biomedical and Environmental Sciences的其它文章
- The Pathogenesis and Treatment of COVID-19:A System Review
- Analysis of Health Service Utilization and its lnfluencing Factors among Patients with Pneumoconiosis in China*
- Omics Approaches for Exploring Pneumoconiosis:A Review*
- Potential Function of MMP3 Gene in Degradation of Extracellular Matrix Complex in Colorectal Carcinoma*
- lmpact of Absolute Humidity and Temperature on Eczema*
- Nuclear Factor-κB Signaling Mediates Antimony-induced Astrocyte Activation*
