Traditional Chinese medicine compounds for the treatment of functional dyspepsia: an updated meta-analysis of randomized,double-blind, placebo-controlled trials
LUO Xioying, YANG Yng, MAO Xinyong, SONG Gengqing, LIU Qin, JIANG Tinyun,WEI Wei,b*
a.Department of Spleen and Stomach Diseases, Wangjing Hospital, Chinese Academy of Chinese Medical Sciences, Beijing 100102, China
b.Key Laboratory of TCM Diagnosis and Treatment of Functional Gastrointestinal Diseases, Beijing 100102, China
c.Department of Gastroenterology and Hepatology of Cleveland Medical Center, Case Western Reserve University, Cleveland, Ohio 44106,United States
d.Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China
Keywords Functional dyspepsia Traditional Chinese medicine compounds Placebo Effectiveness Security
ABSTRACT Objective To evaluate the efficacy and safety of traditional Chinese medicine (TCM) compounds for the treatment of functional dyspepsia (FD).Methods The PubMed, Embase, Cochrane Library, Web of Science, Chinese Biomedical Database (CBM), Wanfang Data, China National Knowledge Infrastructure (CNKI), and China Science and Technology Journal Database (VIP) were searched to collect randomized, double-blind, and placebo-controlled trials of FD treated with TCM compounds.The search duration was from the establishment of the database to March 2, 2021.After two researchers independently screened the literature, extracted the data, and evaluated the bias risk included in the study, they used RevMan 5.4 software for meta-analysis.Results A total of 23 clinical trials were included, including 2 898 patients.Meta-analysis showed that the overall remission rate of FD treated with TCM compounds was significantly higher than that of placebo (73.8% vs.46.2%) [RR =1.50, 95% CI (1.29, 1.76), P <0.000 01].Among the single symptoms, TCM compounds were superior to the placebo in improving epigastric pain [MD = -0.99,95% CI (-1.37,-0.61), P <0.000 01], epigastric burning sensation [MD = -0.32, 95% CI (-0.59,-0.05), P =0.02], postprandial fullness discomfort [MD = -1.59, 95% CI (-1.96, -1.21),P <0.000 01], and early satiety symptoms [MD = -0.93, 95% CI(-1.32, -0.54), P <0.000 01].Compared with the placebo, TCM compounds treatment can obviously improve TCM syndrome in patients with FD [MD =-5.58, 95% CI (-7.55,-3.61), P <0.000 01],gastric emptying rate [MD =12.22, 95% CI (8.90, 15.55), P <0.000 01],and helped to improve patients' quality of life [MD = 11.27, 95% CI(0.10, 22.43), P =0.05].No severe adverse events were reported between the two groups [RR =1.34, 95% CI (0.91, 1.96), P =0.14].Conclusion Our results showed that TCM compounds treatment could significantly alleviate FD symptoms, improve gastric emptying in FD patients, and help to improve their quality of life.No severe adverse reactions have been reported in clinical applications.Due to the limitation of the quantity and quality of the included studies, the above conclusions need to be verified by more high-quality studies.
1 Introduction
Functional dyspepsia (FD) is a common digestive system disease.In 2016, the Rome Committee defined functional gastrointestinal diseases, including FD, as abnormal brain-intestinal interactions[1].The Rome III standard defines FD as having one or more of the following symptoms: postprandial fullness discomfort, early satiety, epigastric pain, epigastric burning sensation, and lack of any organic,systemic, or metabolic diseases that can explain these symptoms.In addition, the symptoms should occur six months ago and have symptomatic activity in the last three months[2].According to the correlation between meals and symptoms, the Rome III standard divides FD into epigastric pain syndrome(EPS) and postprandial distress syndrome (PDS).The Rome IV standard still uses this definition and slightly changes the definition of PDS[3].
The prevalence rate of FD is approximately 16%,and the symptoms are easy to recur.The clinical treatment effect is not good, which is a considerable burden for individuals and society[4].Traditional treatment methods include proton pump inhibitors,histamine H-2 receptor antagonists, prokinetic drugs,central nervous system modulators, and lifestyle regulation.However, clinical efficacy is often difficult to meet clinical needs because of the heterogeneity of symptoms in patients with FD.Presently, there is no single drug that can completely control symptoms.The pathogenesis of FD is complex, and individual symptoms may be caused by different mechanisms.Therefore, patients with similar symptoms caused by different mechanisms may not be treated with the same drugs.Therefore, seeking effective treatment is a key problem that needs to be solved urgently[5].
Traditional Chinese medicine (TCM) compounds have shown evident advantages in the treatment of FD.A previous meta-analysis based on randomized controlled trials (RCTs) showed that TCM is more effective than prokinetic drugs in improving the overall FD symptoms[6].In 2016, the team passed a metaanalysis.The curative effects of TCM compounds and placebo on FD were compared, highlighting the potential of TCM compounds for FD treatment.However, the number of studies included is limited,and the research evidence is not sufficient[7].In recent years, an increasing number of scholars have paid attention to the therapeutic effect of TCM on FD.Therefore, this study is based on randomized,double-blind, and placebo-controlled clinical trials.Based on previous studies, the literature has been updated, and more attention has been paid to improving TCM compounds in objective indicators and specific symptoms.The clinical efficacy and safety of TCM compounds in treating FD were systematically evaluated with the aim of drawing a higher level of evidence and a more objective and comprehensive evaluation conclusion to guide clinical treatment.
2 Data and methods
2.1 Document retrieval
Search scope: the PubMed, Embase, Cochrane Library, Web of Science, Chinese Biomedical Database(CBM), Wanfang Data, China National Knowledge Infrastructure (CNKI), and China Science and Technology Journal Database (VIP).The retrieval time was from the establishment time of each database to March 2, 2021, and the conference papers and dissertations of related clinical trials were simultaneously retrieved in CNKI and Wanfang data resource systems.
2.2 Retrieval strategy
Consider the retrieval strategy in PubMed as an example.The specific retrieval formula is as follows:"(functional dyspepsia [title/abstract]) OR (postprandial distress syndrome [title/abstract]) OR (epigastric pain syndrome [title/abstract]) AND (randomized[title/abstract]) AND (placebo [title/abstract]) AND(blind [all fields]) OR (blind [all fields])" and "(Traditional Chinese Medicine[Title/Abstract])OR(Chinese Medicine [Title/Abstract]) OR (Chinese Herbal Medicine [Title/Abstract]) OR (Medicine,Chinese Traditional [Mesh]) OR (Formula [Title/Abstract]) OR (Decision [Title/Abstract]) OR (Prescription [Title/Abst] RACT) OR (table [title/abstract]) OR(capsule [title/abstract]) OR (granule [title/abstract])".
In the Chinese database, the synonymous Chinese search words are used for replacement, and the logical relationship is the same as above.
2.3 Inclusion criteria
2.3.1 Types of researchRandomized, double-blind,placebo-controlled clinical trials.
2.3.2 Subjects of studyThe subjects were patients with FD over 18 years old who met the diagnostic criteria of Rome II[8], Rome III[9], and Rome IV[10].If the research object is limited to gender and occupation,it will not be included.
2.3.3 InterventionsTreatment group: only oral Chinese medicine compound treatment and does not involve the addition and subtraction of drug taste and dosage; there is no limit to the form of TCM (decoction, granules, capsules, etc.), and the course of medication should not be less than two weeks.
Control group: only oral placebo control, the placebo should meet the form, character, and taste of Chinese medicine similar to the treatment group,and the course of treatment was the same as that of the treatment group.
2.3.4 Curative effect evaluation indexMain outcome evaluation index: overall symptom remission rate.The data are extractable dichotomous variables;if the evaluation outcome index of doctors and patients is involved simultaneously, the evaluation outcome index of patients will prevail.
Secondary outcome evaluation indexes: single symptom score, gastric emptying rate, TCM syndrome curative effect, quality of life evaluation, and adverse drug reactions.
If the main outcome evaluation index does not meet the above requirements, the outcome evaluation index, including TCM syndrome efficacy or quality of life evaluation can also be included; studies reporting different outcome indicators from the same clinical trial were combined and included.
2.4 Exclusion criteria
(1) Non-Chinese and English literature; (2) the original materials are not published publicly; (3) intervention measures are interfered by other treatment measures; (4) the full text cannot be obtained, or the data is incomplete; (5) repeated published literature.
2.5 Data extraction
Two researchers independently read the title, abstract, and full text of the literature, and screened and included the literature according to the standard of arrangement.In the case of objection, the third party intervened to evaluate it.The extracted data included literature title, author, publication date, research source, sample size, western medicine diagnostic criteria, TCM syndrome differentiation, intervention measures, course of treatment and follow-up time, outcome evaluation index and results, and adverse reaction events.
2.6 The quality evaluation of included literature
Two researchers independently conducted the quality evaluation, and a third party evaluated the objections.In this study, RevMan 5.4 software was used to evaluate the bias risk of included literature.
2.7 Statistical analysis
We used RevMan 5.4 for merge effect analysis.Two classification variables were analyzed by relative risk(RR), and numerical variables were analyzed by mean difference (MD), all of which were expressed by 95% confidence intervals (CIs).The formula for calculating the number needed to treat (NNT) is NNT =1/[control event rate ×(1 − RR)].
The heterogeneity among statistics of multiple identical studies was tested by tests for heterogeneity and evaluated using theX2test combined withI2statistics.IfP>0.10 andI2<50%, the heterogeneity was considered acceptable[11].If the included research has homogeneity, the fixed effects model is used; if there is heterogeneity, a random-effect model[12]is used.
The difference between the combined statistics of several similar studies was evaluated using the hypothesis testZtest, and it was considered to be statistically significant whenP<0.05.
3 Results
3.1 Retrieval results
According to the literature retrieval strategy, 132 studies were obtained by preliminary retrieval,including 15 in Cochrane, 10 in Web of Science, 18 in PubMed, seven in Embase, 72 in CNKI, 13 in Wanfang Data, 19 in CBM, and nine in VIP.First, according to the title of the literature, the repetitive literature was eliminated (58 articles).Second, by reading the title and abstract of the literature, 68 articles were excluded, including nine reviews, one experimental article, seven research schemes, three non-randomized double-blind placebo studies, eight non-meeting diagnostic criteria, 15 articles using western medicine, nine oral studies of non-TCM compound,10 inconsistent prescriptions in the treatment group,five experimental studies related to children, and one limited gender.Finally, eight studies were screened out by reading the full text.Among them, eight articles were published repeatedly (in different languages or different article types).Finally, 29 articles were included[13-41].The specific literature screening process is shown in Figure 1.Among the 29 articles,there are six dissertations[13-15,20,23,41]and 23 journal articles[16-19,21,22,24-40], among which six articles are different results published by other similar studies.Therefore, the same studies were merged into information statistics and analysis.Table 1 shows specific combined results, and a total of 23 studies were included in the meta-analysis.
Among the 23 studies included in the analysis, 19 were completed in China[13-23,27-36,38-41], two in Korea[24,25], and two in Japan[26,37].A total of 2 898 patients were included in the analysis, and the number of participants in each study ranged from 36 to 319,among which the proportion of male patients ranged from 29.1% to 45.7%.Twelve studies involved 17 articles[16-19,21,22,24,26-29,33,35-38,40]were multicenter studies,and the number of participating research centers ranged from 2 to 56.The basic information of the 23 studies included in the analysis is shown in Table 1.The specific drug composition of Chinese medicine compounds involved in the study is shown in Table 2.
3.2 Included research quality
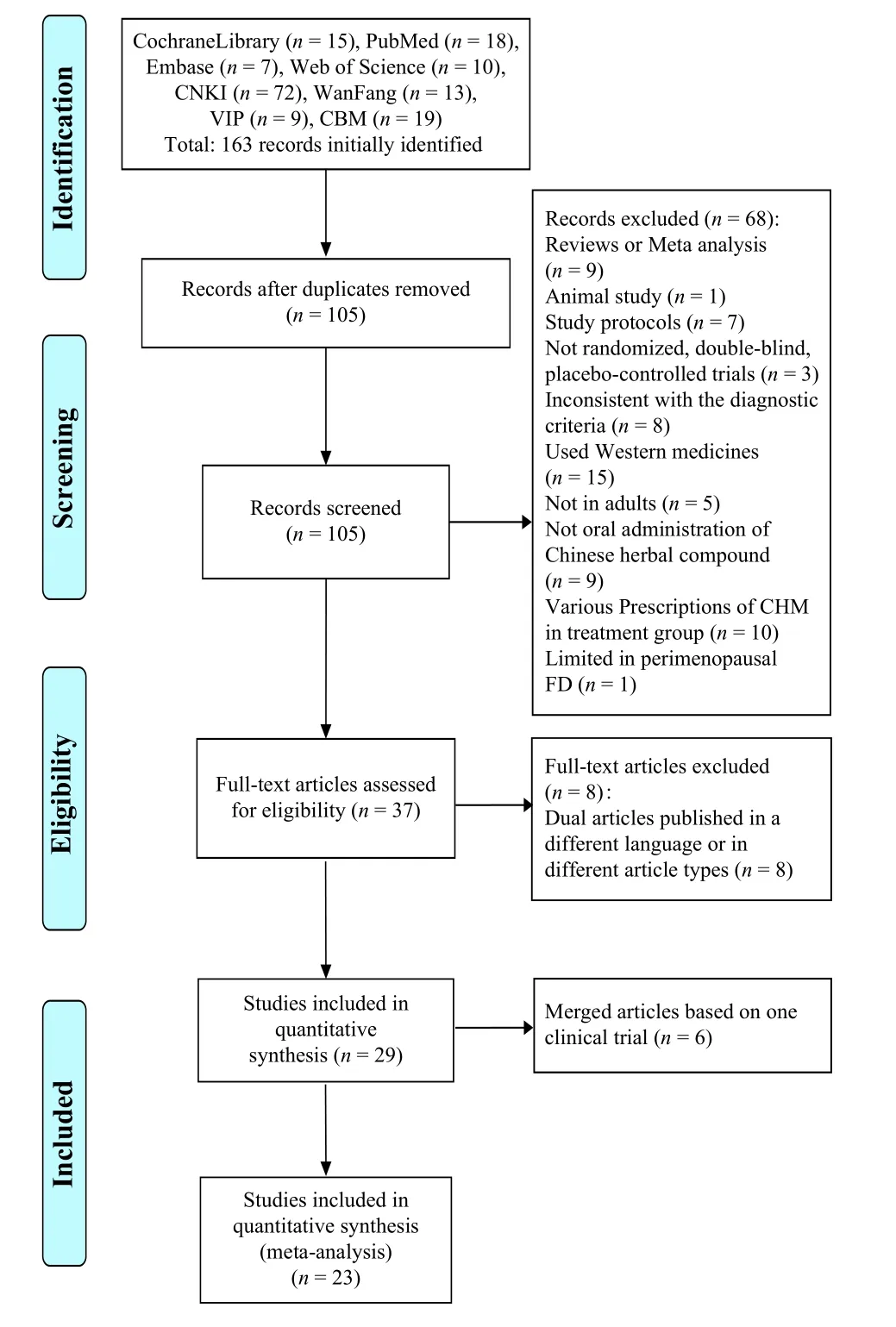
Figure 1 Flow chart of literature screening
The basic information of research methodology is incorporated as follows.(1) Random allocation methods: 15 studies describe the methods of generating random allocation sequences, the remaining three studies are not specifically described, and the contact authors have not responded[13,14,30,31]; (2) concealment of allocation schemes: 16 studies describe specific methods of hiding random allocation sequences, whereas the rest are not explained in detail[13,14,20,25,30-32,36]; (3) blind method (research object and treatment plan implementer): 23 studies all described the blind method for subjects and experimenters; (4) blind method (measurer of research results): seven studies involved 12 articles[16-19,21,22,25-29,35]stated that the data were managed and analyzed by a third party, whereas the rest were not explained in detail; (5) integrity of result data: nine studies involved 12 articles[16-19,21,22,25,26,28,29,36,40]clarified that Intentionality-to-Treat (ITT) was selected in the result analysis process, six studies[15,20,32,35,37,41]had incomplete information but clarified the reasons, and four studies adopted Per-protocol (PP) in the result analysis[23,33,38,39]; (6) results of selective research reports: 10 studies involved 15 articles[16-19,21,22,26-29,35-38,40]provided exact clinical trial registration information,one study[38]published the trial plan before the trial was conducted, and the trial plans of other studies could not be obtained; (7) other sources of bias: all the included studies have comparable baselines between the treatment group and the control group.In addition,eight studies involved10 articles[18,19,21,22,24,26,35,36,38,40]identified possible conflicts of interest.The results of the Cochrane risk bias assessment tool document quality assessment are shown in Figure 2.
3.3 Analysis of statistical results
The statistical results were described from six aspects: overall symptom relief rate, single symptom curative effect, TCM syndrome curative effect, gastric emptying rate, quality of life, and adverse reactions.
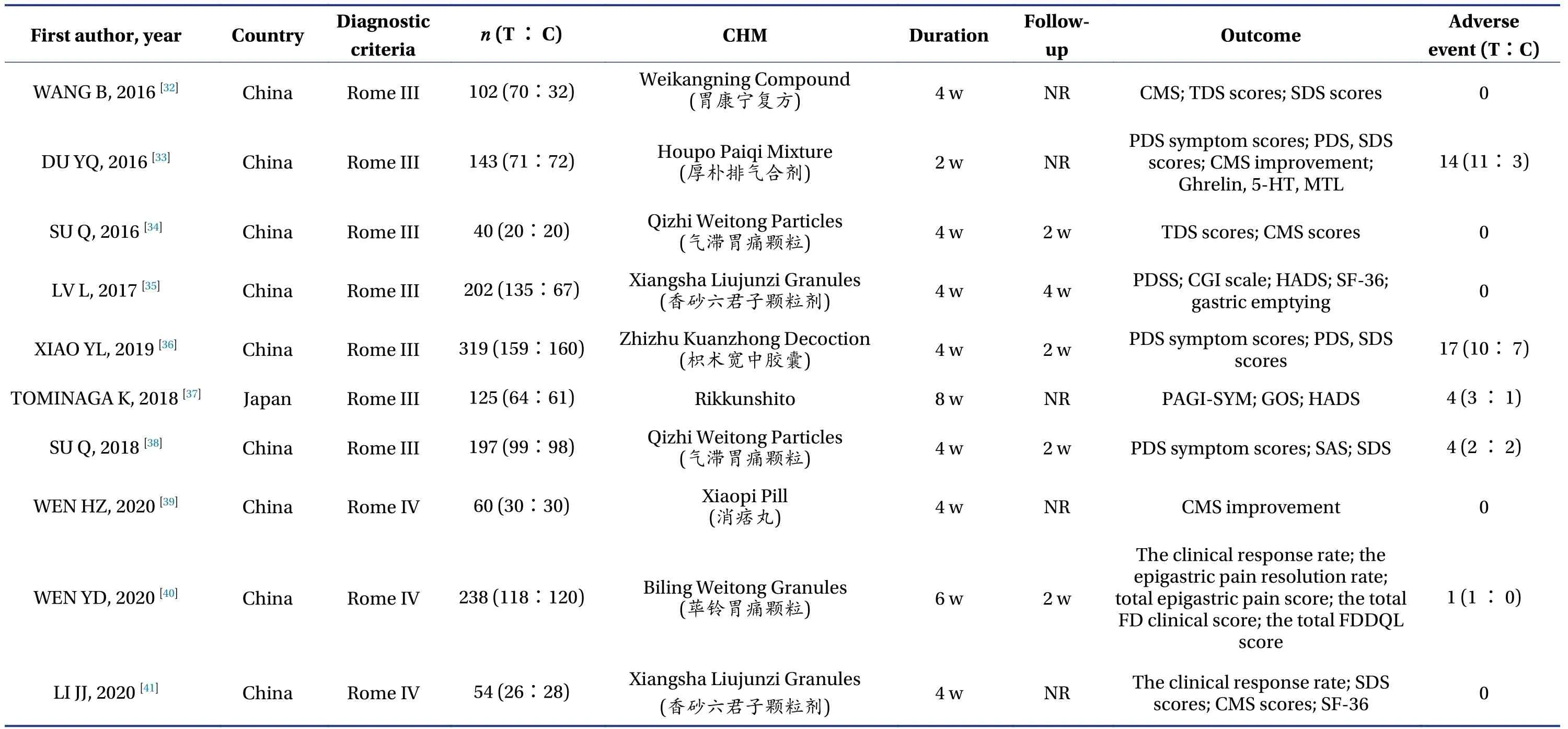
Table 1 Continued
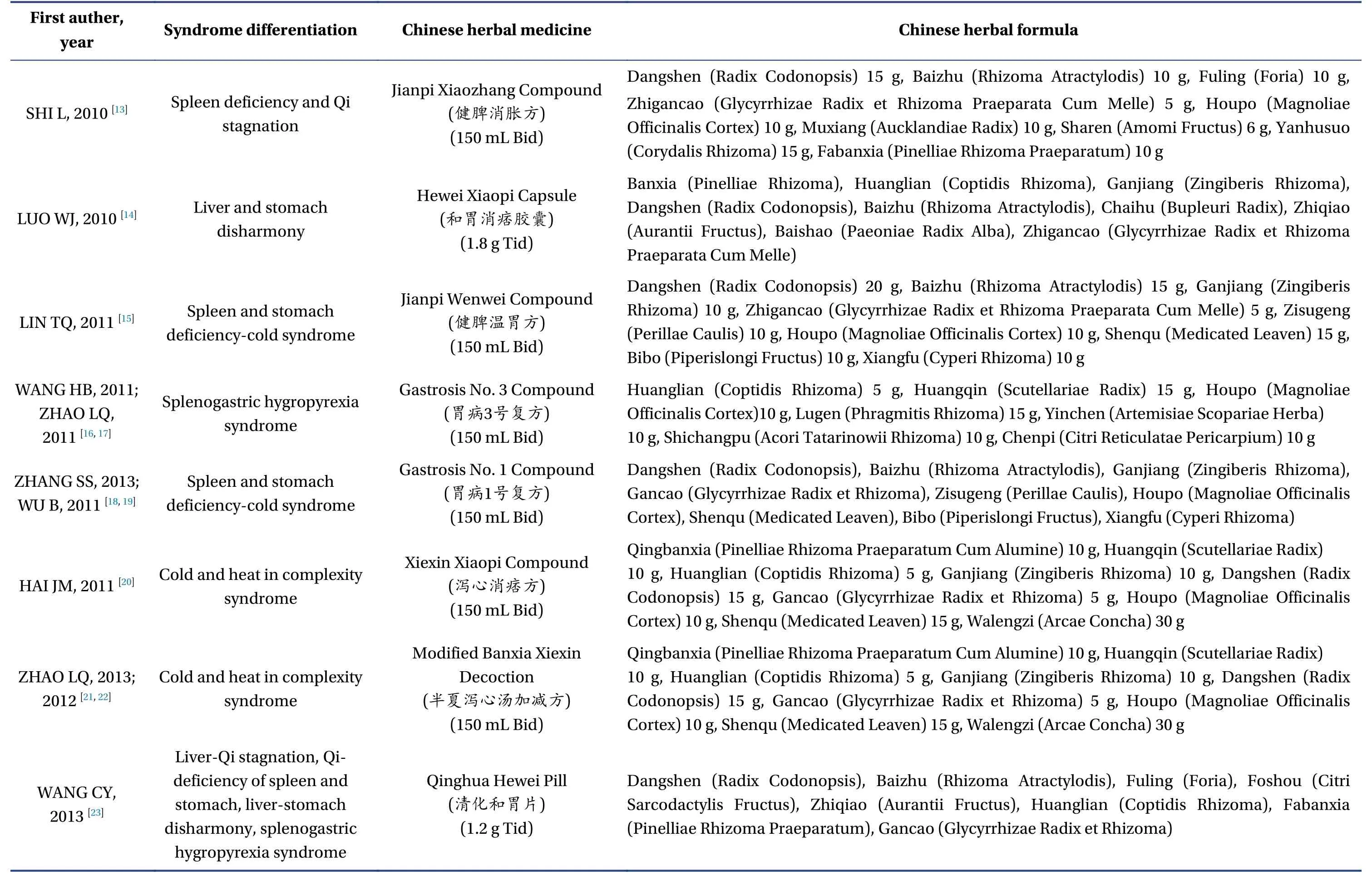
Table 2 Composition of prescriptions included in the literature

Table 2 Continued
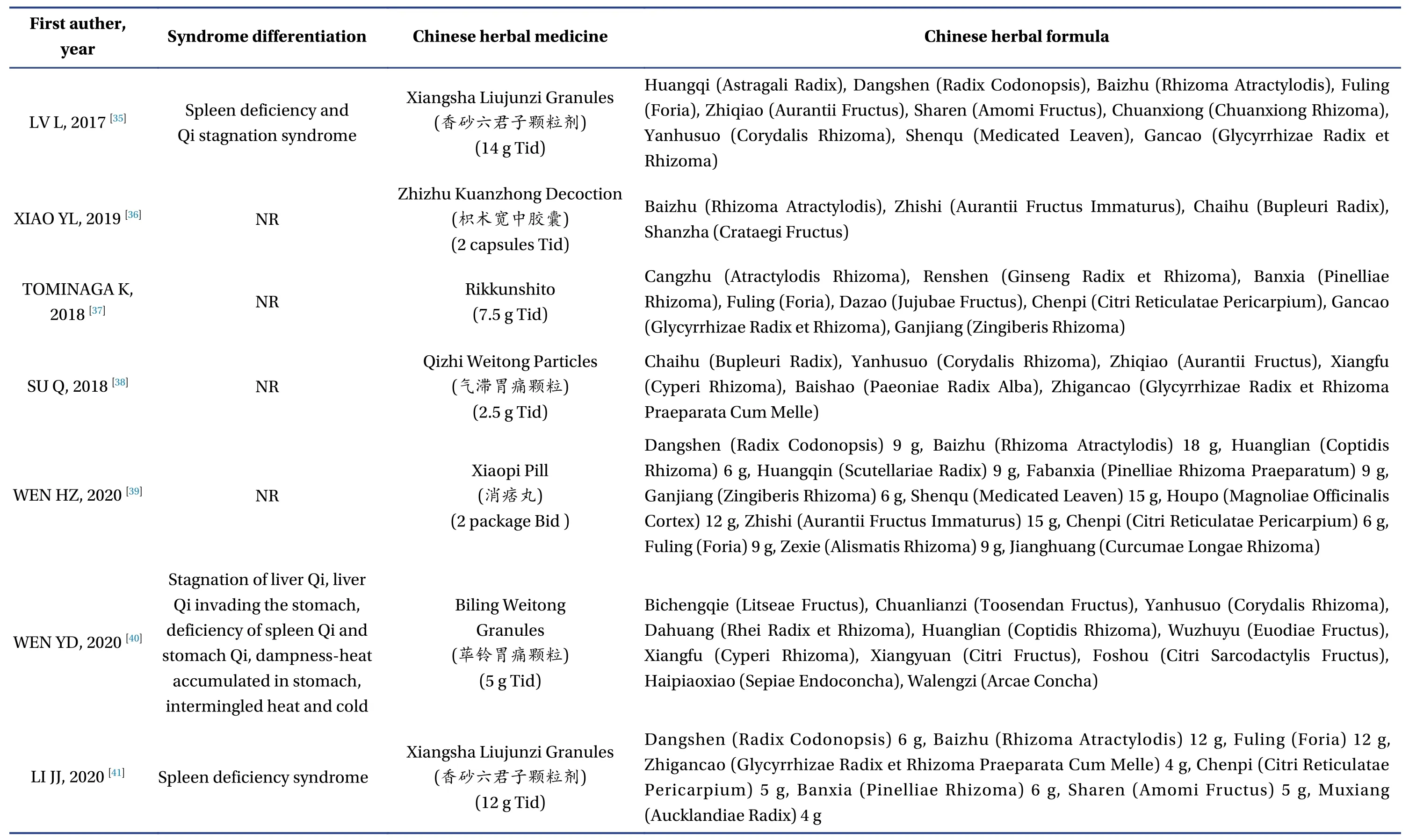
Table 2 Continued
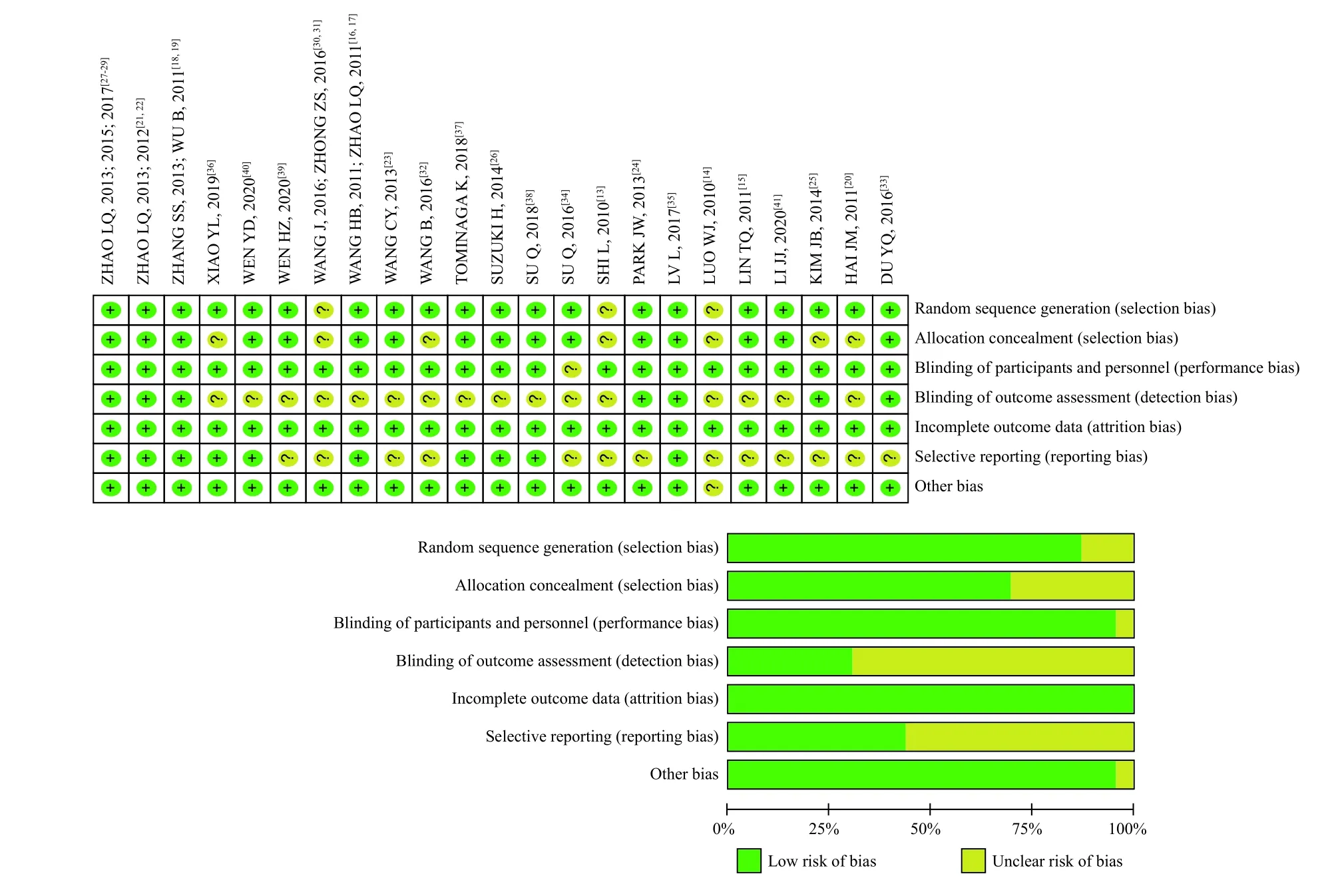
Figure 2 The quality evaluation of included research literatures
3.3.1 Overall symptom remission rateAmong the 23 studies included, 16 studies involved 18 articles reported the overall symptom remission rate[13-22,32-36,38,39,41], totaling 1 911 cases, 1 091 cases in the Chinese medicine compound treatment group.Of these, 805 cases reported overall symptom improvement(73.8%), and 820 cases in the placebo control group,of which 379 cases reported overall symptom remission (46.2%).Compared with the two groups, the synergistic effect of the TCM compound treatment group was 27.6%, and the NNT was calculated to be 4.3, that is, one person benefited from TCM every 4.3 people treated.
The heterogeneity test showed high heterogeneity (X2= 59.40,P< 0.000 01,I2= 75%); therefore, a randomized effect model was adopted.The results showed that the therapeutic effect of TCM compound was significantly better than that of placebo on the opposite side of overall symptom improvement [RR =1.50, 95% CI (1.29, 1.76),P<0.000 01].According to the different evaluation methods of the total effective rate, there was some homogeneity among the five studies using the TDS score (X2=5.13,P= 0.27,I2= 22%).The results of theZtest showed that the effect of the Chinese herbal compound group was more obvious, which was consistent with the overall results [RR = 1.29, 95% CI (1.14, 1.46),P<0.000 1],and there was a significant heterogeneity among the four studies using the PDS scale (X2=23.27,P<0.000 1,I2=87%).The results of theZtest were also consistent with the overall results [RR = 1.89, 95% CI (1.14,3.12),P=0.01].There was some heterogeneity among the five studies using the TCM syndrome scale (X2= 12.83,P=0.01,I2=69%), and the results of theZtest were consistent with the overall results[RR =1.85, 95% CI (1.24, 2.76),P=0.003].The other two studies used other scales, and the results showed that the effective rate of both Chinese medicine compound groups was higher; however, there was no significant difference with the placebo group (P>0.05)(Figure 3).
3.3.2 Efficacy of single symptomAmong the included studies, there were four studies involved nine articles[16-19,21,22,27-29], which evaluated the curative effect of single symptoms of “epigastric pain, epigastric burning sensation, postprandial fullness discomfort,and early satiety”.A total of 527 patients were included, including 351 patients in the Chinese medicine compound group and 176 patients in the placebo group.The differences in single symptom scores between each group after treatment and baseline were analyzed.

Figure 3 Meta-analysis of overall symptom relief rate of TCM compound compared with placebo
(1) Symptom score of epigastric pain: the heterogeneity test showed homogeneity in the combined study (X2=2.31,P=0.51,I2=0%).Using the fixed effect model, theZtest showed that the improvement of epigastric pain symptoms in FD patients after treatment with TCM compound was better than that of placebo [MD = -0.99, 95% CI (-1.37, -0.61),P<0.000 01] (Figure 4).
(2) Symptom score of epigastric burning sensation: the results of the heterogeneity test showed homogeneity (X2= 4.38,P= 0.22,I2= 32%); thus, the fixed-effect model was adopted.TheZtest showed that the effect of the Chinese medicine compound group was better than that of the placebo group in improving the symptoms of epigastric burning sensation in FD patients [MD = -0.32, 95% CI (-0.59,-0.05),P=0.02] (Figure 5).
(3) Postprandial fullness and bloating score: there was homogeneity among the four studies (X2=1.42,P=0.70,I2=0%).Using the fixed effect model, theZtest showed that the Chinese medicine compound group was significantly better than the placebo group in improving the postprandial fullness symptoms of FD patients [MD=-1.59,95%CI(-1.96,-1.21),P< 0.000 01] (Figure 6).
(4) Early satiety symptom score: there was homogeneity among the four studies (X2=1.34,P=0.72,I2=0%).Using the fixed effect model, the results of theZtest showed that the Chinese medicine compound group was significantly better than the placebo group in improving the early satiety symptoms of FD patients [MD = -0.93, 95% CI (-1.32, -0.54),P< 0.000 01](Figure 7).
3.3.3 TCM Syndrome ScoreAmong the 23 studies ,there were four studies involved seven articles[13,27-31,35]with spleen deficiency and Qi stagnation syndrome,three studies involved four articles[20-22,32]with the mixed syndrome of cold and heat, two studies involved three articles[15,18,19]with spleen and stomach deficiency and cold.There was one study with liverstomach disharmony, spleen-stomach damp-heat,and spleen deficiency syndrome, respectively[14,16,41],and three studies[23,33,40]with various syndrome types such as dampness-heat of the spleen and stomach,disharmony between the liver and stomach, etc.,whereas the rest of the studies did not show clear syndrome differentiation.Among the studies on syndrome differentiation, nine studies involved 13 articles[13,16-23,30-32,41]reported TCM syndrome scores.The differences in TCM syndrome scores between the two groups after treatment and baseline were analyzed.As shown in Figure 8, the heterogeneity test showed that the heterogeneity of the combined study was large (X2=36.56,P< 0.000 1,I2=78%); thus, the random effects model was adopted.The results showed that the improvement of TCM syndromes after treatment with TCM compounds was more evident than that of placebo treatment [MD = -5.58,95% CI (-7.55,-3.61),P< 0.000 01].(Figure 8)

Figure 4 Meta-analysis of symptom integral changes of abdominal pain on TCM compound versus placebo

Figure 5 Meta-analysis of symptom score changes of epigastric burning sensation compared with placebo in TCM compound

Figure 6 Meta-analysis of the changes of postprandial fullness and bloating score of TCM compound compared with placebo
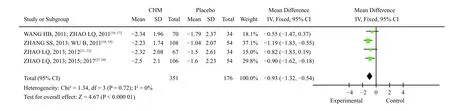
Figure 7 Meta-analysis of early satiety symptom score changes of TCM compound compared with placebo
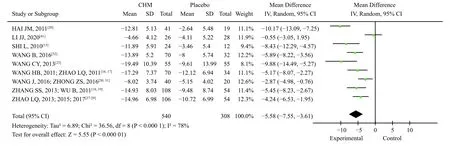
Figure 8 Meta-analysis of TCM syndrome integral changes between TCM compound and placebo
3.3.4 Gastric emptying rateThree studies involved four articles[13,30,31,35]reported the gastric emptying rate.SHI[13]used radioimaging, whereas ZHONG et al.[31]and LV et al.[35]used real-time ultrasound.The heterogeneity test showed that the combined study had some homogeneity (X2=3.18,P=0.20,I2=37%); thus, the fixed-effect model was adopted.Ztest showed that the Chinese medicine compound group could improve the gastric emptying rate of FD patients better than the placebo group [MD =12.22, 95% CI(8.90, 15.55),P< 0.000 01] (Figure 9).
3.3.5 Quality of lifeSeven studies reported an improvement in quality of life, among which two studies[26,32]were evaluated using the FD-QoL scale, and five studies involved 10 articles[16-19,21-23,27-29]were evaluated using the MOS 36-item short-form health survey (SF-36).According to the physical component summary (PCS) score and mental component summary (MCS) score provided by the SF-36, the sum of the two was extracted as the final evaluation result of the SF-36 scale.The differences between the baseline score and the TCM compound group and the placebo group were analyzed.Heterogeneity test results showed that the combined study had a certain heterogeneity (X2=41.32,P< 0.000 01,I2=83%); thus,the random effects model was adopted.Ztest results showed that the improvement trend of quality of life in the Chinese medicine compound group was better; however, there was no significant difference between the two groups [MD =11.27, 95% CI (0.10,22.43),P=0.05].The results of the heterogeneity test showed that there was homogeneity among the five studies using the SF-36 scale (X2=4.41,P=0.35,I2=9%), and the results of theZtest showed that the effect of the Chinese medicine compound group was better than that of the placebo group [MD =30.51,95% CI (8.64, 52.37),P=0.006].There was a significant heterogeneity among the three studies using the FD-QoL scale (X2=32.92,P< 0.000 01,I2=94%).The results of theZtest showed that there was no significant difference between the Chinese herbal compound group and the placebo group [MD =5.39, 95%CI (-7.03, 17.81),P=0.39] (Figure 10).
3.3.6 Adverse eventsAmong the 23 studies included, one study[30,31]did not mention any adverse events, whereas the rest observed and evaluated adverse events.Among them, seven studies[24,26,33,36-38,40]reported the occurrence of adverse drug events, 54(7.8%) of the 690 patients involved in the trial group reported adverse reactions, and 40 (5.8%) of the 688 patients in the placebo group reported adverse reactions.The heterogeneity test showed that the combined study had homogeneity (X2=7.18,P=0.30,I2=16%).Using the fixed effect model, the results showed no significant difference in adverse reactions between the Chinese medicine compound group and placebo group [RR = 1.34, 95% CI (0.91, 1.96),P=0.14] (Figure 11).
4 Discussion
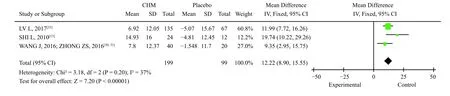
Figure 9 Meta-analysis of gastric emptying rate after placebo treatment compared with TCM compound

Figure 10 Meta-analysis of quality of life after placebo treatment compared with TCM compound
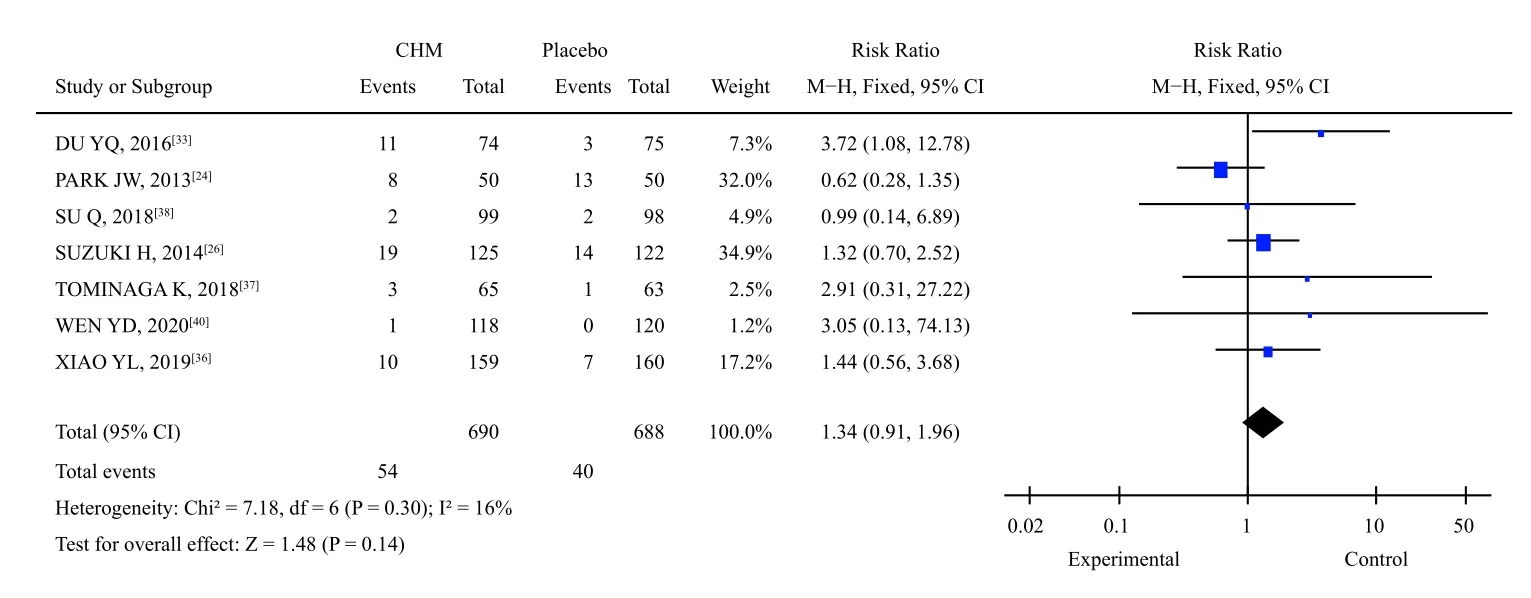
Figure 11 Meta-analysis of adverse reaction events of TCM compound versus placebo
This study collected clinical data from randomized,double-blind, placebo-controlled trials and evaluated systematically and objectively the clinical efficacy and safety of Chinese herbal compounds for FD treatment using evidence-based medicine.In the case of symptom improvement, compared with placebo, TCM compounds can better relieve the overall symptoms of FD patients.The TDS score is a commonly used evaluation method for dyspepsia symptoms, including epigastric pain, burning sensation, postprandial fullness discomfort, early satiety,nausea, vomiting, and belching.There was some homogeneity among the five studies using the TDS score in the subgroup analysis.The results are consistent with the overall study, indicating that the research results are relatively reliable.Patients with FD often exhibit complex digestive tract symptoms.This study showed that TCM compounds have a good curative effect on the four most typical symptoms of FD.In addition, TCM syndromes can reflect the overall performance of a class of patients with similar characteristics, which is a better means of evaluating the overall therapeutic effect of TCM compounds;however, due to the lack of concentration of syndrome differentiation in this study, there is no subgroup analysis of the treatment effect of different syndrome types.However, the current TCM syndrome scale still needs to be standardized and improved,which might also be the reason for the high heterogeneity among various studies in the analysis of TCM syndrome scores in this study.Patients with functional dyspepsia often show depression and decreased quality of life[5].Studies by LV et al.[35]and TOMINAGA et al.[37]showed that TCM compounds can also improve HADS anxiety and depression scores in patients with FD.FD is a chronic disease with a long cycle and easy recurrence.In the literature included in this study, there are four studies[18,21,28,35]showing that the improvement effect of TCM compounds on FD symptoms lasts for at least four weeks; however,few research has reported the recurrence rate of FD.
The pathogenesis of FD is complex and still not completely clear; however, it may be related to visceral hypersensitivity, gastrointestinal motility disorder, and low-grade inflammation of the duodenal mucosa caused by abnormal brain-intestinal interactions.In addition to the improvement of gastric emptying, WANG et al.[30]also found that the treatment of TCM for invigorating the spleen and regulating Qi can significantly increase the levels of serum motilin and ghrelin in FD patients with spleen deficiency and Qi stagnation syndrome, which may be one of the mechanisms for improving gastric motility.HAI[20]tested visceral sensitivity by water load combined with B-ultrasound test and found that the improvement of visceral sensitivity by TCM compound is better than placebo, which may be one of the effective mechanisms of TCM compounds in improving FD symptoms.
The safety of TCM compounds is also worthy of attention, and no severe adverse reactions were reported in 2 898 patients included in this study.Notably, research by DU et al.[33]showed that the incidence of adverse reactions in the Houpo Paiqi Mixture group was significantly higher than that in the placebo group, and its adverse reactions were mainly mild diarrhea.The drug is composed of Houpo(Magnoliae Officinalis Cortex), Muxiang (Aucklandiae Radix), Zhishi (Aurantii Fructus Immaturus), and Dahuang (Rhei Radix et Rhizoma), and the occurrence of adverse reactions is mainly related to the pharmacological effects of the drug.Adverse reactions reported in other studies include nausea, headache, and a mild increase in alanine aminotransferase.In general, Chinese medicine compound treatment of FD has better safety; however, in clinical application, it should be reasonable to differentiate symptoms and signs and clarify the scope of application of drugs.
5 Conclusion
In summary, this meta-analysis shows that Chinese medicine compounds are more effective than placebo in treating FD due to the improved indigestion symptoms, CMS, gastric emptying rate, and quality of life of FD patients.There are no severe adverse reactions.However, due to the limitation of the number and quality of the included studies, the above conclusions should be further analyzed and verified.
Acknowledgements
We thank for the funding support from the National Natural Science Foundation of China Project (No.81820108033) and the Project of Wangjing Hospital,China Academy of Chinese Medical Sciences (No.WJYY2020-18).
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年4期
Digital Chinese Medicine2021年4期
- Digital Chinese Medicine的其它文章
- P-glycoprotein mediated interactions between Chinese materia medica and pharmaceutical drugs
- Systematic review of robust experimental models of rheumatoid arthritis for basic research
- Rational drug design, synthesis, and biological evaluation of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides as potential antimalarial, antifungal, and antibacterial agents
- Study on differential gene expression profile of serum exosomes in patients with acute cerebral infarction
- Effects of Fuke Qianjin Formula on hormones and their receptors and metabonomics study in uterine fibroids model rats
- Protective effects of Fufang Ejiao Jiang against aplastic anemia assessed by network pharmacology and metabolomics strategy
