Could gastrointestinal tumor-initiating cells originate from cell-cell fusion in vivo?
Yang Zhou,Jun-Ting Cheng,Zi-Xian Feng,Ying-Ying Wang,Ying Zhang,Wen-Qi Cai,Zi-Wen Han,Xian-Wang Wang,Ying Xiang,Hui-Yu Yang,Bing-Rong Liu,Xiao-Chun Peng,Shu-Zhong Cui,Hong-Wu Xin
Yang Zhou,Jun-Ting Cheng,Ying-Ying Wang,Ying Zhang,Wen-Qi Cai,Zi-Wen Han,Xian-Wang Wang,Ying Xiang,Xiao-Chun Peng,Hong-Wu Xin,Laboratory of Oncology,Center for Molecular Medicine,School of Basic Medicine,Health Science Center,Yangtze University,Jingzhou 434023,Hubei Province,China
Yang Zhou,Jun-Ting Cheng,Ying-Ying Wang,Ying Zhang,Wen-Qi Cai,Zi-Wen Han,Xian-Wang Wang,Ying Xiang,Hong-Wu Xin,Department of Biochemistry and Molecular Biology,School of Basic Medicine,Health Science Center,Yangtze University,Jingzhou 434023,Hubei Province,China
Zi-Xian Feng,Department of Oncology and Haematology,Lianjiang People's Hospital,Guangzhou 524400,Guangdong Province,China
Hui-Yu Yang,Bing-Rong Liu,Department of Gastroenterology,The First Affiliated Hospital of Zhengzhou University,Zhengzhou 450000,Henan Province,China
Xiao-Chun Peng,Department of Pathophysiology,School of Basic Medicine,Health Science Center,Yangtze University,Jingzhou 434023,Hubei Province,China
Shu-Zhong Cui,State Key Laboratory of Respiratory Disease,Affiliated Cancer Hospital Institute of Guangzhou Medical University,Guangzhou 510095,Guangdong Province,China
Abstract Tumor-initiating cells (TICs) or cancer stem cells are believed to be responsible for gastrointestinal tumor initiation,progression,metastasis,and drug resistance.It is hypothesized that gastrointestinal TICs (giTICs) might originate from cell-cell fusion.Here,we systemically evaluate the evidence that supports or opposes the hypothesis of giTIC generation from cell-cell fusion both in vitro and in vivo.We review giTICs that are capable of initiating tumors in vivo with 5000 or fewer in vivo fused cells.Under this restriction,there is currently little evidence demonstrating that giTICs originate from cell-cell fusion in vivo.However,there are many reports showing that tumor generation in vitro occurs with more than 5000 fused cells.In addition,the mechanisms of giTIC generation via cell-cell fusion are poorly understood,and thus,we propose its potential mechanisms of action.We suggest that future research should focus on giTIC origination from cell-cell fusion in vivo, isolation or enrichment of giTICs that have tumor-initiating capabilities with 5000 or less in vivo fused cells,and further clarification of the underlying mechanisms.Our review of the current advances in our understanding of giTIC origination from cell-cell fusion may have significant implications for the understanding of carcinogenesis and future cancer therapeutic strategies targeting giTICs.
Key Words:Gastrointestinal tumor-initiating cell;Stem cell;Bone marrow-derived cells;In vivo;Cell-cell fusion;In vitro
INTRODUCTION
Tumors are composed of cells with different levels of differentiation,and tumorinitiating cells (TICs) are the least differentiated cancer cells,which are then capable of giving rise to other cancer cells[1,2].TICs are the source of gastrointestinal tumor initiation,progression,metastasis,and drug and radiation resistance.Moreover,they are capable of self-renewal,can differentiate into multiple cell lineages (such as cancer cells),and can undergo asymmetric cell division.TICs are the most carcinogenic subpopulation of cells in most cancer types[3-5],including gastrointestinal cancers[6].The origin of TICs remains unknown;however,many hypotheses[7]have been proposed to explain it,including those involving gene mutations[8],endogenous reprogramming[9,10],and cell-cell fusion[11-14].
Gastrointestinal TICs (giTICs) may originate from gene mutations[15].Some hypothesized that gastrointestinal stem cells,similar to other types of stem cells,have protective mechanisms that reduce tumorigenesis.These mechanisms include asymmetric cell divisionviachromosomal segregation and relatively slow cell cycles[16],which can protect cells from DNA damage and cellular stress[17].To form giTICs,these mechanisms must be circumvented.The development and progression of colorectal cancer (CRC) are associated with a number of identified gene mutations,in genes such asKRAS,adenomatous polyposis coli (APC),andp53,that promote the conversion of normal epithelial mucosal tissue to cancerous tissue[18,19].The tumor suppressor genep53ensures the genomic stability of stem cells,and can therefore act as a barrier to the formation of TICs[20].Wild-typep53can be experimentally replaced with a mutant version ofp53 viaPCR,CRISPR/Cas9,and knock-in techniques.When a related gene mutation occurs,p53loses its tumor-suppressing ability and acquires additional carcinogenic capabilities.This process is termed as mutantp53gain of function (GOF).Experimental evidence suggests that mutantp53GOF can mediate cancerous properties,such as cell death resistance,sustained proliferation,metastasis and invasion,and tumor-promoting inflammation[21-23].Mutantp53is highly expressed in colorectal TICs and CRC tissues[8].Most evidence that supports this hypothesis arises from the observation that common mutations in CRC would affect normal stem cell behavior.For example,deletion or inactivation of theAPCgene is often the initiating step in colorectal carcinogenesis[18]and as such,acts as a gatekeeper in CRC.The absence ofAPCis rare andAPCis commonly found in gastrointestinal cells,including normal populations of gastrointestinal stem cells,as it plays a major role in regulating normal stem cell function[24].There is little direct evidence demonstrating that giTICs originate from gene mutations in stem cells.Regardless,it is generally believed that giTICs originate from mutated stem cells because stem cells are longlived gastrointestinal cell types.Thus,there is sufficient time for them to accumulate oncogenic mutations[19].In addition,TICs and normal stem cells have many identical or similar properties,indicating that they have a common source or originate from the same ancestor.
Another hypothesis is that giTICs may originate from endogenous reprogramming.A specific combination of transcription factors can reprogram differentiated cells into pluripotent stem cells[25].Following the same reasoning,gastrointestinal epithelial cells can be dedifferentiated into progenitor/stem cellsviaspecific matched signal transduction pathways.Notably,bidirectional transformation between TICs and non-TICs was observed in intestinal tumors.Nuclear factor kappa-B (NF-κβ) induces the stabilization of β-catenin and activation of the β-catenin/T-cell factor transcription complex,which,together with the cancer-causing Kras,can induce dedifferentiation of non-stem colon cancer cells into stem-like cancer cells[9,26]or TICs[27,28].However,the mechanisms underlying their regulation remain unclear[28].Epithelial-mesenchymal transition (EMT) may also be involved in endogenous reprogramming[29]by inducing overexpression of the transcription factors Snail[30-33]and zinc finger E-box-binding protein 1 (Zeb1)[34-37].It is worth noting that Zeb1 activation is associated with Slug(Snai2) in TICs[36].Zeb1,a transcription factor known to be involved in EMT,is necessary for the conversion of non-TICs to TICs.EMT in TICs also induces the expression of CD44,which was shown to be highly expressed in giTICs[36].
Cell-cell fusion can be easily inducedin vitroby physicochemical or biological molecules but also occursin vivo,such as the fusion of sperm and egg cells.Cell fusion is an essential physiological process,which plays a role in fertilization,virus entry,muscle differentiation,and placenta development.It was also reported to be closely associated with the occurrence and development of cancer.Fused cells display the genotype and phenotype of the maternal cells,and hybrids produced by the fusion of different cell types have distinct properties.Cell-cell fusion can be identified by cell size and shape,karyotypes,DNA,gene expression,cell-specific markers,and other properties.Both fused cells and TICs display aneuploidy,such as being tetraploid,and chromosomal instability,as well as have the ability to induce metastasis and drug resistance[38],which suggests that cell-cell fusion may produce TICs.In other words,cell-cell fusion may be a better explanation of TIC generation than the aforementioned conventional gene mutation and endogenous reprogramming hypotheses.In addition,cell-cell fusion may play a role in giTIC formation by introducing endogenous reprogramming,as cell fusion hybrids retain transcripts from both parental cells and also express a unique subset of transcripts[39].
Cell-cell fusionin vivoand tumor-initiating capacityin vivoshould be the criteria used to determine whether giTICs originate from cell-cell fusion.Non-tumor initiating cancer cells can also proliferate and generate tumors when enough of such cells are used.However,theoretically,only TICs can initiate tumor formation using a limited number of cells.Generally,unsorted cancer cells contain both TICs and non-TICs.Therefore,it is difficult to determine which cells are responsible for tumor initiation.Here,we review giTICs that can initiate tumorsin vivousing 5000 or fewerin vivofused cells,as well as tumor-initiating like cells (TILCs) that can generate tumors using more than 5000 fused cells.However,we do not exclude the possibility that more than 5000 hybrid cells may be needed to initiate tumors when cell-cell fusion occasionally induces rare genetic changes that lead to tumor development.
GiTICs originate from cell-cell fusion:Supporting and opposing evidence
The fusion of human cellsin vivowas confirmed by reports describing the fusion of melanoma cells and osteoclasts in 2007 and the fusion of BRAF mutated melanoma and stromal cells in 2016[40,41].The fusion of macrophages and peripheral blood melanoma cells,which was discovered in 2015,also provided evidence for human cell fusionin vivo.Moreover,studies have shown that fusion cells exhibit high expression levels of cell fusion factors,including the cell fusion molecule chemokine receptor 4(CXCR4),as well as that fusion cells may cause tumor metastasis.However,in the tumorigenic experiments described,5 × 106fusion cells were inoculated into mice to generate primary tumors at a number that was much higher than the 5000 cells criteria used forin vivotumor generation.Therefore,the fusion cells were not concluded to be TICs when using the aforementioned restrictions[42].
Currently,there are no reports of giTICs originating from cell-cell fusionin vivo[43].We summarize the reports regarding giTICs originating from cell-cell fusion,including the fusion of gastrointestinal cells with various cell types,the study methods used (in vitroorin vivo),evidence of cell-cell fusion,tumorigenic and tumor-initiating properties of the fused cells,and possible mechanisms of cell-cell fusion (Table 1).
Cell fusion between gastric cells and stem cells may generate giTICs
Xueet al[44]fused DIO-labeled (green) HGC-27 gastric cancer cells with DID-labeled(red) human umbilical cord mesenchymal stem cells (hucMSCs) using polyethylene glycol (PEG) 1500in vitro.The fused cells with double nuclei were then stained with Hoechst 33342 (blue) and DIO-GC and DID-hucMSC double labels (yellow) were observed after 7 d by sortingviaflow cytometry.Then,20 male BALB/C nude mice were injected subcutaneously with 2 × 106gastric cancer cells or fused cells.Mice in the fusion group exhibited tumor nodules at 4 d post-injection,while mice in the gastric cancer group showed no tumor nodules.Moreover,the fusion cells were shown to form more colonies than their parental cells and had higher Cyclin D1 and proliferating cell nuclear antigen (PCNA) expression levels.Cyclin D1 and PCNA expression in tumor tissues is usually positively correlated with cancer cell proliferation.The expression levels of the stem cell transcription factors Sox2,Oct4,Nanog,and Lin28,as well as those of the cancer cell markers CD133 and CD44,were also shown to be increased in the fused cells[44].In addition,real-time RT-PCR analysis revealed that E-cadherin mRNA expression was decreased in fused cells,whereas that of mesenchymal markers,such as α-SMA,FAP,vimentin,snail,N-cadherin,slug,and twist,was significantly increased,indicating that the fused cells underwent EMT.EMT is associated with the metastatic ability and invasiveness of cancer cells.As such,the obtained fusion cells were shown to have EMT properties,which is similar to TICs[44-46].Cell fusionin vitrobetween gastric epithelial cells and MSC also resulted in fusion cells with tumorigenic capabilities that underwent EMT[47].However,these hybrid cells were formedin vitroand the number of cells used for the tumorigenic experiments was much higher than 5000 cells.
In a report by Yanet al[48],the bone marrow of green fluorescent protein (GFP)transgenic female C57BL/6 mice was transplanted into irradiated male homologous mice (68/68),all of which survived.Then,the transplanted bone marrow-derived cells(BMDCs) became the main bone marrow cells of the chimeric mice.Tumors were induced using the tumor-causing drug 3-methylcholanthrene.Three of the 12 treated mice successfully developed tumors.Hematoxylin &eosin staining showed two diffuse-type carcinomas in the glandular stomach and one squamous cell carcinoma(SCC).Analysis of CK-18 (mostly expressed in epithelial cancer cells and determinedviaimmunohistochemistry staining) and GFP expression (fluorescence microscopy)showed that cells derived from both cancer types were positive for CK-18 and GFP expression,indicating that they are epithelial tumors originating from BMDCs.Moreover,co-expression of the Y chromosome and GFP in the cytoplasm was detected in a large number of adenocarcinoma cellsviafluorescencein situhybridization (FISH)and immunofluorescence microscopy.In SCC tissues,GFP expression was mainly detected in the interstitium and keratin pearl,but FISH did not detect the presence of the Y chromosome.Instead,the Y chromosome and GFP were co-expressed in the epithelial cells surrounding the SCC.Gastric cancer may originate from the BMDCs of transplant donors and develop initiallyviatrans-differentiation and then cell-cell fusion[48-50].These authors revealed that BMDC-gastric epithelial cell fusion may contribute to the renewal of the gastric mucosa and lead to increased carcinogenesis potential.Additionally,the aforementioned experiments demonstrated that fusion cells exhibit stem cell and cancer cell markers in chemical-induced tumor tissuesin vivobut did not prove that fusion cells can initiate tumors.As such,it is not possible to distinguish between drug-induced tumors or fusion cell-induced tumors.
Cell fusion between CRC cells and stem cells
In a previous study[51],researchers directly co-cultured PM7 cells,which are eGFPlabeled bone marrow-derived MSCs,with the DsRED-labeled colon cancer cell line HT-29.After co-culture,some cells showed eGFP and DsRED double-positive labels and these fused cells were shown to be positive for epithelial-specific antigen (ESA)and cytokeratin expression.However,the authors did not investigate the tumorigenic capacity and other stemness properties of the fused cells.Other reports have found similar results[52,53].
Notably,a study revealed that the fusion of intestinal epithelial cancer cells and macrophages from BMDCsin vivoleads to nuclear reprogramming and the authors suggested that the fusion cells may play a role in tumor development and metastasis[39].

Table 1 Tumor initiating cell origination from cell-cell fusion
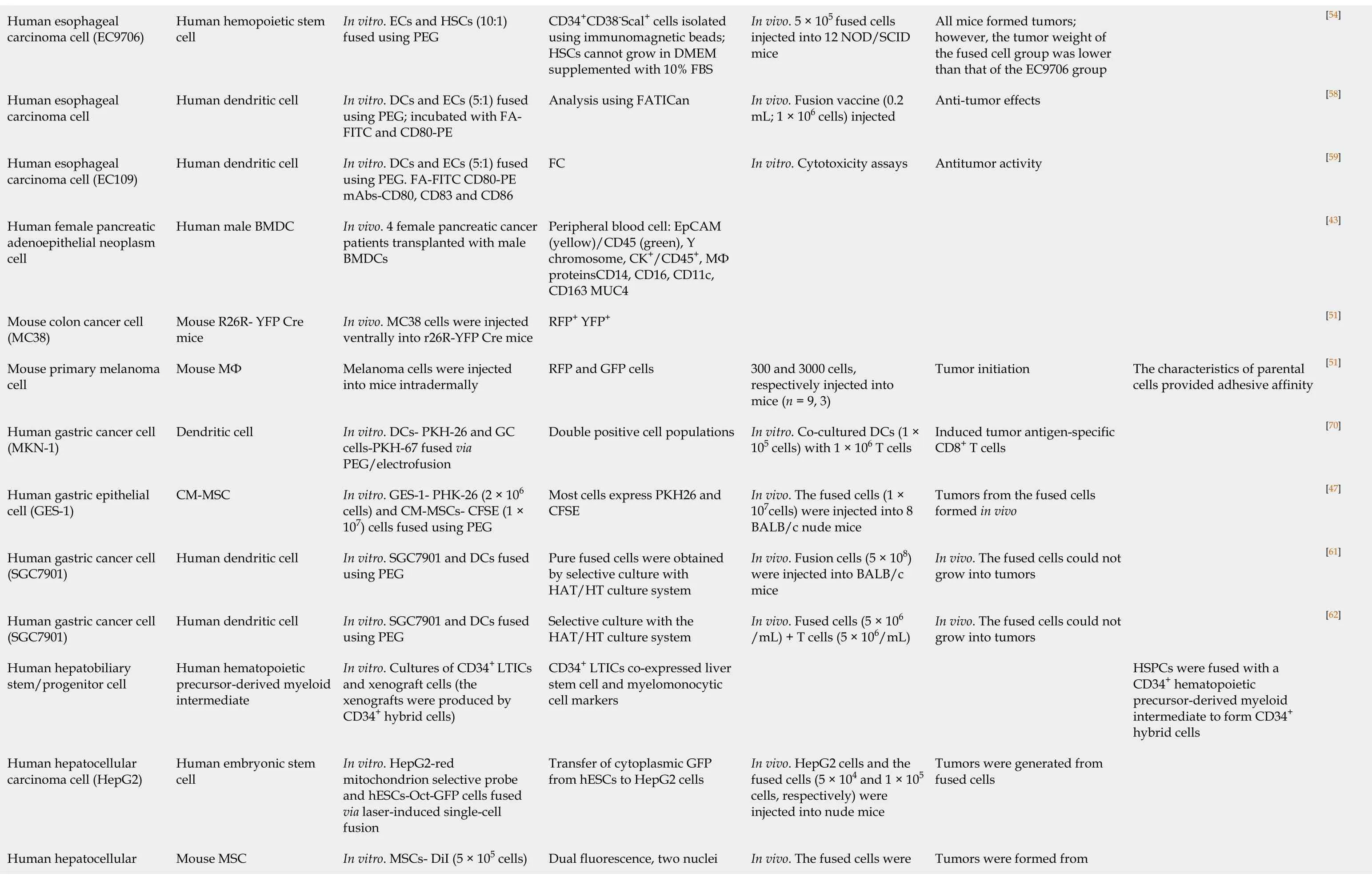
[54][58][59][43][51][51][70][47][61][62]The characteristics of parental cells provided adhesive affinity HSPCs were fused with a CD34+ hematopoietic precursor-derived myeloid intermediate to form CD34+hybrid cells All mice formed tumors;however, the tumor weight of the fused cell group was lower than that of the EC9706 group Anti-tumor effects Antitumor activity Tumor initiation Induced tumor antigen-specific CD8+ T cells Tumors from the fused cells formed in vivo In vivo. The fused cells could not grow into tumors In vivo. The fused cells could not grow into tumors Tumors were generated from fused cells Tumors were formed from In vivo. 5 × 105 fused cells injected into 12 NOD/SCID mice In vivo. Fusion vaccine (0.2 mL;1 × 106 cells) injected In vitro.Cytotoxicity assays 300 and 3000 cells,respectively injected into mice (n = 9,3)In vitro.Co-cultured DCs (1 ×105 cells) with 1 × 106 T cells In vivo. The fused cells (1 ×107cells) were injected into 8 BALB/c nude mice In vivo. Fusion cells (5 × 108)were injected into BALB/c mice In vivo. Fused cells (5 × 106/mL) + T cells (5 × 106/mL)In vivo. HepG2 cells and the fused cells (5 × 104 and 1 × 105 cells, respectively) were injected into nude mice In vivo. The fused cells were CD34+CD38-Scal+ cells isolated using immunomagnetic beads;HSCs cannot grow in DMEM supplemented with 10% FBS Analysis using FATICan FC Peripheral blood cell: EpCAM(yellow)/CD45 (green),Y chromosome,CK+/CD45+,MФ proteinsCD14,CD16, CD11c,CD163 MUC4 RFP+ YFP+RFP and GFP cells Double positive cell populations Most cells express PKH26 and CFSE Pure fused cells were obtained by selective culture with HAT/HT culture system Selective culture with the HAT/HT culture system CD34+ LTICs co-expressed liver stem cell and myelomonocytic cell markers Transfer of cytoplasmic GFP from hESCs to HepG2 cells Dual fluorescence, two nuclei In vitro.ECs and HSCs (10:1)fused using PEG In vitro.DCs and ECs (5:1) fused using PEG;incubated with FAFITC and CD80-PE In vitro.DCs and ECs (5:1) fused using PEG.FA-FITC CD80-PE mAbs-CD80, CD83 and CD86 In vivo. 4 female pancreatic cancer patients transplanted with male BMDCs In vivo. MC38 cells were injected ventrally into r26R-YFP Cre mice Melanoma cells were injected into mice intradermally In vitro.DCs- PKH-26 and GC cells-PKH-67 fused via PEG/electrofusion In vitro.GES-1- PHK-26 (2 × 106 cells) and CM-MSCs- CFSE (1 ×107) cells fused using PEG In vitro.SGC7901 and DCs fused using PEG In vitro.SGC7901 and DCs fused using PEG In vitro.Cultures of CD34+ LTICs and xenograft cells (the xenografts were produced by CD34+ hybrid cells)In vitro.HepG2-red mitochondrion selective probe and hESCs-Oct-GFP cells fused via laser-induced single-cell fusion In vitro.MSCs- DiI (5 × 105 cells)Human hemopoietic stem cell Human dendritic cell Human dendritic cell Human male BMDC Mouse R26R- YFP Cre mice Mouse MФ Dendritic cell CM-MSC Human dendritic cell Human dendritic cell Human hematopoietic precursor-derived myeloid intermediate Human embryonic stem cell Mouse MSC Human esophageal carcinoma cell (EC9706)Human esophageal carcinoma cell Human esophageal carcinoma cell (EC109)Human female pancreatic adenoepithelial neoplasm cell Mouse colon cancer cell(MC38)Mouse primary melanoma cell Human gastric cancer cell(MKN-1)Human gastric epithelial cell (GES-1)Human gastric cancer cell(SGC7901)Human gastric cancer cell(SGC7901)Human hepatobiliary stem/progenitor cell Human hepatocellular carcinoma cell (HepG2)Human hepatocellular

[51][86][52][63][64][72][65][87][48][66][39][78]In mice, hematopoietic fusion with non-hematopoietic cell the absence of disease types occurs endogenously in Inflammation and proliferation act together to mediate intestinal cell fusion Chronic inflammation(adenocarcinoma, glandular stomach, not squamous cell carcinoma)epithelium origin at the natural Fusion between circulating blood-derived cells and tumor course of tumorigenesis fused cells Acquired epithelial characteristics Cell fusion is dispensable for tissue homeostasis The fused cells could not generate tumors The fused cells did not generate tumors.CTL anti-tumor effects Anti-tumor effects in vivo fusion causes genetic reprogramming in vivo Bone-marrow/epithelial cell Tumor formed from fused cells In vitro.Activated cytotoxic T lymphocytes Tumor formed from fused cells in vivo Nuclear reprogramming Tumor formed from the fused injected into 7 nude mice/group with 2.4 × 107 cells/group In vivo. BALB/c mice injected with 5 × 105 cells In vivo. Injection of 1 × 104,105,or 106 cells/mouse In vivo. 1 × 106 fused cells and 5.0 × 105 CT26CL25 cells In vivo. Parabiosis surgery(GFP and ROSA mice)In vivo. GCs were induced with a carcinogen In vitro.CTL assay In vivo. 1 × 106 ES-cancer fused cells injected into nude mice In vivo. The IEC-6 fused cells eGFP and DsRED double positive cells Stained for X- (green) and Y-(red) chromosomes and Lamin B1 (white)Co-staining for GFP and EpCAM.GFP+ cells in the intestine EGFP expression in all principal intestinal epithelial lineages Assessedvia the trypan-blue exclusion test Analyzed by FC Analyzedby FC Co-expression of GFP and the Y chromosome Direct.Positive for the Y chromosome and expressed GFP as determined by FM The fusion cells were yellow under the confocal microscope Double fluorescence-positive Co-localization of GFP (green)and β-galactosidase (red)The fused cell emits both CFSE and HepG2-eGFP (1 × 105 cells)fused using PEG In vitro.PM7-eGFP and HT-29-DsRED cells were cocultured In vitro.X- and Y-chromosome determined by FISH. Female recipients of hematopoietic cell transplant from male donors In vivo. CMV-CreGFP+ mice BM were transplanted into iDTR mice In vivo. Donor female mice BMDCs-GFP, male recipient mice In vitro.Tumor cells- PKH67-Green and DCs fused using PEG In vitro.DCs-anti-CD11cmAb and tumor cells- CFSE fused using BM-GFP (5 × 106 cells)PEG In vitro.DCs-PKH26-red andCT26CL25-PKH67-green fused using PEG In vivo. Female mice BMDCs-GFP(1 × 107 cells) transplanted into irradiated male mice In vivo. Male irradiated C57BL/6 mice received female C57BL/6 mice BMDC-GFP In vitro.HCCs PKH-26-red and DCs-PKH-2-green fused using PEG In vitro.Cancer cells-GFP and ES cells-RFP fused using PEG In vivo. W injected in recipient mice (male WT,ApcMin/+,ROSA26,ROSA26/ApcMin/+). Parabiosis In vitro.IEC-6- CFSE andIEC-6-Human MSC Human hematopoietic cell Mouse bone marrowderived cell Human bone marrowderived cell Mouse dendritic cell Mouse dendritic cell Allogeneic and semi allogeneic dendritic cells Mouse BMDC Mouse BMDC Mouse dendritic cell Mouse embryonic stem BM cell Mouse W(macrophage)Mouse intestinal epithelial carcinoma cell (HepG2)Human intestinal cancer cell (HT-29)Human intestinal epithelial cells Mouse intestinal epithelial cell Mouse intestinal stem cell Mouse colon adenocarcinoma cell(CT26)Mouse colon adenocarcinoma cell line(CT26)Mouse colon carcinoma cells (CT26CL25)Mouse colon epithelial cell Mouse gastric epithelial cell Mouse hepatocellular carcinoma cell Mouse hepatoma cell line(Hepa1-6)Mouse intestinal epithelial cancer cell Mouse intestinal epithelial

BMDC:Bone marrow-derived cell;CAM:Cell adhesion molecules;CM-MSCs:Cord matrix-derived mesenchymal stem cells;CTL:Cytotoxic lymphocytes;DC:Dendritic cell;DMEM:Dulbecco's modified eagle medium;EC:Esophageal carcinoma;FACS:Fluorescence-activated cell sorting;FBS:Fetal bovine serum;FM:Fluorescence microscope;FC:Flow cytometry;GC:Gastric cancer;hucMSCs:Human umbilical cord mesenchymal stem cells;HSPC:Hematopoietic stem and progenitor cell;hESC:Human embryonic stem cell;HSC:Hematopoietic stem cell;HCC:Hepatocellular carcinoma;HLA:Human leukocyte antigen;LTICs:Liver tumor-initiating cells;MSC:Mesenchymal stem cell;MNU:N-methyl-N-nitrosourea;PEG:Polyethylene glycol;WBM:Whole bone marrow;W T:Wild-type;IEC:Intestinal epithelial cell;HGC:Human gastric cancer cell;GFP:Green fluorescent protein;MDA:Malonaldehyde;TGF:Transforming growth factor;FITC:Fluorescein isothiocyanate;PE:Physical examination;CM:Chylomicron.
Cell fusion between liver cancer cells and stem cells may generate giTICs
In a previous study,human embryonic stem cells (hESCs) were labeled with Oct-GFP and HepG2 hepatocytes and stained with a mitochondrial (red) probe,and the cells were then fusedvialaser-induced fusion.Later,it was shown that GFP was transferred from hESCs (green) to liver cancer cells (red),confirming the successful generation of fusion cells.Subsequently,different amounts of 5 × 104- 1 × 106fused cells were injected subcutaneously into nude mice,and mice injected with normal liver cancer cells were used as controls.The fused cell group exhibited a tumor incidence of 9/12,while the liver cancer cell group had a tumor incidence of only 1/8.Moreover,a lower number of fused cells were necessary for tumor generation when compared to the liver cancer cell group.These results demonstrated thatin vitrocell fusion between liver cancer cells and stem cells could generate cells with giTILC properties.The tumorigenicity of the fusion-generated giTILCs was also shown to be significantly higher than that of the maternal cancer cells.However,the number of cells used for tumorigenicity experiments was higher than 5000 and cell-cell fusion was inducedin vitro.Similar reports have also shown thatin vitrocell-cell fusion produces tumorigenic hybrid cells or giTILCs.
CD34+hybrid cells extracted from liver cancer cell lines were shown to express high levels of hepatic stem cell and bone marrow mononuclear cell markers.The cells were also shown to be drug-resistant and express some TIC markers.As such,these results suggested that liver TILCs may be formed by the fusion of hepatobiliary stem/progenitor cells and hematopoietic precursor-derived myeloid cells.
Evidence opposing or not supporting the hypothesis that giTICs originate from cellcell fusion
The fusion of gastrointestinal cells and dendritic cells does not produce giTICs or giTILCs,which is generally used to generate tumor vaccines[54-73].In addition,it was shown that cell fusion in the pancreas and esophagus did not generate giTICs or giTILCs[54,74].Moreover,in vitrofusion cells obtained from human colon adenocarcinoma cells and metastatic human cervical cancer HeLa cells were shown to possess cancer cell properties but were not considered to be giTICs or giTILCs[75].Currently,cell-cell fusion between tumor cells and tumor cells has not been shown to lead to the generation of TICs[76].Notably,cell-cell fusion between human colon epithelial cancer cells and normal colon cells not only fail to induce TICs or TILCs but also to generate tumorigenic hybrids in some cases[77].Similarly,cell-cell fusion between intestinal epithelium cells did not generate giTICs or giTILCs[78].
TIC generation from cell-cell fusion in other tissue types
Gastet al[43]intradermally injected mouse primary melanoma cells (RFP+,actin–green fluorescent protein,5 × 104cells) into mice with GFP+macrophages (actin–green fluorescent protein,n= 12).This resulted in tumor formation and mouse macrophages(MФ,GFP) and melanocyte fusion cells (RFP+GFP+) were detected in the tumors.The researchers then implanted 300 RFP+GFP+cells,which were isolated by fluorescence activated cell sorting (FACS),into 19 recipient mice (300 cells per mouse) and found that the fusion cells led to tumor growth.Then,3000 fusion cells per mouse were implanted into three mice for time-dependent analysis of tumor growth characteristics.It was found that the fusion hybrids obtainedin vivohad different tumor growth rates,which indicated that the obtained hybrid cells had heterogeneous growth abilities.Therefore,the authors demonstrated that melanoma TICs originate from cell-cell fusionin vivo[43].Notably,MФ-tumor fusion cells were found in the peripheral blood and were shown to have a stronger ability to metastasize and proliferate.Moreover,the authors showed that the presence of hybrid cells in the peripheral blood of female pancreatic cancer patients with bone marrow transplants from male donors was correlated with disease stage and patient survival.
Xieet al[79]reported that glioma stem cells reorganized the inflammatory microenvironment at the implanting site in mice.Cell-cell fusion between glioma cells and immunoinflammatory cells was also demonstratedin vitroand the fusion cells were shown to be tumorigenic in nude mice and have TILC characteristics.
The formation of cancer cell/MSC hybrids was observed in breast and prostate cancers.Researchers transplanted stem cells into experimental mice and identifiedin situCK8+prostate tumors derived from GFP-labeled transplanted stem cells.This demonstrates that 1 × 106fusion cells formed from stem cells and breast cancer cells can generate tumors.However,due to the excessive number of cells used for the tumorigenesis experiment,these fusion cells may not be TICs[79,80].
GiTICs originate from cell-cell fusion:Possible mechanisms
Cell-cell fusion is a process involving cell chemotactic trafficking,membrane fusion,intramembrane structure fusion (including nucleus),and formation of functional fusion cells[81,82].Moreover,it requires two or more cells to undergo cell membrane merging.However,nuclear fusion is not necessary for the formation of functional fused cells.After the first mitotic division,the binuclear hybrid may undergo nuclear fusion to produce mononuclear cells[43,48,83].Membrane fusion involves the physical merging of membranes from different cells into a single bilayer,allowing for the exchange of cellular contents[84,85].Generally,cells undergo cell fusion as an adaptation to unfavorable environments or factors and for the acquisition of favorable phenotypes.
Here,we summarize and hypothesize the mechanisms of giTIC generation from cell-cell fusion (Table 2 and Figure 1).The generation of TICsviacell-cell fusion may involve several fusion partners:(1) BMDCs fusing with local differentiated cells;(2)BMDCs fusing with local stem cells;(3) Local differentiated cells fusing with local stem cells;and (4) Migratory cells from different tissues fusing with local stem cells.In all cases,fusion cells may inherit the self-renewal ability of stem cells[7,11].
The mechanisms of giTIC generationviacell-cell fusionin vivoare very rarely elaborated.Cell-cell fusion between gastrointestinal cells and stem cells may be spontaneous[39,86],unexplained[44],or induced by carcinogens (carcinogenic chemicals)or carcinogenic factors[48,87],such as chronic inflammation and body damage.It is hypothesized that stem cells may initiate changes in the local microenvironment,which then recruits differentiated cells and leads to the fusion of local stem cells with differentiated cells,thereby generating TICsviacell-cell fusion.Similarly,Helicobacter pylorican cause chronic inflammation leading to gastric epithelial mucosal damage,which may recruit BMDCs.These BMDCs can differentiate through cell fusion with local gastric epithelial cells,leading to giTIC formationviacell-cell fusion and adenocarcinoma development[49].

Table 2 Molecules potentially involved in gastrointestinal tumor-initiating cell generation from cell-cell fusion

Figure 1 Origins of gastrointestinal tumor-initiating cells.
Fusion proteins,also called fusogens,play an important role in mediating membrane fusion[84,85].Cell adhesion molecules (CAMs) and cell membrane glycoproteins can mediate cell fusion.Most CAMs are involved in the process of membrane fusion and some in cell transfer[88,89].CAMs,such as CD44,EpCAM[89,90],and cell membrane glycoprotein CD133,are highly expressed in gastrointestinal tumors,especially in giTICs or giTILCs[3,91].
CXCR4,which is a receptor of the chemokine CXCL12,is preferentially expressed in gastrointestinal tumors and promotes invasion and metastasis of gastrointestinal cancer cells[92-103].The binding of CXCL12 to CXCR4 promotes the directed migration and homing of BMDCs[104,105].Moreover,CXCL12 was shown to attract organ-specific metastases of CXCR4-expressing tumor cells[106,107]and CXCR4-positive MSCs were shown to migrate to the destination area,such as the stem-cell initiated tumor microenvironment[108].Moreover,CXCL12 was shown to attract organ-specific metastases of CXCR4-expressing tumor cells and CXCR4-positive MSCs were shown to migrate to the destination area,such as the stem-cell initiated tumor microenvironment,thereby clarifying the mechanism of the induction/activation-cell migration-adhesion-cell fusion process[82,109].Fujitaet al[110]found that diffuse-type gastric cancer-derived CXCR4-positive stem-like cells penetrate into the gastric wall and migrate to the CXCL12-expressing peritoneum,resulting in the formation of peritoneal tumor lymph nodes and malignant ascites in an immunodeficient mouse model[110],which were also found to contain tumorigenic hybrid cells.Many factors,such as inflammatory factors,exosome secretion,cancer-related signal transduction pathways,and chemokines (such as CXCR4/CXCL12),can promote or cause cell chemotaxis;however,no report has shown that these factors are actually involved in the membrane fusion process[111].
In 2019,the cell-cell fusion of two colon cancer cell lines (HCT116 and LoVo) using cobalt chloride showed that syncytin 1,CD9,CD47,and c-Jun were overexpressed in the polyploid giant cancer cells (fusion cells),while PKARIα and JNK1 expression was decreased.Molecules that mediate cell fusion are usually highly expressed in fusion partner cells and hybrid cells.These highly expressed molecules or molecular pathways may be further studied as candidate cell fusion molecules that mediate cellcell fusion.
The molecules or molecular pathways summarized in Tables 1 and 2 are likely involved in cell-cell fusion processes and the properties of TICs.As such,they may have potential as cell-cell fusion and TIC markers[112-118].
CONCLUSION
Understanding giTIC generation from cell-cell fusion may have significant implications for the understanding of carcinogenesis and the development of future cancer therapeutic strategies targeting giTICs.Under the aforementioned restrictions for giTICs and TILCs,to date,there is little evidence demonstrating that giTICs originate from cell-cell fusionin vivo,although there are reports showing that giTILCs and mouse TICs can formin vivo viathe cell-cell fusion of melanoma cells and macrophages[4,5,43].Human cell-cell fusionin vivohas also been reported,namely,the fusion of stem cells with microglia and mature neurons after the transplantation of bone marrow-derived stem cells[119].In addition,the mechanisms of giTIC generationviacell-cell fusion are poorly understood.As such,we propose potential mechanisms involving a multi-step cell fusion process of different cell fusion partners,which is mediated by chemokine and fusogen molecules.Studies onin vitrocell-cell fusion may promote our understanding of the possible mechanisms of giTICs generationviacellcell fusionin vivo.We suggest that future research should focus on giTIC generationviacell-cell fusionin vivo,isolation of giTICs that have tumor-initiating capabilities when using 5000 or lessin vivofused cells,and the understanding of their underlying mechanisms.
 World Journal of Gastrointestinal Oncology2021年2期
World Journal of Gastrointestinal Oncology2021年2期
- World Journal of Gastrointestinal Oncology的其它文章
- Rare common bile duct metastasis of breast cancer:A case report and literature review
- Outcomes of curative esophageal cancer surgery in elderly:A metaanalysis
- Total neoadjuvant therapy vs standard therapy of locally advanced rectal cancer with high-risk factors for failure
- Influence of the heat irrigating effect of radiofrequency ablation on regional liver tissue in Bama miniature pigs
