Dynamic Changes in the Physicochemical Properties and the Volatile Flavor Compounds in ‘Merlot’ and ‘Vidal’ Icewines during Storage
ZHOU Xueyan, CHEN Yu, LIN Ke, ZHANG Dandan, GE Zhengkai, SU Congyan, WANG Bin, SHI Xuewei
(Food College of Shihezi University, Shihezi 832000, China)
Abstract: In our study, the quality changes of ‘Merlot' and ‘Vidal' icewines produced in Yili, Xinjiang Uygur Autonomous Region were monitored during two-year storage at intervals of one year. The physicochemical properties and aroma compounds of icewines were determined by high performance liquid chromatography (HPLC), titration, spectrophotometry and headspace solid phase microextraction combined gas chromatography (HS-SPME-GC-MS). According to the results of SPSS statistical analysis, good discrimination was achieved among icewines based on the physicochemical indexes. After two-year storage, the contents of total acid, volatile acids and malic acids in ‘Merlot' icewine decreased by 5.9%, 13.2% and 39%, respectively, and lactate content increased by 57.2%. Browning index (BI), wine color (WC), color intensity (CI), color hue (CH), and the proportions of yellow (Ye) and red (Rd) changed significantly after one year of storage; CI, CH and Ye showed increasing trends with storage time, while Rd showed an opposite trend. After two-year storage, the contents of total acid and malic acid in ‘Vidal' icewine increased by 9.8% and 33.1%, respectively, while the content of volatile acid decreased by 29.8%. WC, BI, CI, CH, and Ye changed significantly after one year of storage; WC and CI increased with storage time,whereas there was no significant difference in Rd or the proportion of blue (Bl). The results of principal component analysis(PCA) showed clear discrimination among icewine samples of different ages and between the two icewines. Two-year-old icewines contained the largest number of volatile compounds such as isopropyl alcohol, isoamyl acetate, ethyl hexanoate,hexyl acetate, decanal, citronellol, ethyl phenylacetate, phenethyl acetate, and β-damascenone in ‘Merlot' icewine; and 1-pentanol, isoamyl alcohol, ethyl formate, isopropyl alcohol, furfural, 1-butanol, isoamyl acetate, acetaldol, hexyl acetate,octyl acetate, 3,4-dimethylbenzaldehyde, 5-hydroxymethylfurfural, phenethyl acetate, β-damascenone in ‘Vidal' icewine.The above results showed that storage time had a significant impact the physicochemical properties and aroma compounds of icewines. Most importantly, we found that the volatile flavor composition of ‘Vidal' icewine changed more significantly as compared to ‘Merlot' icewine during storage.
Keywords: icewine; storage period; physicochemical indexes; volatile compounds
Icewine, a dessert wine, is fermented with grapes that have been frozen on the vine and picked at a low temperature(< -8 ℃). The frozen grapes contain higher concentrations of sugar, acids, aroma and flavor compounds because much of the water in the grapes exists in the form of ice, giving a rich flavor and high quality to the frozen grapes[1-2]. According to the International Organization of Vine and Wine (OIV),grapes used to ferment icewine should be harvested and pressed at a low temperature below or equal to -8 ℃, at which, the obtained grape juice should have a minimum value of 25.3° Brix prior to fermentation[3]. After fermentation,icewine should contain residual sugars > 125 g/L and total acidity > 6.5 g/L. Because of the requirement of low temperature, icewine can only be produced in a few countries,such as Austria, Canada, China, Germany and Slovenia,where temperatures can reach -8 ℃ in winter[4].
Furthermore, only the grape cultivars possessing thickskinned berries and resisting cold weather can be used to brew icewine[5]. The varieties of ‘Riesling' and ‘Vidal' grapes are used most commonly in icewine production. Because of the relatively thick skins of the berries and their high resistance to harsh climates, ‘Vidal' grapes have been widely used to produce white icewine, which is praised as one of the highest quality icewines in the world[1,6-7]. ‘Merlot' grapes, as a major red Vitis vinifera species, contain high concentrations of phenolic and bioactive compounds, which are responsible for the high antioxidant properties and nutritional benefits of ‘Merlot' icewine[8]. It is normal to notice that the ‘Merlot'juices and young red wines are fulfill with fruit nuances of cooked plum and dried fig[9].
Like other fermented foods, aroma, as an important part of wine, mostly determines wine quality and price according to the preference of consumers. Head space solid-phase micro extraction with gas chromatography-mass spectrometry (HSSPME-GC-MS) has been successfully applied for analyzing the volatile compounds in grapes and wines because it is a fast, easily used, inexpensive and solvent-free procedure[10-11].Results have shown that fatty acids, esters, alcohols and terpenoids constitute to the main aroma of wine and icewine[12-13]. The aroma of wine is a result of the coordinated and complex balance of volatile compounds that originate from grapes (varietal aromas), secondary products formed during the alcoholic fermentation (fermentative aromas) and aging (post fermentative aromas)[14]. During ageing, chemical reactions modify both chemical compositions and sensory properties of icewine character. In most cases, the aging period of wine is an essential stage in the production process. The effect of ageing is to modify the various organoleptic properties, making some more and others less intense[15]. Most wines are consumed after a period of ageing that may take place in wooden casks, in the bottle, or in both successively. It is expected that this will increase the appeal to the consumers.
The cultivation environment of grapes accounts for the change in sugar concentration, pH, total acidity, ionic strength, viscosity, osmotic pressure and vapor pressure, as well as in their surface and interfacial tensions[16]. The grapeproducing area of Xinjiang Yili River Valley, California of the United States and Bordeaux of France are known as “the world's three largest wine grape paradise-producing areas”.However, only in the special climate of Yining County,Xinjiang, some production areas in the Yili River Valley are suitable for the cultivation and growth of ice wine grapes.Baiano et al.[17]believed that aging time mainly affected limpidity and minerality of Minutolo wines. However, there have been few studies examining the effects of storage time on icewine quality. Considering icewine rare and unique, the roles of both variety and ageing on wine quality, our goal was to study ‘Merlot' ice red wine and ‘Vidal' ice white wine using on the one hand physicochemical analyses and on the other hand volatile flavor compounds analyses. The influences of storage stage on changes in the basic properties as well as volatile flavor compounds data are searched, aiming to gain a more comprehensive knowledge of the composition and changes that occur during the storage period of ‘Merlot' and‘Vidal' icewines.
1 Materials and Methods
1.1 Materials and reagents
The study was carried out with samples from Yili in the west of Xinjiang. A total of 9 icewine samples (Merlot cultivar) and a total of 9 icewine samples (Vidal cultivar)from Yizhu Wine Company (Yining County) were collected.The dates of production of these icewines was 2014, the sampling time was calculated from the end of the alcohol fermentation time, with an interval of one year to obtain the analysis sample. Three well known storage periods of icewines include the end of the alcoholic fermentation and after 1 and 2 years of storage. Particularly, they are kept under similar conditions (16 ℃, 65%) during and after the icewinemaking process.
1.2 Instruments and equipment
5977A-7890B gas chromatography-mass spectrometry,HP-innowax column, Agilent Technology Co. Ltd.; SPME manual injection handle, DVB/CAR/PDMS extraction head, Supelco Co. Ltd., USA; UV-17000 Pharma Spec UV-vis spectrophotometer, Kyoto Co. Ltd., Japan; Acqity arc system high-performance liquid chromatograph (HPLC),Waters wats Co. Ltd., USA; CyberScan pH 510 meter, Eutec Instruments Co. Ltd., Nijkerk, Netherlands.
1.3 Methods
1.3.1 Physicochemical analysis conditions of the icewine samples
The icewines were analyzed for the following parameters: total acidity (pH 8.2) and volatile acidity were determined by titration aliquots and expressed as g tartaric acid/L and g acetic acid/L. The pH values were measured using a Cyber Scan pH 510 meter calibrated with buffer solutions at pH 4.00 and 6.86. The contents of reducing sugar, ethanol, malic acid and lactic acid in the wine were determined by HPLC using a BioRad HPX87H column as described previously[18]. The density were determined as recommended by the OIV[3]. Absorbance measurements were recorded on an UV-17000 Pharma Spec UV-vis spectrophotometer. The Folin-C index assay in icewine samples was performed following the method[19]using 1 cm quartz cuvettes. For color determination, absorbance values at the wavelengths of 420, 520 nm and 620 nm of undiluted icewine were measured; wine color (WC, A520); color intensity(CI, A420+A520+ A620), color hue (CH, A420/A520), and browning index (BI, A420) were determined, and the proportions of yellow color (Ye), red color (Rd) and blue color (Bl) were calculated as the proportions of the respective absorbance at 420, 520, 620 nm to the color density, respectively.Experimental data are presented as the ± s obtained from duplicate analyses of each wine.
1.3.2 Aroma analysis
1.3.2.1 Extraction of Aroma compounds
Aroma compounds were isolated using HS-SPME according to the modified method[20]and analyzed by GCMS. Briefly, a 10 mL icewine sample and 20 μL 20 mg/L 3-octanol standard solution and 3.6 g sodium chloride were placed in a 15 mL glass vial. The vial was sealed with a Teflonlined septum cap, and the sample was equilibrated at 45 ℃ for 20 min. Micro extraction lasted for 60 min at 45 ℃ with stirring(500 r/min). For desorption, the fiber was inserted into the GC-MS injector port for 5 min in splitless mode.
1.3.2.2 Chromatographic conditions
GC-MS analysis was conducted with an Agilent 7890 B-gas chromatography coupled to a 5977A-mass selective detector. The GC was performed by using a HP-innowax column (60 m × 0.25 mm). Helium was the carrier gas at a flow rate of 1 mL/min. The oven temperature program was set as follows: 5 min at 50 ℃, 3 ℃/min up to 125 ℃ (remain 3 min), 2 ℃/min to 180 ℃ (remain 3 min), 15 ℃/min to 230 ℃ and held at this temperature for 15 min. The injector temperature was 230 ℃.
The MS was operated in the electron impact mode with electron energy of 70 eV. The global run time was recorded in full scan mode (m/z40-450 mass range) with a 0.2-sinterval,ion source temperature was 200 ℃, the interface temperature was 280 ℃[21].
Qualitative and quantitative analysis: tentative identification was performed by matching degree of standard compounds in each component peak with NIST14.L library and comparing the obtained mass spectra and retention indices (RIs) with those of reference standards and compounds in the NIST14.L spectrum library. The relative content of each component was calculated by internal standard method[22]. The results were showed in the ±sof three replicates of icewine samples.
1.4 Statistical analysis
Physicochemical and aroma analyses were carried out in triplicate for each icewine sample and expressed as±sto represent the results. SPSS 19.0 (SPSS Inc., Chicago,IL, USA), Origin 8.5 (Origin Lab Inc., Hampton, MS,USA) and Microsoft Office Excel 2016 (Microsoft Corp.,Redmond, WA, USA) were used to process the GC-MS and chemical indicator data. Principal component analysis (PCA)was carried out by SIMCA software to study the potential grouping of volatile flavor substances in the ‘Merlot' and‘Vidal' icewines at different storage stages.
2 Results and Analysis
2.1 Effects of storage times on physicochemical parameters
The physicochemical parameters, as basic characteristics of icewine, were monitored during storage (Table 1). In the‘Merlot' icewine, there were no changes in pH value and density during storage. However, with the prolongation of storage time, the contents of reducing sugars, ethanol and lactic acid were increased. Among them, reducing sugar contents at different storage times showed significant differences (P< 0.05). Meanwhile, the contents of titrate acid, volatile acid and malic acid were decreased. The results showed that the biological stability of ‘Merlot' icewine was good and there was an acid-lowering phenomenon during the storage. Although the pH value was not changed, the compositions of the organic acids were changed significantly,especially the increase of lactic acid content made the icewine taste softer.
While in ‘Vidal' icewine, as times went on, the contents of total acidity, ethanol and malic acid were increased, but the contents of Folin-C index and volatile acidity were decreased.Furthermore, reducing sugars and pH values increased first and then decreased. The change of density was not significant in ‘Vidal' icewines. There were significant differences in the contents of Folin-C index, volatile acidity, malic acid, pH values and reducing sugars at different storage times. The results showed that the biological stability of ‘Vidal' icewine was good. During this period various, acids displayed an increasing tendency to increase acidity, which makes white wine to have a special taste. Furthermore, changes in the Folin-C index indicate a decrease in antioxidant activity,reflecting a loss of nutritional efficacy of ‘Vidal' icewine during storage.
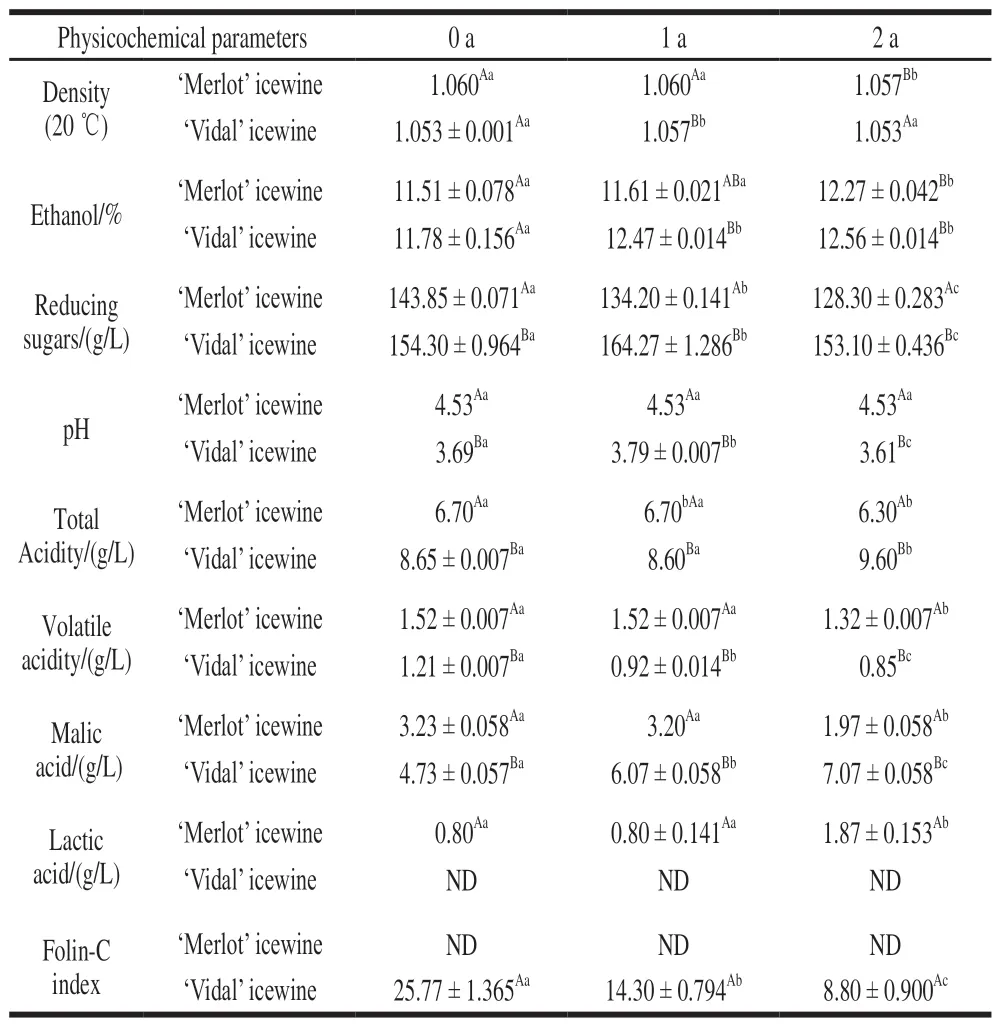
Table 1 Physicochemical parameters of ‘Merlot’ and ‘Vidal’ icewines
2.2 Color change during storage
Color characteristics are an intuitive factor for evaluating icewine quality. The WC, CI, BI and CH of icewine at different storing times were monitored in this study.
The WC reflects the red characteristics of wine. CI is a comprehensive measurement of red, yellow and blue colors in the wine body and can reflect the full color of the wine. In the‘Vidal' icewine, the CI and WC were rapidly increased after 2 years of storage. In the ‘Merlot' icewine, both CI and WC showed a downward trend (Table 2). This result indicated that 2-year storage made no significant difference to the ‘Merlot'icewine color but greatly deepened the ‘Vidal' icewine color.Gómez-Plaza et al.[23]believed that the gradual accumulation of highly colored intermediates contributed to an increase of absorbance in the visible spectrum, especially at 520 nm and 420 nm, which indicates the deepening of the wine color.
The BI represents the degree of browning, which is closely related to a series of complex redox reactions that usually take place during wine aging[24]. The BI (A420) in the‘Merlot' icewine first increased and then decreased, and that of the‘Vidal' icewine gradually increased during storage.Furthermore, the BI of the‘Vidal' icewine apparently increased more after two years of storage. With increasing storage time, the BI of icewine was increased, which is similar to the trend of the WC and CI during storage, as mentioned above for both icewines. Generally, non-enzymatic oxidation browning prevails during wine storage, which is mainly attributed to a series of complex reactions that can produce brown pigments, enrich constituents, and increase the browning index and color intensity[25].
The values of CH, the proportions of Ye, Rd and Bl for the samples (Table 2) followed a similar pattern in both tested icewines. Specifically, the proportion of Rd was decreased throughout storage while the proportion of Bl remained practically constant, CH values and Ye proportion were increased gradually with increasing storage time. Moreover,the CH emphasizes the importance of yellow tones in the red color[26]. Generally, the higher the CH value of icewine was,the higher the proportion of yellow tones was, as compared to that of red tones. With increasing storage time, the CH in the ‘Merlot' icewine was increased, which may be attributed to the quicker increase of yellow tones than that of the red or blue tones. Although the‘Vidal' icewine samples displayed a similar trend for the above color parameters during storage,the color parameters in the ‘Merlot' icewine were better in terms of sensory quality than those in the ‘Vidal' icewine.Therefore, to some extent, the effect of storage time on the color characteristics of the ‘Vidal' icewine is more significant than that of ‘Merlot'.
Overall, during the storage time, the biological stability was high, and the acid contents were changed in both icewines; however, the nutritional efficacy of the ‘Vidal'icewine was declined. The absorbance of the visible spectrum of the different varieties of icewine was increased, the CI, CH and BI of each sample were increased, and the ratio of the blue tones to the overall color was not obviously changed.
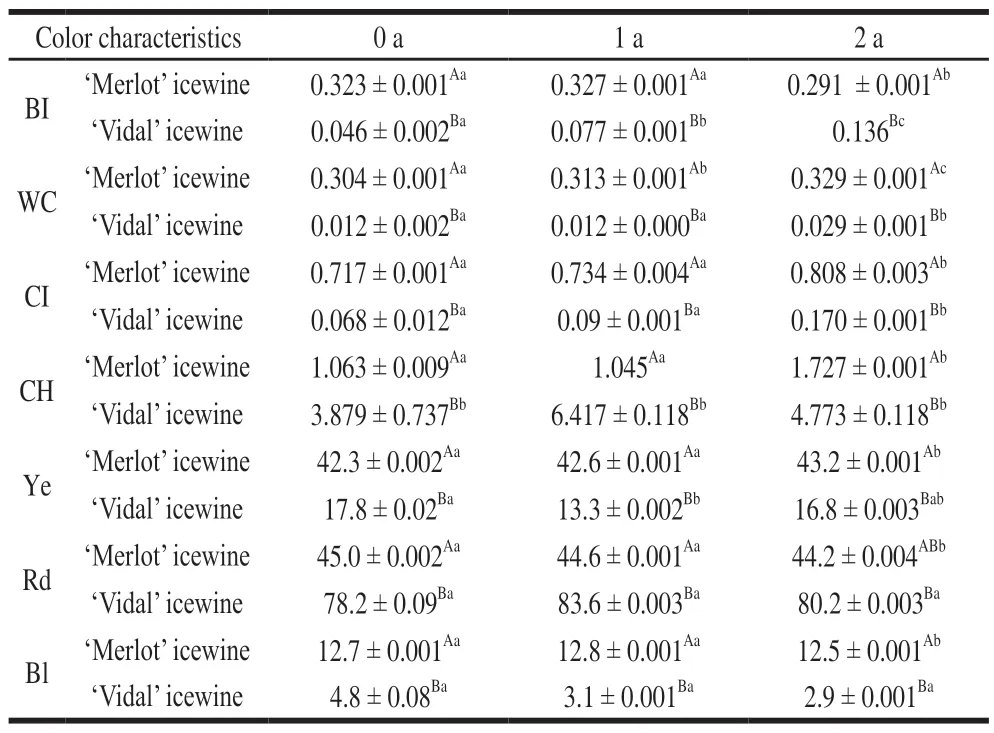
Table 2 Effects of storage time on color characteristics of icewine
2.3 Effect of storage times on icewine volatile compounds
The taste of wine does not exist as an independent entity but is derived from the mutual coordination and presence of alcohols, esters, acids, aldehydes and ketones and terpenes and C13-norisoprenoids, which constitute the structural components of wine aroma[26]. The relative contents of 44 aroma compounds in the ‘Merlot' and ‘Vidal' icewines during storage were identified and quantified (Table 3).
Among the terpenes and C13-norisoprenoids identified,β-damascenone and citronellol were the two most momentous volatile compounds in both icewines, greatly contributing to the icewine aroma. The former aromatic compound exhibited the greatest enhancement in the ‘Merlot' icewine stored for 2 years, while the latter component displayed the greatest decrease in the ‘Vidal' icewine. Both linalool and longifolene presented decreasing trends with increasing storage time in the ‘Vidal' icewine. C13-norisoprenoids have low odor threshold values, and these compounds play an important role in the varietal types of wine[27-28]. Terpene compounds are associated with floral and citric aromas[28].
For volatile aldehydes and ketones, storing time negligibly affected the release of most compounds present in the ‘Merlot' icewine, but benzaldehyde, 2,4-di-tertbutylphenol, furfural, 5-methylfurfural displayed decreasing trends whereas 3,4-dimethyl-benzaldehyde and acetaldol showed the increased levels in the ‘Vidal' icewine with increasing storage time. During the entire storage period,the newly produced substances in the ‘Vidal' and ‘Merlot'icewines were acetaldol and decanal, respectively. Volatile phenolic substances were also detected, and the effects on the final aroma of the icewine were likely positively or negatively depending on their concentrations. Aldehydes,associated with the aroma of fruit or nuts and dried fruits,are commonly metabolized by yeast to form such aromas[29].These oxidation products produced by phenolics, especially nucleophilic aldehydes and ketones, are easily involved in the reactions that occur during wine maturation, which have important effects on the senses and flavor of wine[30].
For volatile esters, a total of 16 compounds were identified and may contribute to fruity nuances to the wine aroma. The extension of storage time in the present investigation remarkably enhanced the production of most detected esters, especially in the case of the ‘Merlot' icewine,in which almost 20% of the total esters increased after 2 years of storage, which was not observed in the ‘Vidal' icewine. In the ‘Merlot' icewine, the major ester compounds that exhibited significant enhancement were ethyl hexanoate, ethyl octanoate,isoamyl acetate, hexyl acetate, phenethyl acetate and diethyl butanedioate, while in the ‘Vidal' icewine, the major compounds that were increased with storage time were ethyl formate,ethyl decanoate, isoamyl acetate, octyl acetate, hexyl acetate and phenethyl acetate. Volatile acetates are among the key compounds that impart the fruity flavor of wines[31].
For the specific volatile acids quantified, it was found that storing time negatively influenced the production of all the determined volatile acids present in the ‘Merlot' icewine,namely, hexanoic acid, octanoic acid and n-decanoic acid.Particularly, after storage for 1 year, the ‘Merlot' icewine exhibited a decrease 40.2% of n-decanoic acid, which was 0.2 times higher than that obtained after 2 years of storage.In contrast, a longer storage duration resulted in a higher production of most of the detected volatile acids in the ‘Vidal'icewine, especially acetic acid, hexanoic acid, decanoic acid and octanoic acid. Malic acid and lactic acid bacteria have been reported to have the capacity to decrease the production of malic acid during high quality red grape wine making[32],which was in accordance with our results in ‘Merlot' icewine.Acids have been related to fatty, cheese and rancid attributes and are produced during the lipid metabolism of yeast[33].These compounds have been attributed to undesirable odors at concentrations higher than 20 mg/L, but lower concentrations made a positive contribution to the quality of the wine by increasing the aroma complexity[34].
Finally, among the group of alcohols, it was found that increasing storage time led to a higher production of 2,3-butanediol, isopropyl alcohol and phenylethyl alcohol in the ‘Merlot' icewine. Similarly, 2-year storage also resulted in a higher production of the three abovementioned volatile alcohols and 1-pentanol. These findings indicate that certain interactions may occur during storage. Of note, the yield of phenylethyl alcohol was evidently increased after 1 year of storage in both icewines, which was more marked in the case of the ‘Merlot' icewine since an increase of this compound was detected in the ‘Merlot' icewine that was 82.68% greater than that observed in the ‘Vidal' icewine. Alcohols were the group with the highest content of volatile components in both the ‘Merlot' and ‘Vidal' icewines, which is in agreement with results reported by other authors for this and other varieties of icewine[35-37]. Contents of higher alcohols below 300 mg/L add desirable complexity to wine, whereas higher concentrations can be unfavorable to wine quality[38]. In contrast, 2-phenylethanol is generally a positive contributor to wine aroma and is characterized by a rose-like aroma[39].
The aroma components of the ‘Merlot' and ‘Vidal'icewines from the different storage years were extracted by HS-SPME. The results are shown in Fig. 1. The detected components were searched and analyzed, and the relative contents of the aroma substances are presented in Table 3.A total of forty-four aroma components were detected in the two icewines subjected to different storage times, including 16 esters, 10 alcohols, 5 organic acids, and 13 aldehydes,ketones and phenols. Many of these volatile substances are found in icewine storage processes. As the aging time increased, the types of volatile aroma substances of both icewines were increased from 13 to 22 and 23 to 27 in the‘Merlot' icewine and the ‘Vidal' icewine, respectively.During the storage period, the increase in the number of ethyl esters of fatty acids and terpenes was more pronounced in the ‘Merlot' icewine, while the number of aldehydes and ethyl esters of fatty acids were more prominent in the ‘Vidal'icewine. The zero-year-old ‘Merlot' icewine had three unique aromatic substances. The one-year aged ‘Merlot' icewine had four unique aromatic substances, while the two-year aged‘Merlot' icewine had nine unique aromatic substances. The aged ‘Vidal' icewine had 4 unique aromatic substances as compared to those detected in the ‘Merlot' icewine. The oneyear aged ‘Vidal' icewine had 2 unique aromatic substances,while the two-year aged ‘Vidal' icewine had 10 unique aromatic substances.
According to Fig. 1A, during the whole storage period,two aroma substances disappeared in the ‘Merlot' icewine.Phenylethyl acetate and ethyl decanoate had peach aroma and coconut aroma, respectively, and their contents were decreased with storage time; that is, the fruit aroma of the‘Merlot' icewine was decreased with prolonged storage time. The newly developed substances were linalool, (R)-citronellol (floral),DL-phenylsuccinic acid,n-propyl acetate,2,3-butanediol (buttery, creamy), andn-decanoic acid (fatty,unpleasant), which are accompanied by floral, creamy and fat flavors. The flavor substances produced during one year of storage are more than those produced during two years of storage. That is, increasing the aging time can promote the accumulation of aroma substances, but in view of the creamy and fat flavors produced after two years of aging, the oneyear aging period is more critical to the accumulation of aroma components, so it is suggested that the storage time of this ‘Merlot' icewine should not exceed two years to avoid the development of unpleasant flavors.
Fig. 1B shows that during the whole storage period, the newly developed volatile flavor substances produced in the‘Vidal' icewine were 2-hexanol (green, grass), octyl butyrate(fruit aromas), glycerin, ethyl phenylacetate (rose blossom,pleasant, floral), phenethyl acetate (peach and rose blossom),isoamyl acetate (fruity, banana, sweet), hexyl acetate(pleasant, fruity, pear), ethyl caproate (fruit aromas), 2-ethyl-1-butanol, citronellol, 5-hydroxymethylfurfural,n-decanoic acid (fatty flavor), ethyl caprate (decanoate has a coconut aroma), 2,4-t-butylphenol, 3-chlorophenyl isothiocyanate,and 4-amino-3-mercaptobenzoic acid. With increasing storage time, the fat flavor of the ‘Vidal' icewine was increased
slightly, but the greatest contribution to the overall flavor wasthe accumulation of fruit flavors and flower flavors. It can be seen that more flavor substances were produced during the second year of storage than those produced in the first year.The prolongation of aging time can promote the accumulation of aroma substances that mainly produce a variety of esters during aging, which often provides more fruit flavor, a light body and a delicious taste, and the two-year aging period is critical for the accumulation of aroma components. Therefore,it is suggested that the storage time of this ‘Vidal' icewine should be at least two years.
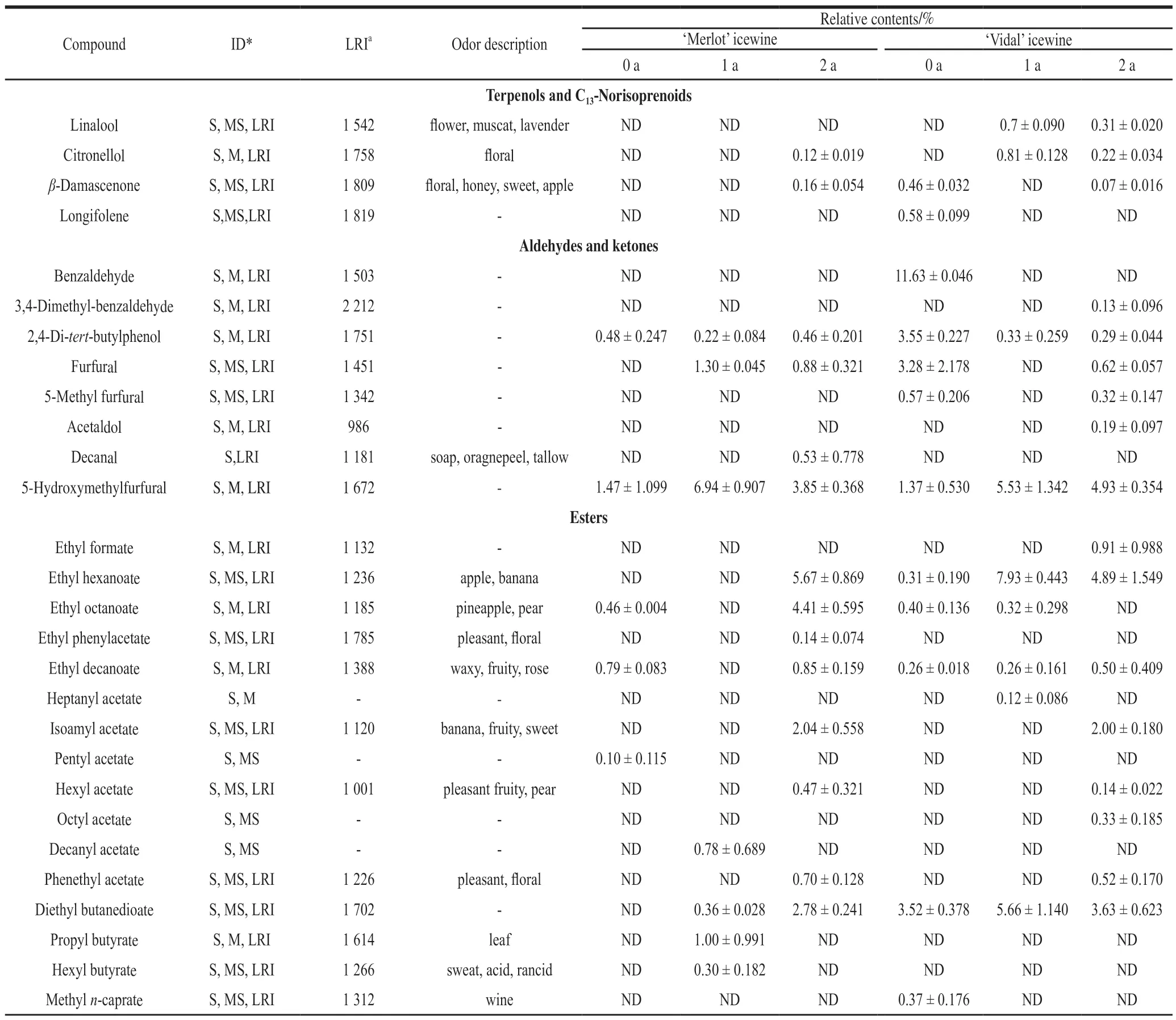
Table 3 Analysis of volatile compounds in icewines of different ages (n = 3)
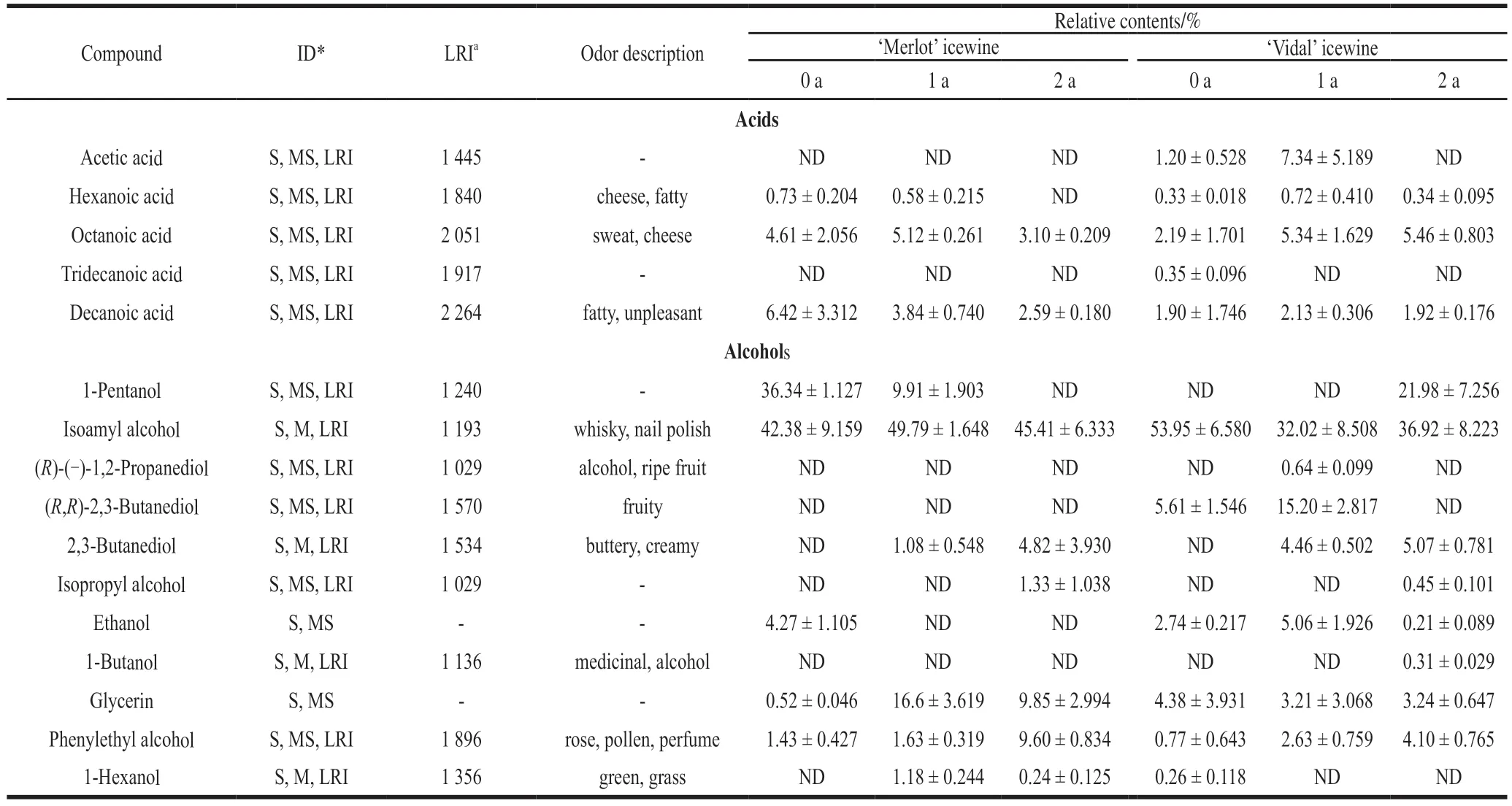
Table 3 continued
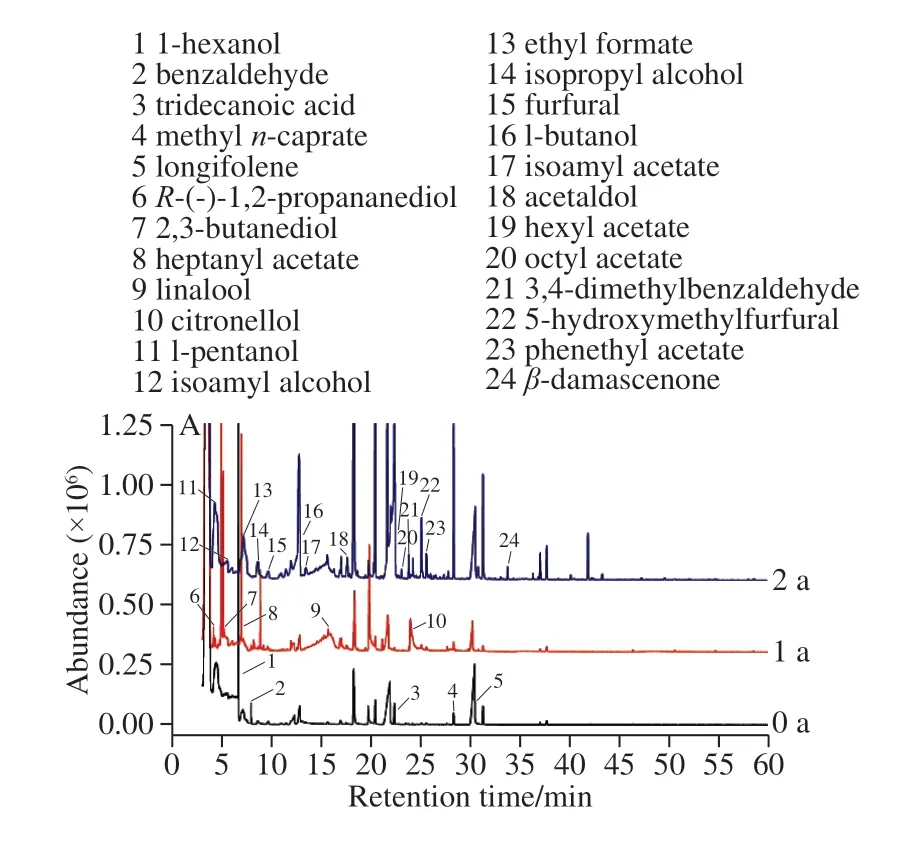
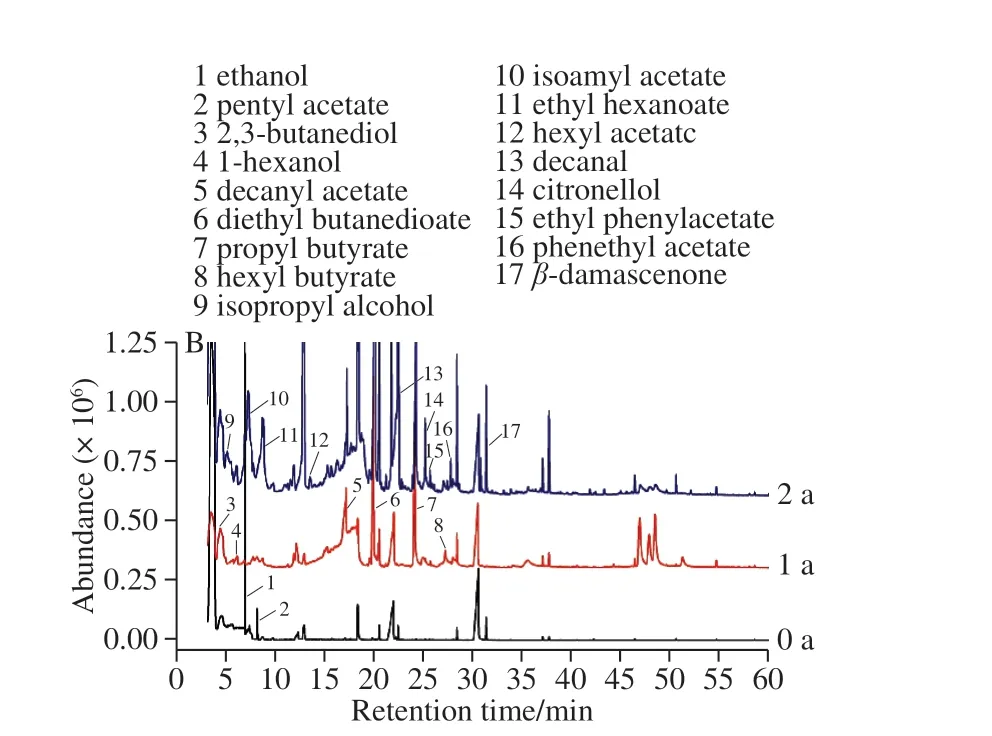
Fig. 1 Gas chromatograms of aroma components in ‘Vidal’ (A) and‘Merlot’ icewines (B) of different ages
Furthermore, the amounts of the volatile flavor compounds were also obviously different. The changes in the main volatile aroma components (alcohols, esters, acids and aldehydes) during different storage times of the ‘Merlot'and ‘Vidal' icewines are shown in Table 2 and Fig. 2. We can obtain the change trend of the total amount of volatile flavor substances during the process of aging (Fig. 2). Regarding the above compounds, the aroma of the varieties composed of C6compounds, volatile phenolic substances and benzene compounds in the ‘Merlot' and ‘Vidal' icewines shows different trends with increasing storage time. The aroma content of the former remained stable during the storage period of two years, while with the prolongation of the storage period, the content of the latter was decreased notably.
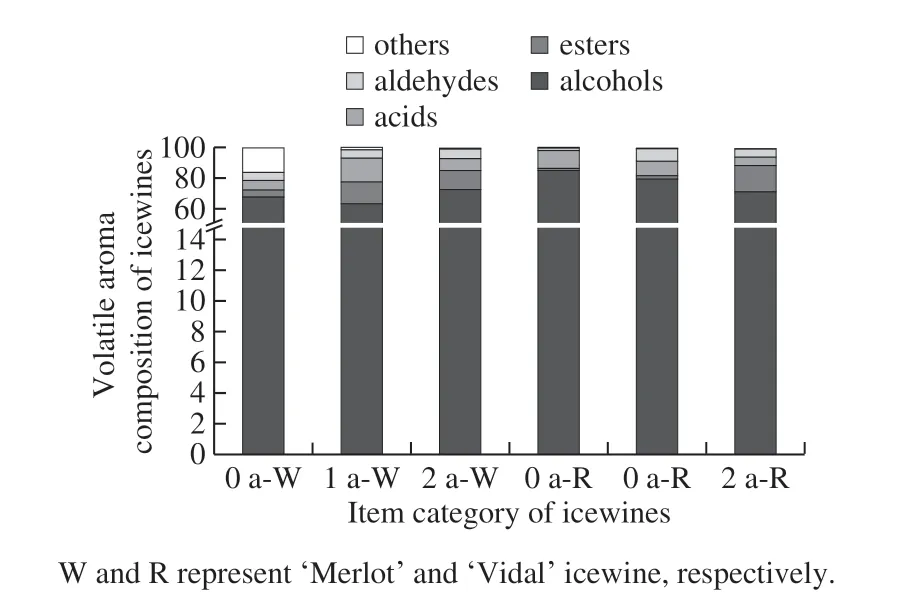
Fig. 2 Proportions of each chemical class of volatile flavor compounds in ‘Vidal’ and ‘Merlot’ icewines
It showed that the storage time of icewine played an important role in the numbers of volatile aroma compounds:the longer the storage time was, the more aroma substances were produced, and the esters in both icewines all presented an increasing trend. In addition, the abundance of volatile substances in the ‘Vidal' icewine was higher than that in the‘Merlot' icewine; however, the types and quantities of esters they produced during storage were not the same.
2.4 Mathematical relationship between storing stage and volatile compounds
To explain the pattern of correlations among the icewine volatile aroma compounds and the storage time, principal component analysis (PCA) was performed in this study.PCA is usually used to explain data and generate predictive models, and its results are usually expressed in terms of a correlation-loading plot. The correlation-loading plot shows the positive and negative relationships of the variables of interest. The set accounting for 70.7% and 61.3% of the total variance in the ‘Merlot' icewine (Fig. 3A) and ‘Vidal'icewine (Fig. 3C), respectively, was generated. The variables were produced by the volatile compounds present in the‘Vidal' icewine and the ‘Merlot' icewine, respectively, and the volatile substances in the three storage stages of the two icewines were well distinguished. Three parallel wellaggregated samples of each icewine were within a certain range. The aroma substances with the highest correlation with the ‘Vidal' icewine with a storage period of two years were 1-pentanol, isopropyl alcohol, phenylethyl alcohol,ethyl formate, isoamyl acetate, hexyl phenylacetate, octyl acetate, diethyl succinate, phenethyl acetate, octanoic acid,and 5-methyl-2-furancarboxaldehyde. The aroma substances correlated with the one-year storage period were [R-(R*,R*)]-2,3-butanediol, ethanol and acetic acid. The highest correlated aroma substances with the zero-year storage period were 3-methyl-1-butanol, ethyl octanoate, decanoic acid, methyl ester, tridecanoic acid, benzaldehyde and longifolene.
Similarly, there were significant differences in aroma substances between the ‘Merlot' icewine samples at different storage times. The substances that contribute greatly to the aroma of the ‘Merlot' icewine stored for zero year were as follows: 1-pentanol, ethanol and pentyl phenylacetate.1-hexanol, decanyl acetate, propyl butyrate, octanoic acid and 5-hydroxymethylfurfural were the substances that contributed greatly to the aroma of the ‘Merlot' icewine stored for one year. However, the main components of the aroma of the‘Merlot' icewine stored for two years were phenylethyl alcohol, isoamyl acetate, hexanoic acid, ethyl ester, hexyl phenylacetate, ethyl octanoate, citronellol, ethyl phenylacetate and phenethyl acetate.
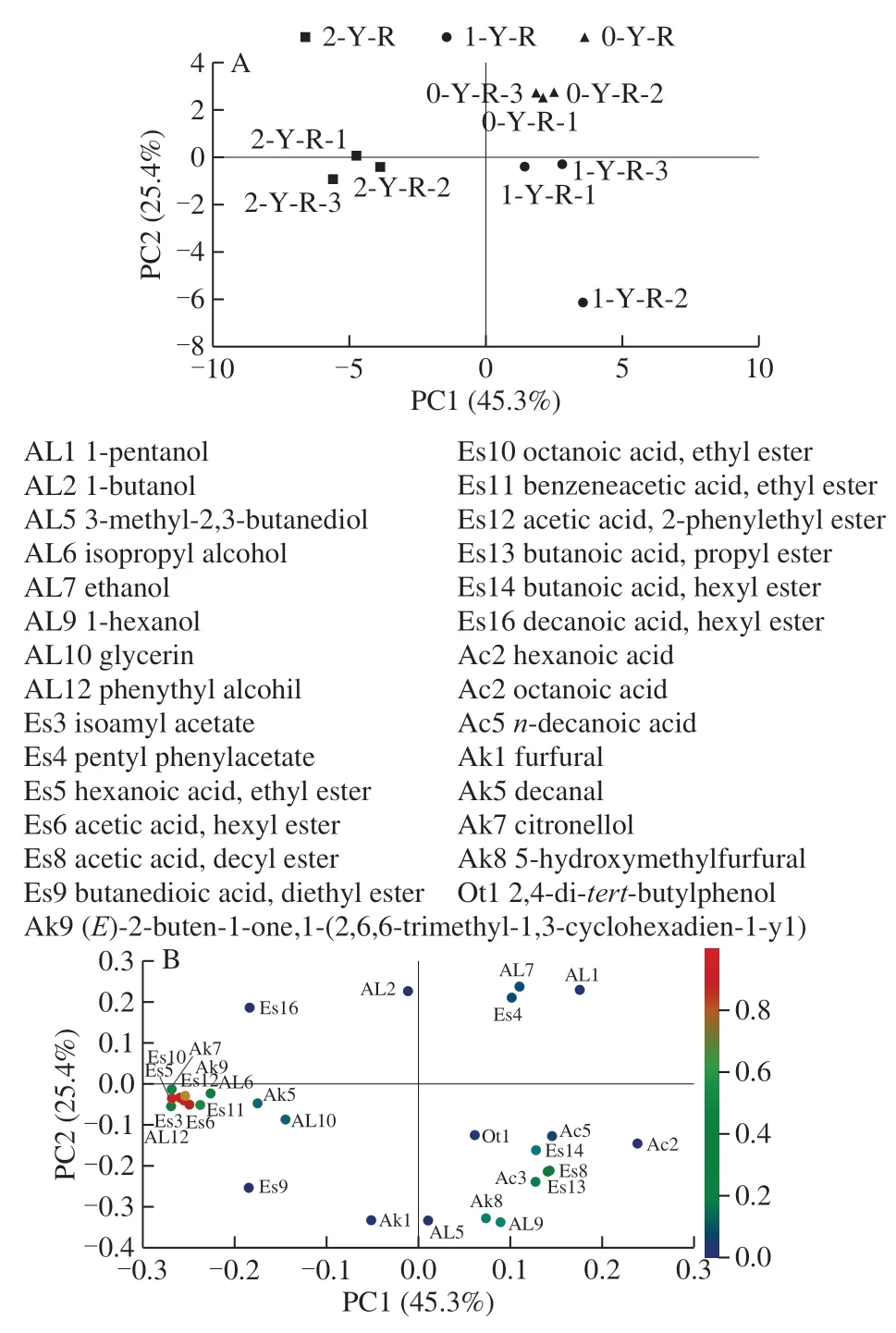
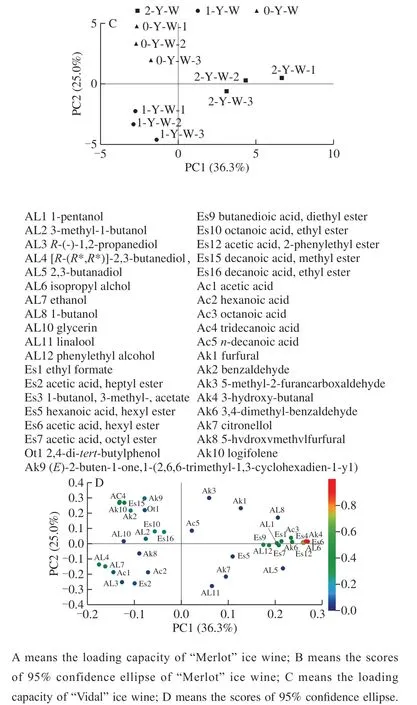
Fig. 3 PCA plots of volatile compounds in nine ‘Merlot’ icewine samples and in nine ‘Vilal’ icewine samples
In addition, principal component analysis (PCA) was used to visualize the differences in volatile composition among different samples and to identify the volatile aroma that provided greatest discrimination between different storage period (Fig. 4). The first two PCs were extracted by PCA, and the cumulative contribution of variances of those components was up to 37.2%. PC1 represents 20.4% of the total variance and PC2 represents 16.8% of the total variance(Fig. 4). The score scatter plots of the PCA of all icewines are shown in Fig. 4A and its corresponding load plot is shown in Fig. 4B. As shown in Fig. 4A, 18 icewines can be divided into three groups according to the location of plotted data.Fig. 4B provides information on the relationships between the volatiles and the PCs. PC1 was closely related to the esters,acids and aldehyde compounds, PC2 correlated with all kinds of compounds. All the variances were generated by volatile compounds, which enabled differentiation of those samples and separated them into 3 groups, by Duncan test[40]. The first group, obtained for the ‘Vidal' icewine and the ‘Merlot'icewine from the two-year storage period, formed close clusters (group 1) in the fourth quadrant of the plot, strongly and positively loaded upon PC1. This finding reflected the fact that the parallel samples of both icewines obtained from the same storage period had very similar patterns for many volatile compounds, such as isopropyl alcohol, phenylethyl alcohol, ethyl formate, isoamyl acetate, hexyl acetate, octyl acetate, ethyl phenylacetate, phenethyl acetate, 3-hydroxybutanal and 3,4-dimethyl-benzaldehyde, which are ranked from high to low regarding percentage content and are consistent with their concentrations reported in Table 2. The‘Vidal' icewine and the ‘Merlot' icewine stored for 0 and 1 year are grouped together, forming close clusters (group 2)in the junction of the second quadrant and the third quadrant of the plot. The negative direction of PC1 was driven by relatively high contents of acetate (decanyl acetate, heptanyl acetate, and phenylacetate), esters (propyl butyrate and hexyl butanoate), an alcohol (R-(-)-1,2-propanediol), an acid (n-decanoic acid) and an aldehyde (3-hydroxy-butanal),which characterized the features of the icewines comprising group 2. The ‘Vidal' icewine obtained from zero-year storage constituted a distinct group (group 3), which was scattered throughout the first quadrant near the positive axis of PC1,reflecting extremely different patterns of ‘Vidal' icewine volatile compounds than that of the other samples. These compounds were characterized by a strong association with terpenols and C13-norisoprenoids (longifolene), benzene compounds (benzaldehyde), acids (tridecanoic acid), other compounds (2,4-di-tert-butylphenol) and esters (hexanoic acid ethyl ester) that had important contributions to the differentiation of the ‘Vidal' icewine samples according to storage time. Furthermore, the percentage contents of volatile acids (acetic acid), esters (ethyl formate, octyl acetate and methyl n-caprate), aldehydes (5-methyl furfural) and benzene compounds (3,4-dimethyl-benzaldehyde and benzaldehyde)contributed to the distinction of the ‘Vidal' white icewine from the two-year and zero-year storage periods.
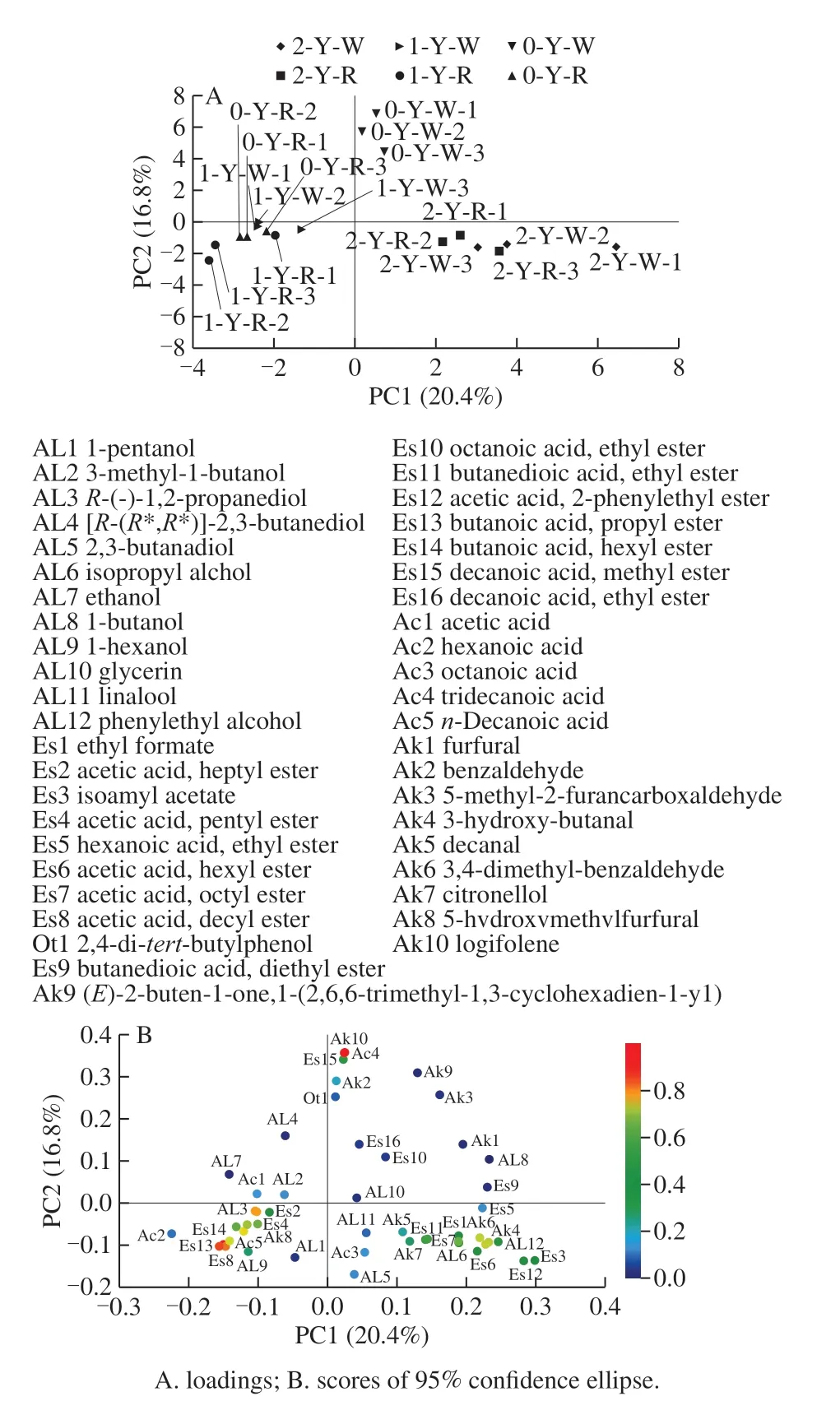
Fig. 4 PCA loading and score plots of volatile compounds in six icewine samples
Finally, from the PCA results, it should be highlighted that the stored period had an obvious influence on the aroma of both icewines, and the variable effect on the ‘Merlot'icewine stored for 0 and 2 years appeared to be smaller as compared with that of the ‘Vidal' icewine samples. In other words, the ‘Merlot' icewine samples with varying storage times showed higher similarity; nevertheless, the ‘Vidal'white icewine samples exhibited a contrasting trend. This may be related to the characteristics of the ‘Vidal' grape,which often has a strong young and unrestrained aroma unique to this variety, and the ‘Merlot' grape does not have distinctive variety characteristics.
3 Conclusions
There were significant differences in visible spectrum and color parameters (wine color/chromaticity, browning index and blue color ratio) between ‘Merlot' icewine and‘Vidal' icewine stored for 0—2 years. The wine color,chromaticity and browning index of each sample were increased, and the ratio of blue color was not changed obviously. When the aging time was prolonged, the types of volatile aroma substances of both icewines were increased, furthermore, the contents of alcohols and acids were decreased, the contents of esters were increased,aldehydes were increased at first and then decreased, and the proportions of aroma of varieties remained the same in the ‘Merlot' icewine. In ‘Vidal' icewine, the content of alcohols was first decreased and then increased, the contents of acids were first increased and then decreased, ester content was increased. Aldehydes basically remained stable, and the aroma of varieties was decreased with the extension of storage time. PCA showed that the aroma components of‘Merlot' and ‘Vidal' icewines preserved for two years were very similar and could be divided into the same category,followed by ‘Vidal' icewine which had been stored for zero year. The ‘Merlot' icewines that preserve the one year and the zero year are very similar to the ‘Vidal' icewines of preserved for one year, they can be divided into the same category.This is the first study on physicochemical compositions and aroma compounds of ‘Vidal' and ‘Merlot' icewines from three different vintages (China). However, from the result of this study, the impact of storage on the ‘Merlot' and ‘Vidal'icewines ought by through sensory evaluation and others evaluation measures and collection more abundant samples from more stored years in the country to further confirm in order to have a more comprehensive and systematic theoretical understanding.

