Critically ill patients with cancer: A clinical perspective
Frank Daniel Martos-Benítez, Caridad de Dios Soler-Morejón, Karla Ximena Lara-Ponce, Versis Orama-Requejo, Dailé Burgos-Aragüez, Hilev Larrondo-Muguercia, Rahim W Lespoir
Frank Daniel Martos-Benítez, Caridad de Dios Soler-Morejón, Karla Ximena Lara-Ponce, Versis Orama-Requejo, Dailé Burgos-Aragüez, Hilev Larrondo-Muguercia, Rahim W Lespoir, Intensive Care Unit 8B, Hermanos Ameijeiras Hospital, Havana 10300, Cuba
Abstract Cancer patients account for 15% of all admissions to intensive care unit (ICU) and 5% will experience a critical illness resulting in ICU admission. Mortality rates have decreased during the last decades because of new anticancer therapies and advanced organ support methods. Since early critical care and organ support is associated with improved survival, timely identification of the onset of clinical signs indicating critical illness is crucial to avoid delaying. This article focused on relevant and current information on epidemiology, diagnosis, and treatment of the main clinical disorders experienced by critically ill cancer patients.
Key Words: Acute respiratory failure; Cancer; Cardiotoxicity; Chemotherapy; Critical care;Infection; Mechanical ventilation; Neutropenia; Postoperative; Sepsis
INTRODUCTION
Cancer is one of the main causes of morbidity and mortality worldwide[1]. Overall cancer-death rates have decreased in both men and women due to reduced tobacco use, improved early detection (e.g., colorectal, breast, and cervix) and enhanced treatment options[2-4]. Currently, critical care medicine contributes as a supportive care for patients with cancer.
In the past decades, patients with advanced hematological or oncological disease were not candidates for entry to intensive care unit (ICU) due to low survival rates;however, expectancy in life-span has changed over the last 25 years because of a breakthrough in terms of new anticancer therapies and organ support methods[5].Cancer patients account for 15% of all admissions to ICU[6]and 5% will experience a critical illness resulting in ICU admission[7]. These frequencies may grow considering the current global burden of cancer and demographic features. Analysis from large databases suggests that a higher proportion of cancer patients survive at ICU discharge[7,8]. Since the five-year survival rate is 41%[9], reluctance to admit cancer patients to the ICU must be avoided from medical practice. In fact, most recent evidences support an increased ICU admission because of improved ICU and hospital outcomes[8-11].
Patients with hematological malignancy or solid tumor are at higher risk for ICU admission as a consequence of acute respiratory failure due to pulmonary infiltrates or pneumonia, healthcare associated infection by multidrug-resistant pathogens related or not with immunosuppression, postoperative care, cardiovascular complications,and neurological disorders[6,12-14].
The aim of this article was to provide critical care clinicians with an overview on relevant and current information on epidemiology, diagnosis, and treatment of the main clinical disorders experienced by cancer patients with a critical illness.
ADMISSION CRITERIA TO INTENSIVE CARE UNIT
The main reasons for admitting cancer patients to ICU are postoperative care, acute respiratory failure (ARF), and sepsis. Other clinical situations are cardiac complications, neurological disorders, acute kidney injury, bleeding, and oncological emergencies[15]. Mucositis, acute graft-versus-host disease, diffuse alveolar hemorrhage, cardiac dysfunction, hypertension and hepatic venoocclusive disease are other causes in hematological malignancies[16](Table 1).
Several studies indicate that early ICU admission is associated with higher survival rates[17-21]; thus, timely identification of patients at onset of clinical signs indicating critical deterioration is crucial to avoid delayed organ support[6]. The ability of physician to identify what patient is expected to benefit from ICU management is limited. As proposed by Ñamendys-Silvaet al[22], the following criteria may help for this purpose: Sequential Organ Failure Assessment (SOFA) score between 7 and 10 or< 3 organ failures, recent diagnosis of oncohematological disease, cancer-related medical emergencies (e.g., tumor lysis syndrome, pulmonary infiltrates in patients with leukemia or leukostasis as the initial manifestation of leukemia), likelihood of cure or disease control, Eastern Cooperative Oncology Group scale between 0 and 2,and postoperative intensive care for patients undergoing complex surgical procedures who require hemodynamic monitoring and/or ventilatory support.
As decision-making for ICU admission and management may be challenging, the following strategy is recommended: (1) Full-code ICU management: Full organ support methods (e.g., invasive mechanical ventilation, vasopressors, renal replacement therapy, nutritional support) without limitations of ICU resources for patients with curative therapeutic options, patients in remission, and those with expected life-span ≥ 1 year[5]; (2) Time-limited ICU trial: ICU management with fullcode status for a limited period. Although the time of full-code should be judged in accordance with the patient´s clinical course rather than with a fixed time, a reasonable interval could be 2 wk in hematology patients (1 wk if multiple organ failure) and 1 wk in patients with solid tumors (4-5 d if multiple organ failure)[5,6,23]; (3) Patients with poor performance status not eligible for further anticancer therapy, dying patients, and those rejecting critical care treatment should not be admitted to the ICU in general[5];and (4) Other indications: Exceptional ICU admission (same as time-limited ICU trial)for patients in whom new drugs (approved or not) are available; prophylactic ICU admission with full-code for high-risk patients (e.g., patients at risk of tumor lysis syndrome or acute respiratory failure after chemotherapy); palliative ICU admissionfor optimizing medical care with noninvasive strategies (e.g., noninvasive ventilation,vasopressors without invasive hemodynamic monitoring, electrical cardioversion,pneumothorax decompression, optimizing pain relieve)[6].
ACUTE RESPIRATORY FAILURE
ARF is the leading cause of unplanned ICU admission in cancer patients[24]. The incidence is higher in patients with acute lymphoblastic leukemia, acute myeloid leukemia, hematopoietic stem cell transplant (HSCT), neutropenia, and lung cancer[25-29]. Ventilatory support is used in 35%-50% of critically ill cancer patients[11,30,31]; the associated ICU and hospital mortality rate is as high as 50% and 65%, respectively[31,32](Table 2). Mortality-related factors in patients with ARF can be grouped into five categories: (1) Respiratory failure associated with organ dysfunction;(2) Factors inherent to delayed ICU admission; (3) Factors associated with chronic underlying comorbidities; (4) Factors involved with the initial treatment of respiratory failure; and (5) Factors related to the etiology of respiratory failure.
Five major pathophysiological mechanisms can explain ARF in cancer patients(Table 3). Ventilation/ perfusion mismatch is the most common mechanism, usually caused by pulmonary infiltrates, pneumonia, atelectasis or pulmonary embolism.Increased intrapulmonary shunt occurs in primary or secondary acute respiratory distress syndrome. Drug-associated interstitial lung disease and high-degree metastasized lungs explain disorders of oxygen diffusion[33].
Primary tumor location, clinical stage, admission from the emergency department,medical admission, malignancy-unrelated ICU admission, sepsis, adverse event to chemotherapy, and Acute Physiology And Chronic Health Evaluation (APACHE) II score have been found as risk factor for severe ARF requiring invasive mechanical ventilation[34]. Causes of ARF in patients with cancer are depicted in Table 4[35].
Direct actions focused on the best therapeutic options are required in cancer patients with ARF. Clinical examination is crucial since pulmonary infiltrates and respiratory symptoms (e.g., increased respiratory rate, cough, sputum, rales, thoracic pain, and hemoptysis) are associated with increased ventilatory support and mortality rates[13].DIRECT approach may suggest the cause of ARF[36]: Identification of the type and duration (D) of respiratory symptoms, assessment of immunosuppressive (I) therapy,interpretation of X-ray (R) pattern, clinician’s experience (E), clinical (C) finding, and high-resolution computed tomography scan (T)[36,37]. Figure 1 depicts a diagnostic algorithm for ARF.
Bacterial infection, usually in immunocompromised patients, is the main cause of ARF[30]. It is common in early stages of lymphoproliferative disorders[38]. Opportunistic infections have been reported prior to initiation of anticancer therapy in patients with T-cell diseases[39]. Noninfectious causes (e.g., lung infiltrates, leukemic infiltrates,diffuse alveolar hemorrhage, drug-related interstitial lung diseases, and noninfectious lung diseases after HSCT) are difficult to identify, therefore more invasive diagnostic studies are needed for reaching diagnosis such as bronchoscopy and bronchoalveolar lavage (BAL)[25,26,40]. Thrombocytopenia, bleeding disorders, and hypoxemia may preclude bronchoscopy and/or lung biopsy. Pattern of computed tomography (CT)scan may help to identify the cause of ARF[37]. Table 5 summarizes invasive and noninvasive diagnostic procedures in cancer patients with ARF. Infectious and noninfectious causes of pulmonary complications following HSCT are depicted in Figure 2[41].

Table 2 Incidence and mortality of acute respiratory failure in cancer patients[25]

Table 3 Mechanisms and features of hypoxemia
Etiology of ARF may be identified using the information of clinical, laboratory,imagenological, and invasive investigations as following[25,42-44]: (1) Acute or subacute onset; symptoms of upper respiratory tract with fever plus centrilobular nodules or ground-glass opacities on CT scan: Viral infection or atypical pneumonia. Exclude bacterial co-infection; (2) Acute onset; suspect bacterial infection plus alveolar consolidation on X-ray o CT scan: Bacterial infection. Consider bronchoscopy and BAL if sputum cannot be obtained; (3) Subacute onset; T-cell deficiency without prophylaxis forPneumocystis jiroveciiplus diffuse ground-glass opacities on CT scan:Pneumocystis pneumonia; (4) Subacute onset; risk factors for invasive aspergillosis (e.g., prolonged neutropenia, allogeneic HSCT, graft versus host disease, T-cell deficiency) plus consolidation or cavitation on X-ray or CT scan: Invasive pulmonary aspergillosis; and (5) Acute or subacute onset; variable clinical presentation: Diseaserelated infiltrates, diffuse alveolar hemorrhage, alveolar proteinosis, drug-related pulmonary toxicity.
Hydrostatic pulmonary edema (Biomarkers: Natriuretic peptide or N-terminal pro-B-type natriuretic peptide; echocardiography), pulmonary embolism (Biomarkers: Ddimer, high-sensitive cardiac troponin; echocardiography; CT pulmonary angiogram),pleural effusion/ pneumothorax (X-ray; ultrasound; CT scan), and cardiac tamponade(Echocardiography) should be ruled out.
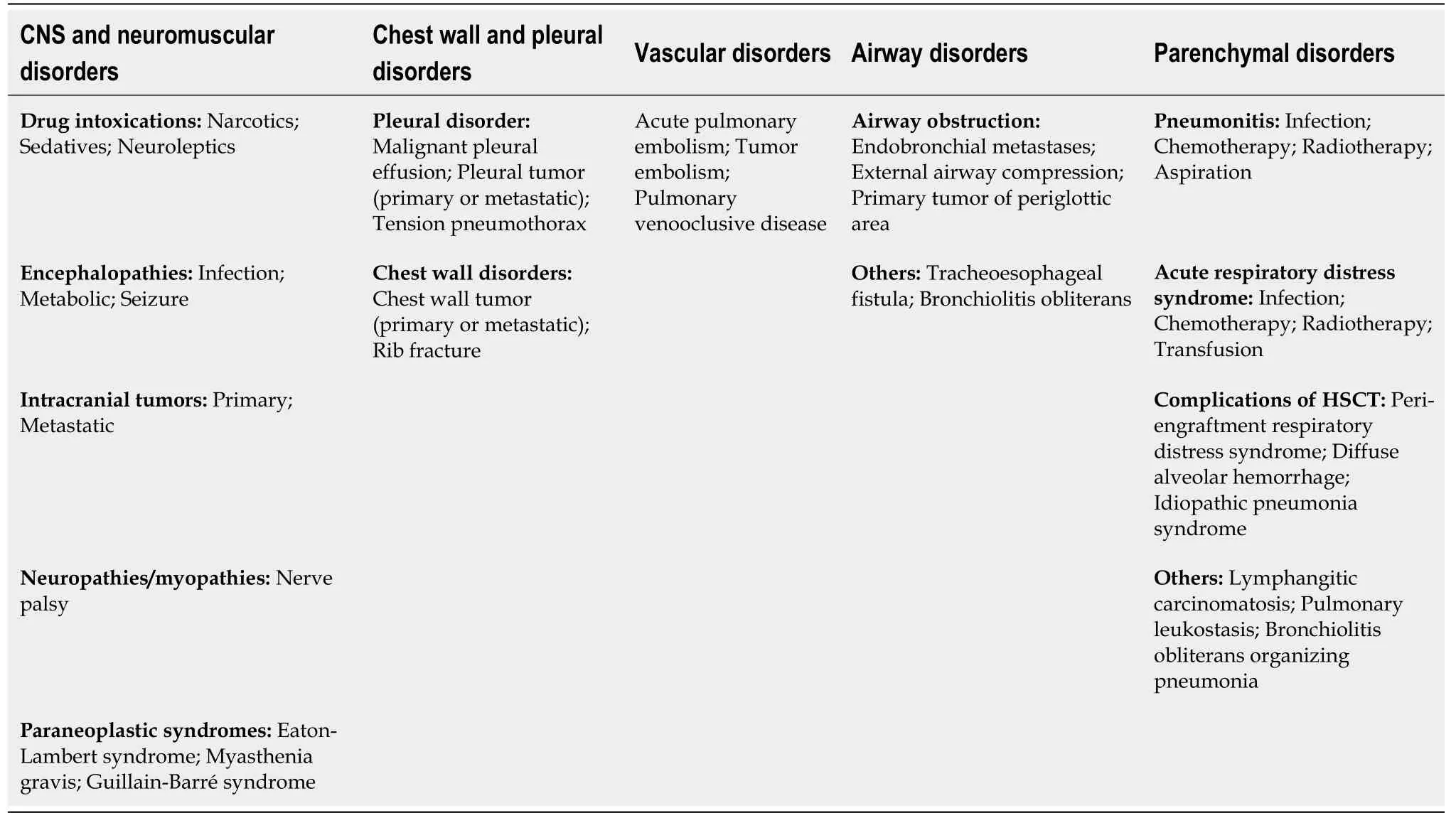
Table 4 Causes of acute respiratory failure in patients with cancer[35]
Treatment of cancer patients with ARF is focused to restore oxygenation, relieve dyspnea and respiratory distress, and improve patient comfort[25]. Mortality rates remain high[17,34]; thus, noninvasive devices are preferred. Although early noninvasive ventilation (NIV) was associated with improved survival rates[45], failure of NIV or high-flow nasal oxygen therapy (HFNO) was associated with increased mortality[24,32].The most challenging issue is choosing those patients in which a specific respiratory strategy is beneficial over others. Physicians need to consider the following risk factors for NIV failure[5]: (1) Prior to NIV: Vasopressor need, multiple organ failure, airway involvement by malignancy, acute respiratory distress syndrome, unknown etiology of ARF, and delayed-onset ARF; and (2) During NIV: Patient not tolerating NIV, not improvement of arterial blood gases within 6 h, respiratory rate > 30 breath per minute, NIV dependency ≥ 3 d, clinical or respiratory deterioration, and unknown etiology of ARF.
A trial of NIV is recommended for most patients with ARF by reversible underlying cause[32,45,46]; however, HFNO is a promising alternative to NIV[47-50]. In a France-Belgium 28-center-randomized controlled trial of 374 immunocompromised patients with ARF, Lemialeet al[51]found no difference in primary and secondary outcomes between intermittent NIV and standard oxygen therapy. A recent meta-analysis of immunocompromised patients showed that intubation rate was lower in the HFNO group than those in the conventional oxygen therapy group and NIV group; however,HFNO did not improve survival or length of stay[52]. The ongoing FLORALI-IM randomized controlled trials may contribute to clarify these findings[53].
NEUTROPENIA AND SEPSIS
Chemotherapy in patients with cancer has resulted in improved survival, although increased the number of cases with neutropenia. Hematological malignancies and myelodysplastic syndromes are other causes of neutropenia[54,55]. Neutropenia is related to severe invasive infections, septic shock, multiple organ dysfunction, and increased mortality[56,57]. Mokartet al[57]found a hospital mortality rate of 45.3% in patients admitted to ICU. In 7512 critically ill patients with cancer included in a recent systematic review, neutropenia was independently associated with unfavorable outcomes; nevertheless, granulocyte colony-stimulating factor was related to reduced mortality rate[58].

Table 5 Invasive and noninvasive diagnostic procedures in cancer patients with acute respiratory failure[5]
According to the absolute neutrophil count, neutropenia is classified as mild (1000-1500 cells/mm3), moderate (500-999 cells/mm3), severe (100-499 cells/mm3), and deep(< 100 cells/mm3)[54]. Infection usually appear with severe or deep neutropenia[59].Febrile neutropenia (FN) is defined as a single oral or axillary temperature > 38.3 °C(101 °Fahrenheit) or a temperature > 38.0 °C (100.4 °Fahrenheit) sustained over 60 min in patients with severe neutropenia[56].
Fever may be the earliest and only sign of infection in neutropenic cancer patient.The incidence of FN varies between 10% and 50% in patients with solid tumors receiving antineoplastic therapy and up to 80% in patients with hematological malignancies[54]. The risk of infection is high in severe neutropenia, moderate neutropenia expected to decline to severe within 48 h, and moderate neutropenia lasting more than seven days.
The main independent prognostic factors for mortality in neutropenic patient are age > 60 years, APACHE scores, Simplified Acute Physiology Score scores, SOFA score, need for mechanical ventilation, high serum procalcitonin, need for renal replacement therapy, and allogeneic HSCT[10,55,57].
Risk-stratification scores allow a quick and objective risk assessment. Several risk scores have been validated to evaluate the risk of complications in patients with FN(Table 6[54,60-62]). Because increased complication and mortality rates, high-risk patients are the following: Group 1-3 of the Talcott classification system, < 20 points in the Multinational Association for Supportive Care in Cancer risk index, and ≥ 3 points in the Clinical Index of Stable Febrile Neutropenia score. High-risk patients generally require in-hospital treatment and intravenous administration of broad-spectrum antibiotics[60-62].
Other risk factors in high-risk patients are the following[55,63-65]: (1) Planned deep neutropenia for more than 7 d; (2) Evidence of liver failure: Abnormal aminotransferases > 5-fold upper limit of normal value or hyperbilirubinemia; (3)Renal impairment: Serum creatinine increase > 50% or > 26.5 μmol/L within 48 h,urine output < 0.5 mL/kg/h for 6 h, or increased concentration in newer biomarkers for sepsis associated-acute kidney injury (e.g., insulin like growth factor binding protein-7, kidney injury molecule-1, neutrophil gelatinase associated lipocalin, tissue inhibitor of metalloproteinase-2); and (4) Pathophysiological imbalance and comorbidities such as, but not limited to: (a) Hemodynamic instability: Hypotension,decreased capillary refill or mottling, hyperlactatemia, central venous oxygen saturation < 70%, and central venous-to-arterial carbon dioxide difference > 6.0 mmHg; (b) Oral or gastrointestinal mucositis interfering with swallowing; (c)Gastrointestinal symptoms: Ileus, severe diarrhea, pain, nausea, and vomiting; (d)Neurological disorders or changes in mental status; (e) Intravascular catheter-related infection; (f) New pulmonary infiltrates or hypoxemia, or decompensated chronic lung disease; and (g) Coagulation abnormalities: International normalized ratio > 1.5,activated partial thromboplastin time > 60 s, or platelet count < 100000 cells/mm3.
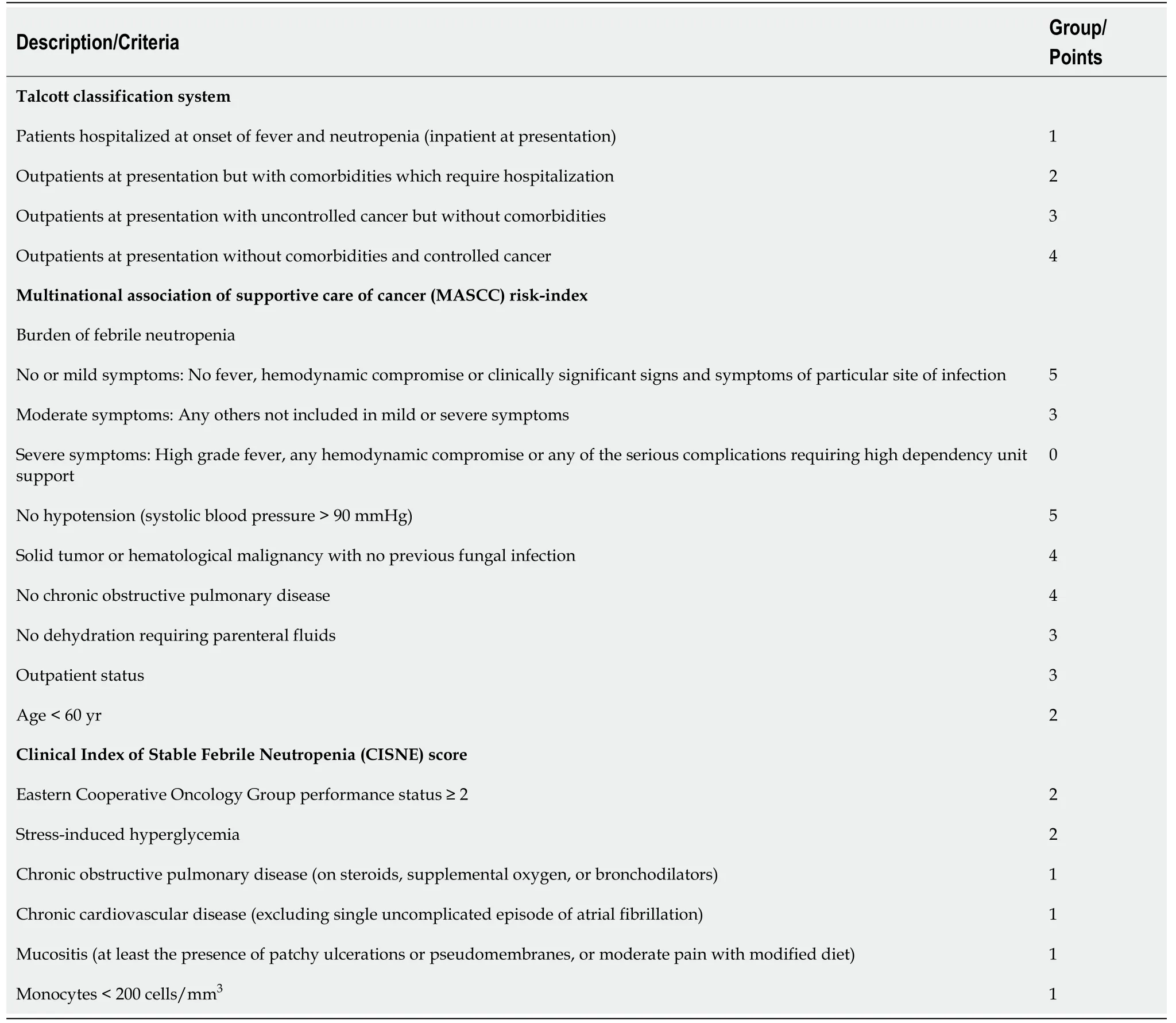
Table 6 Risk-stratification tools for patients with febrile neutropenia[54,60-62]
Most patients with FN have scarce clinical features. Clinically documented infection is only reported in 20%-35%[54]. Thus, the International Immunocompromised Host Society recommends three categories of patients[66]: (1) Microbiologically documented infection: Clinical site of infection and the associated pathogen is identified; (2)Clinically documented infection: Clinical site of infection is identified, but without isolation of the pathogen; and (3) Unexplained fever: Clinical site of infection and pathogen are not identified. The most patients with FN have unexplained fever.
Since the risk of infection is related to the intensity and duration of neutropenia, the risk for developing FN and its severity must be anticipated for an early diagnosis and treatment of unexplained fever; underlying disease, immune status, co-morbidities,and type of intervention (e.g., chemotherapy scheme, intrinsic hematological toxicity,dose and duration) need to be evaluated. Qualitative disorders of neutrophil function may also increase the risk of infections even with normal neutrophil count[55].
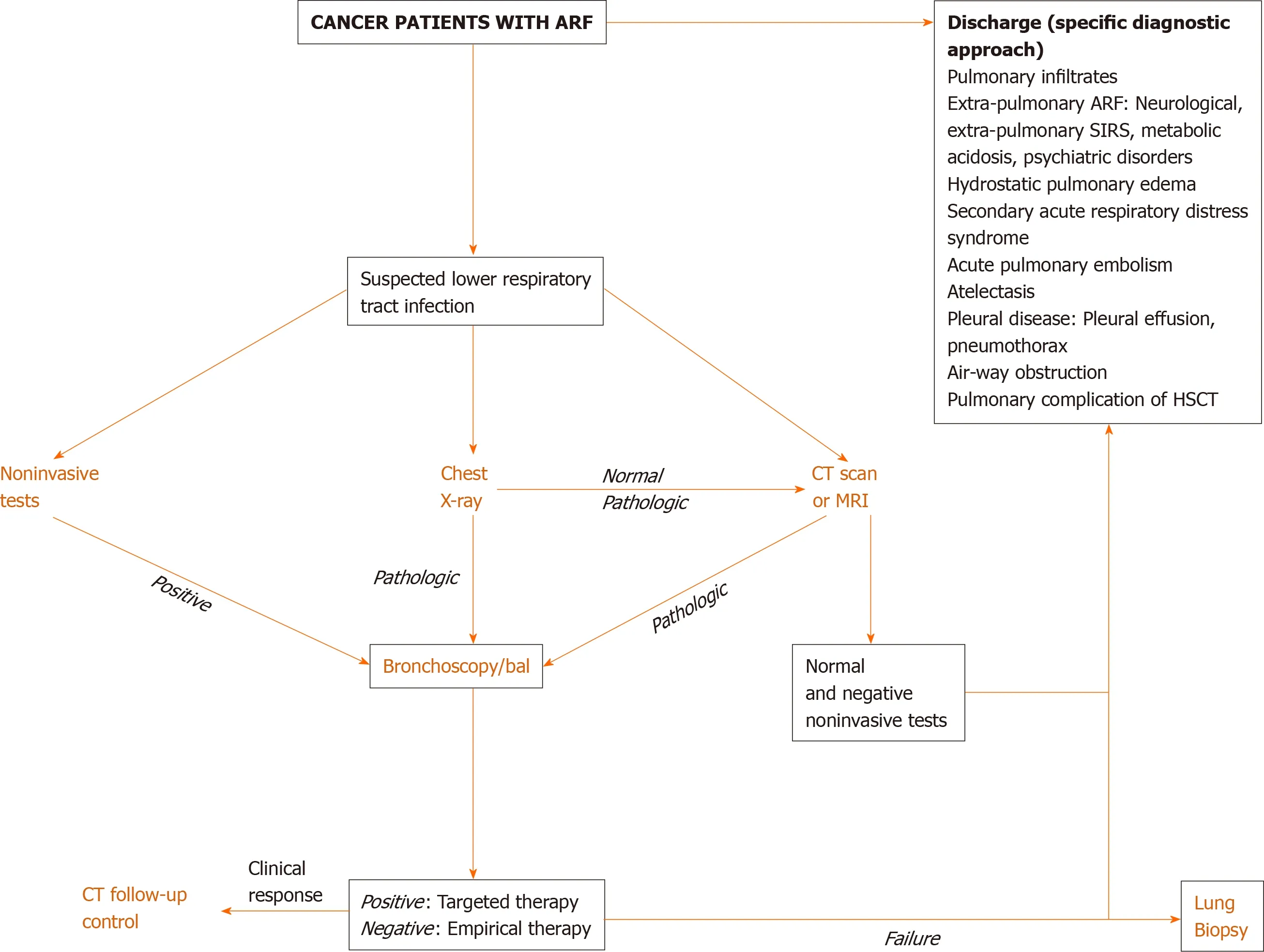
Figure 1 Diagnostic approach for cancer patients with suspected pulmonary infection. ARF: Acute respiratory failure; BAL: Bronchoalveolar lavage; CT: Computed tomography; HSCT: Hematopoietic stem cell transplant; MRI: Magnetic resonance image; SIRS: Systemic inflammatory response syndrome.
There has been a change in the epidemiological patterns of infections because of a wide spread multidrug-resistant bacteria amongst humans, animals and environmental reservoirs[67]. Microorganisms causing infection mostly come from the normal flora of the skin, oropharyngeal cavity, and gastrointestinal tract. Infection is localized in approximately 30% of cases, mainly in the upper respiratory tract or skin,but only 20%-40% are microbiologically documented[59]. Among gram-negative bacteria, carbapenem-resistantEnterobacteriaceaesuch asEscherichia coli[67-74]andKlebsiella pneumoniae[67-69,72-75],Pseudomonas aeruginosa[68,69,72-74]andAcinetobacter baumannii[67,68,76]prevail. The most common gram-positive pathogens are methicillin-sensible[68]and methicillin-resistantStaphylococcus aureus[67,68,77],Streptococcus viridians[68,71]andStreptococcus pneumonia[68]; vancomycin-resistantEnteroccocus faeciummay be found[67,69]. The main fungi identified areCandidaandAspergillusspecies[55,78].Approximately 50% of invasive aspergillosis are found in patients with hematological malignancy or imunocompromissed patients with prolonged severe neutropenia[78].Mortality rates of invasive fungal infection exceeds 30%[79].
The Third International Consensus Definitions for Sepsis and Septic Shock is recommended to use in FN patients (Figure 3)[80]. In a large meta-analysis, neutropenia was independently associated with poor outcomes[81]; therefore FN should be treated as infectious disease until proven otherwise and must be considered as medical emergency. Therapeutic approach is based on the risk of complications and death,presence of life-threatening infection and magnitude and duration of neutropenia.High-risk patients are vulnerable to develop septic shock; early intravenous administration of broad-spectrum antibiotics against gram-negative and gram-positive bacteria is obligatory. Low-risk patients could be treated in-hospital with intravenous antibiotics or as outpatient with oral antibiotics depending on the clinical picture and comorbidities.

Figure 2 Pulmonary complications in patients with hematopoietic stem cell transplant[41]. BOS: Bronchiolitis obliterans syndrome; COP:Cryptogenic organizing pneumonia; DPTS: Delayed pulmonary toxicity syndrome; HSV: Herpes simplex virus; PERDS: Peri-engraftment respiratory distress syndrome; PTLD: Post-transplant lymphoproliferative disorder.
Culture samples must be taken before the onset of antibacterial agents. Information of the general/local epidemiology and resistance profiles is of paramount importance to guide empirical antibiotic therapy[82]. Broad-spectrum antibiotics coveringPseudomonas spp. and methicillin-resistantStaphylococcus aureusare used (Table 7[40,54,55,79,83]). Empirical antimycotic therapy must be promptly started if invasive fungal infection is suspected[79].
Treatment needs to be reassessed within 48-72 h; clinical and microbiological data help to modify therapy. In patients with documented infection, duration of therapy is based on the isolated organism and the site of infection. It is usually continued until recovering severe neutropenia; granulocyte colony-stimulating factors may be used[84].In patients with FN of unidentified etiology, antibiotic therapy should be discontinued after 72 h of apyrexia and clinical recovery irrespective of absolute neutrophil count[56,85].
CARDIOVASCULAR DISORDERS
Several cardiovascular disorders may be developed in cancer patients such as sepsis/septic shock, chemotherapy-associated cardiotoxic disease (CACD), pulmonary embolism, and cardiac tamponade. In this section we refer to CACD since sepsis/septic shock is treated in other section, and pulmonary embolism and cardiac tamponade are nonspecific complication of cancer patients described in other highquality articles[86,87].
Cardiovascular diseases and cancer are interwoven because of increased cancer survival and cardiotoxic anticancer therapy[88]. Up to 33% of cancer survivors may die due to heart disease[89]. Mortality rates in patients with CACD are 3.5-fold higher than those in patients with idiopathic cardiomyopathies[90]. Cardiovascular effects of chemotherapy may also affect the quality of life and compromise survival expectation.
Left ventricular dysfunction is the most common and serious consequence of CACD, usually secondary to cardiomyopathy or myocarditis (Table 8)[91,92]. EarlyCACD may be detected in up to 48% while late-onset disorders may be seen in up to 30%[93]. The highest incidence of CACD is reached by anthracyclines such as doxorubicin (3%-26%), alkylating agents such as cyclophosphamide (7%-28%), and monoclonal antibody such as trastuzumab (2%-28%)[94].

Table 7 Empiric antibiotic therapy in high-risk patients with febrile neutropenia[40,54,55,79,83]

Table 8 Main cardiovascular complications of oncological therapy[91,92]

Figure 3 Sepsis diagnosis and treatment in neutropenic patients. ANC: Absolute neutrophil count; CISNE: Clinical Index of Stable Febrile Neutropenia score; CGS: Coma Glasgow Scale; MAP: Mean arterial pressure; MASCC: Multinational association of supportive care of cancer risk-index; SBP: Systolic blood pressure; UO: Urine output.
CACD is classified as type 1 or type 2 depending on the administered therapy[95].CACD type 1 is typically related to anthracycline drugs, not reversible with cessation of therapy, and dose-dependent; necrosis, vacuoles and disruption of sarcomeres are seen as histopathological findings. CACD type 2, usually associated with monoclonal antibodies such as trastuzumab, is reversible with cessation of therapy, doseindependent, and no ultrastructural disruption in cardiomyocyte cell is found.
There is no consensus to define CACD, but there is convergence regarding clinical or echocardiographic left ventricular dysfunction as the main condition[95]. Diagnosis of CACD can be made if at least one of the following criteria is reached[95,96]: (1)Cardiomyopathy with compromised left ventricular function; (2) Symptoms or signs of heart failure linked to the presence of third noise, tachycardia, or both; (3) Left ventricular ejection fraction (LVEF) less than 55% with a symptomatic decrease of 5%,or an asymptomatic decrease of 10%; and (4) Reduced LVEF > 10% from baseline or LVEF < 53% (the normal reference value for 2D echocardiography), confirmed in two consecutive echocardiography assessments within 2-3 wk apart.
Echocardiography was widely disseminated due to its easy availability, low cost,free of radiation, and information concerning the hemodynamic status and valvular diseases. There is no agreement regarding the time and frequency to achieve echocardiography in cancer patients on chemotherapy, although it is suitable before starting therapy (especially if there are cardiovascular risk factors or history of cardiac disease), during treatment, and 6 to 12 mo after completion[97].
Measurement of LVEF alone may overlook small changes. A variation in myocardial deformation, assessed by myocardial strain image, may precede significant decline in LVEF[98-100]. Magnetic resonance image (MRI) is very useful to determine the size of heart chambers and their function, but cannot be used at bedside for critically ill patients in the ICU. Thus, it should be used when other tests are inconclusive[91,95].
The high-sensitive cardiac troponins and N-terminal pro-B-type natriuretic peptide are cardiac biomarkers without general recommendation for diagnosis of cardiotoxicity; however, these noninvasive diagnostic methods are cheaper than other imaging studies or myocardial biopsy. Cardiac troponins are associated with prognosis in patients on anticancer therapy; thus, higher plasma concentration requires closer monitoring and a possible therapy modification[91,101]. Cut-off points have not been established.
The treatment of adverse-side effects of antineoplastic therapy should be individualized depending on the risk factors for cardiotoxicity, severity, and prognosis(Figure 4[92,102,103]). The International Cardio Oncology Society-One trial found beneficial effects of prophylactic enalapril in patients on anthracycline therapy[104]. A recent study conducted in Brazil showed that carvedilol administered during chemotherapy reduced troponin levels and the risk of systolic dysfunction[105]. A recent meta-analysis showed the usefulness of β-blockers to preserve left ventricular function during anthracycline therapy[106]. Consequently, we would expect an increased cardiac tolerability with higher doses of chemotherapy with little or no interruption.
β-blockers may have further positive effects on malignancy. Since β-adrenergic receptors are overexpressed in malignant breast tissue, propranolol was tested on early-stage breast cancer patients[107]. Molecular analysis showed reduced Ki67 protein expression and decreased phosphorylation of mitogenic signaling regulators;additionally, reduced tumor proliferative indices, metastases rate, and mortality rate were also found[107]. Propranolol also modifies mitogenic and apoptotic signaling in late-stage breast cancer[108]. Long-term β-blockers improved survival outcomes in older ovarian cancer patients with cardiovascular disease[109].
Statins are drugs commonly used in patients with cardiovascular diseases and cancer. Statins regulates cell membrane integrity, cell signaling, protein synthesis, and cell cycle progression; they also modify angiogenesis and tumor growth[110]. Several studies demonstrated that statins are associated with reduced mortality rates in patients with breast cancer, renal cell carcinoma, and colorectal cancer[111,112]. A recent meta-analysis showed that statins was associated with improved outcomes in patients with lung cancer[113], but it was not supported by powered randomized controlled trials. Conversely, other meta-analysis of randomized controlled trials evidenced that statins did not improve overall survival rates or progression-free survival rates in patients with active cancer[114].
For patients with severe heart failure or cardiogenic shock, inotropic drugs and left ventricular mechanical support devices must be considered[115,116]. Glucocorticoids are the first-line therapy, and tumor necrosis factor-α inhibitors as second choice, for myocarditis with lymphocyte infiltration in patients treated with immune checkpoint inhibitors[91]. Cardiac transplant may be an option in selected patients. As expected, the criteria for transplanting these patients differ according to institution and country because active malignancy is generally considered as an absolute contraindication;nevertheless, it is interesting that survival rates after cardiac transplant in cancer patients is similar to those in noncancer patients[102,117].
Recently, cardio-oncology has emerged as a clinical (and scientific) area dedicated to diagnose and treat anticancer therapy-related cardiovascular complications to avoid interruption of treatment. This new discipline combine together cardiologists,oncologists, and hematologists in specialized units[118,119]. In institution without cardiooncology unit, cancer patients with potentially fatal cardiovascular complications must be admitted to the ICU. Thus, it is necessary to adopt clinical guidelines according to the center resources to provide the best care, especially in cases with acute decompensated heart failure, cardiogenic shock, hypertensive emergency, and arrhythmias.
PERIOPERATIVE CARE
ICU is commonly required for cancer patients in the postoperative period because of the complexity of surgical procedure and potential complications. Topics regarding anesthetic management and surgical issues were addressed in this review.
General considerations
Effects of chemotherapy and radiotherapy on respiratory system must be recognized before orotracheal intubation. Severe mucositis lead to pseudomembranous material,edema, and bleeding, which compromises the airway and increases the risk of aspiration during endotracheal intubation. Radiation on the head and neck region may produce permanent tissular fibrosis limiting mouth opening and tongue mobility.Radiotherapy-associated airway fibrosis and tracheal stenosis, usually not recognized on physical examination, may affect intubation and ventilation[120]; thus, monitoring of pulse oximetry and arterial blood gases in perioperative period is mandatory.

Figure 4 Pathogenic, diagnostic and therapeutic approach of chemotherapy-associated cardiac dysfunction[92,102,103]. ACEI: Angiotensinconverting enzyme inhibitor; ECG: Electrocardiography; DM: Diabetes mellitus; hs-cT: High-sensitive cardiac troponins; HTN: Arterial hypertension; LVMS: Left ventricular mechanical support; MRI: Magnetic resonance image; NT-ProBNP: N-terminal pro-B-type natriuretic peptide; ROS: Reactive oxygen species.
Excessive perioperative fluid administration has been correlated with surgical and pulmonary complications; therefore, fluid administration need to be monitored using dynamic indexes to optimize volume status[121,122]. Chemotherapy drugs such as bleomycin and mitomycin may cause lung toxicity[123]. However, in a large cohort of patients from the cancer registry of the Mayo Clinic, only seven patients receiving systemic bleomycin developed acute respiratory distress syndrome after surgery[124].
Transfusion-related immunomodulation is associated with decreased survival rates in cancer patients. This is a secondary phenomenon produced by multiple immunomodulatory mediators derived from white blood cells, red blood cells, and platelets of the donor[125]. Transfusion of red blood cells in the perioperative period affects the survival of cancer patient; thus, reducing blood transfusions could have a positive impact on outcome[126]. On the other hand, Manning-Geistet al[127]observed that perioperative transfusion of red blood cells after debulking surgery in ovarian cancer was not related with wound complication and thrombosis.
Anesthetic topics
Cancer surgery induces neuroendocrine and immune stress response, which may be reduced by regional anesthesia. Surgical manipulation is associated with spreading of tumorigenic cells and releasing cancer-growth factors[128]; thus, immune system modulation may contribute to reduce the incidence of metastases[129].
Changes in immune system has been reported with anesthetic gases[130]. Volatile anesthetics inhibit leukocyte activity and stimulate angiogenesis and metastases[131];however, evidence is not conclusive because most studies were carried outin vitro. In ovarian cancer cells, isoflurane was related to cell cycle progression and cell proliferation, and increased expression of tumorigenic markers such as insulin-like growth factor 1 within the first 24 h[131]. In breast cancer surgery, preserved natural killer (NK) cells activity was found with propofol-paravertebral anesthesia while reduced NK activity was demonstrated using sevoflurane[132].
Honget al[133]found that cancer patients treated with volatile inhaled anesthesia had a 5-year overall survival rate similar to those on total intravenous anesthesia. The ENIGMA-II trial did not show negative effects of nitrous oxide on cancer recurrence or mortality[134]. Further randomized controlled trials are required.
Recent studies suggest that opioids inhibit the cellular and humoral immunity,promote proliferation and migration of tumor cells, and facilitate angiogenesis[129,135].Opioid-induced immunomodulation is manifested in two ways: (1) Direct effects on immune cellsvia μreceptor and toll-like receptor 4 expressed in the surface of NK cells, macrophages and T-cells (peripheral effects)[136]; and (2) Indirect effects through the sympathetic nervous system and hypothalamic-pituitary-adrenal axis, which suppress lymphocyte proliferation and NK cell cytotoxicity in lymphoid organs(central effects)[137,138]. Nonetheless, the type of drug and the administration period may modify the immunological effects of opioids[139,140](Table 9[131,140-142]).
Propofol, a sedative drug commonly used in operating room and ICU, has been associated with tumor growth inhibition and reduced risk of metastasis. In patients undergoing hepatectomy for hepatocellular carcinoma and colon cancer surgery,propofol-based total intravenous anesthesia was associated with improved survival rates and reduced postoperative metastases compared with desflurane anesthesia[143,144]. Instead, Huanget al[145]observed no significant difference in locoregional recurrence or overall 5-year survival rates using desflurane or propofol anesthesia in patients undergoing breast cancer surgery.
Surgical topics
High-local chemotherapy concentration is reached using the hyperthermic intraperitoneal chemotherapy method. Systemic toxicity, including hematological toxicity, is less common than those with systemic administration of chemotherapy[146];nonetheless, hematological and pulmonary toxicity may be occasionally produced with potentially fatal outcomes[147].
The Enhanced Recovery After Surgery (ERAS) program reduces surgical stress and improve recovery for an early hospital discharge. This approach includes three components[148,149]: (1) Preoperative: Preadmission counseling, early discharge planning, reduced fasting duration, carbohydrate loading, no/selective bowel preparation, antibiotic prophylaxis, thromboprophylaxis, pre-warming, and no premedication; (2) Intraoperative: Short-acting anesthetic agents, mid-thoracic epidural anesthesia/ analgesia, surgical techniques, no drains, avoidance of fluid overload, and maintenance of normothermia; and (3) Postoperative: Mid-thoracic epidural analgesia, no nasogastric tube, prevention of nausea and vomiting, avoidance of salt and fluid overload, early removal of catheters, early oral nutrition, nonopioid oral analgesia/nonsteroidal anti-inflammatory drugs, early mobilization, stimulation of gut motility (e.g., chewing gum), defined discharge criteria, and audit of compliance and outcomes.
ERAS program has become a widely accepted surgical practice worldwide. Positive outcomes have been found in several surgical locations including elective and emergency surgery[149-151]. ERAS protocols have led to decreased length of hospitalization by 30% to 50%, as well as reduced complications, readmission rates,and health costs[149,142].
Laparoscopic surgery within ERAS protocols in cancer patients has also shown optimistic outcomes[153,154]. ERAS program resulted in improved outcomes, reduced hospitalization cost, and enhanced quality of life as shown by Wanget al[155]in a metaanalysis of elective gastric cancer surgery.
NEUROLOGICAL DISORDERS
Neurological symptoms and signs are commonly seen in cancer patients. Neurological symptoms may be the initial expression of undiagnosed cancer, emerge during the course of disease, or appear linked to treatment[156]. Cancer patient may also develop nonmalignancy-related neurological disorders, which require a rational approach to exclude cancer-related complications.
Neurological disorders require early diagnosis and treatment to reduce functional loss. Surgical treatment is often required, for which the multidisciplinary approach is mandatory[157]. Neurological disorders in cancer patient is produced by[158]: (1) Direct effects of tumor: Brain metastases, cerebral edema, seizures, spinal cord compression,hydrocephalus, leptomeningeal carcinomatosis; (2) Indirect effects of tumor:Paraneoplastic syndromes, stroke, cerebral venous thrombosis, infection, metabolic and electrolytic disorders; and (3) Treatment effects: Convulsions, cerebrovascularaccident (e.g., intracranial hemorrhage due to thrombocytopenia, venous sinus thrombosis), leukoencephalopathy, loss of vision or hearing, peripheral neuropathy,aseptic meningitis, opportunistic infections, acute or late post-radiation necrosis.
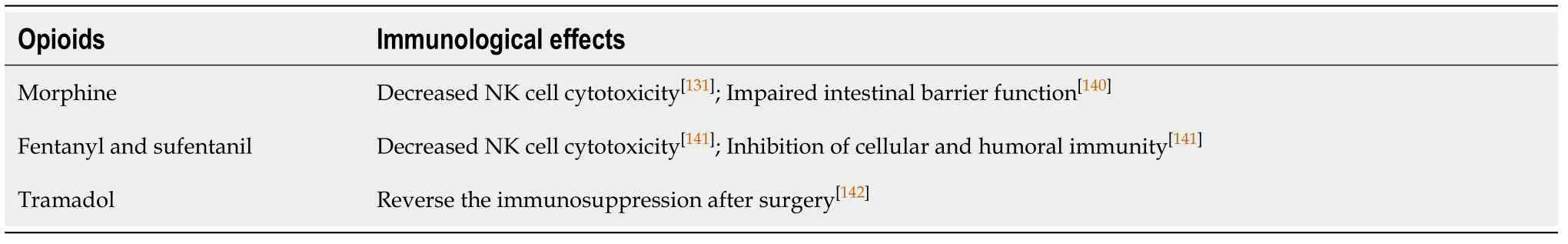
Table 9 Immunological effects of opioids
The most common neurological emergencies are brain metastases, seizures, and obstructive hydrocephalus[156]. Intracranial hypertension related to cerebral edema,hydrocephalus or mass effect is commonly seen.
Brain metastases
Brain metastases complicate up to 20% of cancer patients and are 10-fold more frequent than primary brain tumors[159]. Cancer producing metastases are lung (e.g.,nonsmall cells), breast (e.g., HER-2), kidney and melanoma[158,160]. Fifty percent of metastases are solitarylesion. Distribution of brain metastases are cerebral hemispheres (80%), cerebellum (15%) and brainstem (3%). Cerebral edema associated with metastases produces intracranial hypertension. Pathogenesis of edema is complex, including vasogenic edema secondary to capillary leakage, venous stasis,and cerebrospinal fluid obstruction[159,160].
Cerebral metastases may be the initial feature of cancer in 8-10% of cases. It may be characterized by intracranial hypertension with alterations in level of consciousness,headache, and vomiting; focal neurological deficit such as sensory or motor defects,speech disorders, instability, and cognitive impairment; or asymptomatic. Seizures almost always occur when there are multiple metastases, intralesional bleeding,herniation, hydrocephalus, or sudden-onset ischemia of large vessels[158,160]. Diagnosis is made by contrast enhanced MRI. Contrasted CT is useful if MRI is contraindicated or intracranial hemorrhage is suspected, but it is less sensitive for posterior fossa or small tumors[159,160].
Treatment is discouraging. Several factors such as type and location of primary tumor, age, and extracranial disease are involved in prognosis. Therapeutic options include surgical resection, radiotherapy, stereotactic radiosurgery and chemotherapy[159-167]. In patients with good performance status and known primary tumor, surgical resection of unique lesions of noneloquent areas followed by radiation therapy is recommended[159]. In eloquent area lesions, radiation therapy is preferred.Radiation of the entire skull is chosen for multiple and symptomatic metastases,although prognosis is not improved and almost half of patients die due to neurological progression[162,163]. Traditional cytotoxic chemotherapy is not routinely used in the treatment of brain metastases because of low response rates[164]; however, germ-cell tumors and non-Hogdkin lymphoma with nervous system involvement are treated with chemotherapy[166,167]. Metastases secondary to melanoma and renal carcinoma do not respond to chemotherapy[158]. Targeted therapies and immunotherapy are promising oncospecific therapies[168-172].
Despite aggressive treatment, many patients develop malignant cerebral edema and seizures. Cerebral edema occurs through disruption of blood-brain barrier by direct effect of metastases, as well as released several cytokines and growth factors by the tumor cells including the endothelial vascular growth factor with promoting angiogenesis[159]. These factors favor endothelial clefts formation with fragmentation and fenestration of endothelium, and consequently, injury to the basement membrane[159]. Vasogenic edema with fluid leakage and increased interstitial fluid pressure is then developed. Peritumoral edema eventually leads to symptoms and signs of mass effect and increased intracranial pressure.
Glucocorticoids are indicated for all symptomatic patients with metastasesassociated cerebral edema. Dexamethasone is the most used due to its long half-life and lower mineralocorticoid activity. Recommended dose is 4-8 mg/d (up to 16 mg/d in very severe symptomatic patients). Higher doses have not additional benefits and side effects may occur. Dexamethasone doses should be progressively decreased in 2 or more weeks to avoid complications of chronic steroid administration (e.g.,immunosuppression, hyperglycemia, increased risk of opportunistic infections)[173].Other therapies include hyperventilation, hypertonic sodium chloride or mannitol 20%in severe intracranial hypertension to prevent herniation in neurocritically ill patients[174].
Seizure
Seizure complicating brain tumor is commonly found as simple, complex-partial or generalized epilepsy[158]. Status epilepticus may also be developed. Seizures depend on the type and location of brain tumor, as well as cancer-related complication. The reasons of cancer-related seizure are listed in Table 10. Diagnosis is made by clinical feature and electroencephalography showing epileptic changes on brain waves.Epileptiform waves could be present even in absence of clinically visible seizures[175].Hemiplegia and other focal symptoms may appear up 75% of patients depending on tumor location; infratentorial disease is related toataxia, vomiting, dysarthria and nystagmus[158].
Seizure prophylaxis is not recommended for patients with brain tumor; however,this is a controversial recommendation because of improved accurate diagnosis and prognosis using the current continuous electroencephalography, and the introduction of newer and less toxic anticonvulsants (e.g., levetiracetam, lamotrigine, and lacosamide)[176]. Once airway, breathing, circulation, and “dextrose” (the “ABCDs”)have been addressed, acute seizure is treated with parenteral benzodiazepines (e.g., IV lorazepam or diazepam; intramuscular midazolam) as first-line agents. Second-line therapy (e.g., phenytoin/fosphenytoin, valproic acid, phenobarbital, or levetiracetam)should be initiated within 30 min if first-line treatment failed. If second-line agents are ineffective, treatment is escalated to anesthetic agents such as continuous infusion of midazolam, propofol, or pentobarbital[177]. When mass effect or worsening edema is present, dexamethasone can be effective in controlling seizures. For metastasis-related seizures, chemotherapy, radiotherapy and surgery are alternative therapies. Surgical resection is recommended for patients with tumor located in the posterior fossa. Early diagnosis and urgent correction are required for metabolic or electrolytic imbalanceinduced seizures.
Maintenance anticonvulsant medication requires more careful evaluation since the old anticonvulsants (e.g., phenytoin, carbamazepine, and phenobarbital)simultaneously induce CYP coenzymes. These coenzymes accelerate the metabolism of steroids and chemotherapeutic agents. Newer antiepileptic drugs such as levetiracetam, without CYP metabolism, are recommended in these circumstances.Other options are gabapentin, pregabalin, lamotrigine, and lacosamide[178].
Acute hydrocephalus
Acute hydrocephalus is a medical emergency caused by a stopped cerebrospinal fluid(CSF) flow or an increased CSF content. Table 10 lists the reasons of acute hydrocephalus in cancer patients. Since 80% of maximum ventricular dilation is reached in almost 6 h, acute hydrocephalus may be rapidly developed. Clinical diagnosis is suspected in patients presenting headache, blurred vision, transient loss of visual field, ataxia, vomiting, and impaired consciousness. Headache is present in 50%of cases (on occipital region if increased intracranial pressure), exacerbates with the Valsalva maneuver, and is associated with nausea and vomiting[158,160]. Papilledema and focal neurological signs may be present. Tumor interference the CSF flow with a valve-way mechanism at the level of third or fourth ventricle may result in periodic increased intracranial pressure[160]. Noncontrasted CT scan allows identifying the size of ventricles. Obstructive hydrocephalus is classically characterized by ventriculomegaly proximal to the site of obstruction and periventricular edema.
The treatment of acute hydrocephalus should be early and effective. Several procedures have been described such as emergency ventriculostomy, ventricular bypass, endoscopic ventriculostomy, aqueductoplasty (due to aqueduct stenosis),septostomy (in isolated lateral hydrocephalus), and in some cases tumor debunking.Radiotherapy and chemotherapy are options in patients with hydrocephalus secondary to leptomeningeal carcinomatosis or metastatic CSF seeding. Patients with nonsevere obstructive hydrocephalus could be treated with osmotic agents to reduce intracranial pressure (e.g., manitol 20% or hypertonic sodium chloride) and/ or drug interfering with CSF production such as acetazolamide, furosemide, and glucocorticoids[179].

Table 10 Causes of cancer-related seizure and cancer-related acute hydrocephalus[158]
CHEMOTHERAPY IN ICU
Chemotherapy in ICU may be an option for patients with critical illness driven by the oncological disease, scheduled or ongoing chemotherapy in absence of contraindications, and requirement for monitoring or preventing potentially severe chemotherapy-side effects in high risk patients. Particularly, anticancer chemo or radiotherapy is necessary in cases of acute respiratory failure due to high grade non-Hodgkin lymphoma or hyperleukocytosis[5]. Antineoplastic drugs used by ICU team may be challenging due to little experience; indeed, associated sepsis or organ support methods at the time of chemotherapy onset are erroneously considered as a contraindication[6].
Organ support therapies accompanied by chemotherapy may be beneficial in critically ill patients with cancer-related organ dysfunction[180]. Patient’s consent,comorbidities, performance status, cancer-related life expectancy, and life-spanexpanding treatment are necessary to be evaluated to improve outcome. A close collaboration with the attending oncologist or hematologist is mandatory.Organizational issues should be assured for success, including clinical protocols,securing of the medication circuit, consultation with pharmacist and experienced nurses, and daily rounds with the attending oncologist or hematologist[181].
Studies identifying prognostic factors and outcomes of patients receiving chemotherapy in the ICU are scarce and have several limitations such as retrospective design, small sample size, and several nature of cancer[182-184]. Additionally, the following period and subgroup of analyzed patients may have an impact on clinical response (e.g., solid tumor vs. hematological malignancy; traditional chemotherapy vs.targeted immunotherapy; urgent vs. maintenance chemotherapy), which need to be considered to state prognosis.
ICU and hospital mortality rates for patients with solid tumors who received chemotherapy in ICU range from 25% to 54%, and 58% to 77%, respectively[182-184]. Oneyear survival rates are as low as 7%-12%[182,183]. Lung cancer and acute respiratory failure due to airway compression or pulmonary infiltrates may explain the high mortality rates[182-184]. In patients with hematological malignancy 25%-40% die in the ICU; 30-d, 6-mo and 1-year mortality rates is 40%, 51%-77% and 50%,respectively[10,185]. Risk factors for mortality are degree of organ dysfunction and lifesupport methods such as ventilatory support, vasopressors, and renal replacement therapy.
CONCLUSION
Patients with cancer and organ dysfunction need to be early admitted to ICU for improving survival. Clinical and pathophysiological condition, cancer status, and expected life-span must be collectively evaluated to decide full or time-limited organ support methods. Specific disorders require a specialized and well-trained medical staff to optimize diagnosis, enhance treatment, and improve outcomes.

