Key enzymes of glutamate metabolisms in the brain of neonatal and adult rats exposed to monosodium glutamate
Department of Biochemistry,Faculty of Basic Medical Sciences,University of Calabar P.M.B 1115,Calabar,Cross River State,Nigeria
Abstract Background and objectives:Despite the effective role of monosodium glutamate as a food additive,there are claims indicating that monosodium glutamate consumption increases the level of glutamate an excitatory neurotransmitter which can be toxic to the brain in accumulated level.The present study attempted to understand the differential effect of monosodium glutamate on key enzymes of glutamate metabolisms in rat brain exposed either as neonate or adult to monosodium glutamate.Methods:The rat neonates were divided into six groups with seven animals per group and exposed to different concentrations of monosodium glutamate as neonates only(normal saline or monosodium glutamate 4 mg/g),neonate plus adults(monosodium glutamate 5 or 10 mg/g)and adult only(monosodium glutamate 5 or 10 mg/g).Key enzymes of glutamate metabolisms were measured in whole brain homogenates.All experiments were approved by the Faculty of Basic Medical Sciences University of Calabar and ethics committee-04/11/2018.Results:Except neonate plus adult 5 mg/g group,glutamate dehydrogenase and glutamate synthetase activities were significantly higher in administered groups than in the control group(P<0.05).There was no significant difference in glutamate synthetase activity among monosodium glutamate administered groups(P>0.05).The glutamate carboxylase activity was significantly higher in all monosodium glutamate administered groups than in the control group(P<0.05).The brain alanine aminotransferase and aspartate aminotransferase activities of rats in each monosodium glutamate administered group increased in a dose-dependent manner(P<0.05).Conclusion:Exposure to monosodium glutamate can increase the activities of key enzymes of glutamate metabolism in the brain of neonate and adult rats similarly,which is not determined by age difference.
Key words:brain homogenate;decarboxylase;enzyme activities;glutamate dehydrogenase;glutamate metabolism;monosodium glutamate;neurotransmitter;synthetase
INTRODUCTION
The addition of some consumable substances into foods to enhance flavor,taste and texture has been a long time human practice(Koppula et al.,2017;Fernstrom,2018;Tennant,2018).These substances,commonly called food additives,are usually approved by specific regulatory agencies/bodies,such as the Joint Food and Agricultural Committee,World Health Organization Expert Committee on Food Additives,after series of toxicity tests via animal models mostly(Nnadozie et al.,2019).The aim of this test is to determine the potential of these additives to cause harmful or toxic effect and to establish a safe condition or dose for use in humans(Sukhorukova et al.,2018).Among these food additives,the commonly used monosodium glutamate(MSG)is popularly packaged and known commercially as White maggi(Sukhorukova et al.,2018).
MSG is a white crystal salt of glutamic acid consisting of 78% glutamate,20% sodium,and 2% water(Akataobi,2020).It was commercially used as a food additive to enhance flavor,especially in Chinese and Japanese foods in the early days,but are employed around the world in present day(Inuwa et al.,2011).The addition of MSG to food gives it a flavoring functional taste similar to that of free glutamate occurring naturally in foods(Akataobi,2020).It is available in a good number of commercially packaged foods,foods served in restaurants as well as in house whole cooking(Fernstrom,2018).Examples include sausages,snacks,vegetable burgers,flavored tuna and prepared meals,frozen foods meals,seasoned chicken,hams,soup and sauces(Sukhorukova et al.,2018),and other easily consumed products,such as soft drinks,chewing-gum,bread,biscuits,and energy drinks(Manal and Nawal,2012).
MSG enhances food flavor by the stimulation of orosensory receptor and by improving the palatability of foods,influencing appetite positively,which in turn results in consumption of more of the food and weight induction/gain as well as increase in the concentration of available neurotransmitters in the central nervous system(Fernstrom,2018).In some industrial foods and other consumable products,MSG in allowable limits may be listed and/or described on the food label as flavoring agent or as a hydrolyzed vegetable protein(Kanamori et al.,2017).However,when present in higher than recommended concentration,the quantities of MSG are usually concealed or given unconventional names for disguise in the labels of the products.Such names include glutamic acid,glutaccyl,autolyzed yeast extract,glutarene,calcium caseseinate,sodium caseinate,E621 and no added MSG(Inuwa et al.,2011).
Despite the effective role of MSG as a food additive,there are claims indicating that MSG consumption is toxic and increases the level of glutamate an excitatory neurotransmitter which can be toxic to the brain in accumulated level(Fernstrom et al.,2018).It has also been reported that MSG causes brain damage by increasing the concentration of neurotransmitters,which excite the brain cells to death,leading to conditions such as attention deficit disorder,attention deficit hyper active disorder,Asperger’s syndrome and autism(Kanamori et al.,2017).MSG also stimulates an undetected brain cell growth,resulting in overweight of certain areas of the brain,causing damage in them or destroying them entirely,while promoting growth and normal activity of other areas of the brain in the early stages of life(Kouzuki et al.,2019).Neurotransmission involves the transfer of signals from one neuron to the other(Fernstrom et al.,2018),and this is performed by chemical substances known as neurotransmitters.These neurotransmitters are small endogenous chemical messengers responsible for transmitting chemical or electrical signals across chemical synapses like the neuromuscular junctions,generated from one neuron(nerve cell)directed to a target neuron such as the muscle or heart cells(Hohnholt et al.,2018).These neurotransmitters act on extracellular transmembrane proteins with binding sites to cause conformational changes.
Neurotransmitters can either function as excitatory signal neurotransmitters,for example glutamate,epinephrine,dopamine or as inhibitory signal neurotransmitters,such as glycine,nitric oxide,norepinephrine,or as both excitatory and inhibitory signal neurotransmitters,e.g.acetylcholine(Thomas et al.,2017).
The neurotransmitters are classified into four major groups,the biogenic amines,the neuropeptides,other ligands and the small molecule neurotransmitters,which are low molecular weight substances synthesized in the presynaptic terminals,including acetylcholine,glycine,glutamate,and γ-amino butyrate acid(GABA)(Duman et al.,2019).Studies have proven that consumption of diets containing amino acid,protein,lipid,and added additives,e.g.MSG promote the biosynthesis and in most times accumulation of these neurotransmitters in different areas of the body including the brain(Blaylock et al.,1997).The safety usage of MSG has recently become a more controversial issue globally(Hawkins and Viña,2016).In Nigeria for instance,individuals from most communities often employ MSG as a bleaching agent for stain removal;a practice believes that MSG bleaching properties may be toxic or injurious to the body,or worst still may be able to induce terminal disease to users if ingested(Cynober,2018).In scientific studies,MSG is used as an agent at different doses to induce model disease conditions to be studied in experimental animals.
High doses of MSG cause neuronal necrosis in hypothalamic arcuate nuclei in neonates(Airaodion et al.,2019).On postnatal days 1,3,5 and 7,4 mg/g MSG subcutaneously administered causes prefrontal cerebral cortex changes;at the same dose,MSG when injected subcutaneously on postnatal days 2,4,6,8,and 10 leads to 30% and 40% reduction in weight of pituitary at ages of 6 and 12 months,respectively(Fernstrom,2018).Moreover,when administered intraperitoneally on postnatal days 2,4,6,8 and 10,MSG induces increased adepogenicity and obesity in adult Wistar rats(Rotimi et al.,2012).
In polar medium,MSG dissociates into sodium cations and glutamate anions(Zanfirescu et al.,2019).The glutamate portion is used by the brain in its natural form as a neurotransmitter(Thomas et al.,2017).The brain receives and makes use of blood from the body(peripheral system),thus the brain is exposed to toxic chemical substances normally found in the blood as non-toxic(Sukhorukova et al.,2018).Glutamate is one of such,at high and/or accumulated concentrations glutamate excites brain cells to death or causes wide spread destruction(Akataobi,2020).Considering the amount of MSG(glutamate)added in diets as additives,studies have shown that consumption of these diets leads to elevated levels of glutamate in extracellular space,thus the nervous system(Gasem,2016).
GLUTAMATE TOXICITY EFFECT
Glutamate(C5H5O4NH2),a naturally occurring acidic amino acid,and a major component of MSG(C5H5O4NH2Na),is one of the many amino acids the body uses and are connected to the chain of body protein,and found to be the most common excitatory neurotransmitter located almost in all parts of the brain(Sukhorukova et al.,2018).Glutamate is synthesized in the presynaptic terminal from glutamine,by the phosphate activated enzyme glutamase,which if not immediately utilized,converted and/or removed from the synaptic cleft of the central nervous system(CNS)leads to accumulation of the released glutamate,which then causes the postsynaptic neurons to repeatedly undergo excitation,resulting in neuronal degradation followed by death,a process known as excitotoxicity(Thomas et al.,2017).
To prevent this,glutamate synthetase converts glutamate to glutamine which is less toxic to the brain in a reversible reaction which is used to synthesize glutamate when required in the brain(CNS)through glutamate-glutamine cycle involving the enzyme glutamase from which the enzyme glutamate decarboxylase catalyzes the decarboxylation of glutamate to synthesize GABA and CO2(Collison et al.,2012;Hazzaa et al.,2020)or from which glutamate dehydrogenase biosynthesizes α-ketoglutarate and ammonia through a reversible conversion of glutamate involving glutamate oxaloacetate transaminase(Sukhorukova et al.,2018).
MATERIALS AND METHODS
Experimental animals
Male and female adult Wistar rats were obtained from the Animal House of the College of Medical Sciences,University of Calabar P.M.B 1115,Calabar,Cross River State,Nigeria.After being co-habited at the ratio of 2:3,two males and three females,the females were allowed to become pregnant(identified physically by the change in size of their stomach)before they were further separated into different cages where they littered and raised their offspring(neonate).The principles of Laboratory Animal Care(NIH publication No:83-23 reversed 1985)were followed as well as specific national laws where applicable.All experiments were approved by the Faculty of Basic Medical Sciences University of Calabar and ethics committee-04/11/2018.
Distribution of experimental animals
Offspring(neonates)of the co-habited Wistar rats were used as experimental animals.The animals were divided into six groups with seven animals per group and exposed to different concentrations of MSG as neonates only,neonates plus adults and adults only.
MSG administration
Post-natal exposure
Briefly,following the day of delivery,neonates were divided into two groups.In group 1,animals received a single dose of 4 mg/g of MSG purchased from Wat market in Calabar South,Calabar Cross River State(reconstituted in normal saline)via intra-peritoneal route,on postnatal days 2,4,6,8 and 10.In group 2,animals were similarly treated with normal saline to represent normal control animals according to the method of Akataobi(2020).The rats were weaned on day 21 and raised normally for 32 weeks,after which the MSG administered group was further divided into three groups and administered saline,5 mg/g and 10 mg/g MSG orally using an oral gavage.
Adult exposure
Matured Wistar rats of the same age and size as in the section post-natal exposure above,obtained from the same source were divided into two groups and administered 5 and 10 mg/g MSG orally using an oral gavage.
With the exception of the control,all the groups were given MSG orally reconstituted in portable water,twice a day in the morning and the evening(twelve hours apart).The controls were also administered normal saline,in the same manner.Treatment lasted for 6 weeks.All the animals were kept in a room that allowed air to reach them.In night hours,constant light was provided and the animals were giving free access to feed(rat chow)and waterad libitum(Table 1).
Termination of the experiment and collection of sample
At the end of the study,all groups were fasted overnight and sacrificed under ketamine and the animals were decapitated and the skull was opened and whole brain was removed and suspended in an ice cold phosphate buffer saline of pH 7.4 in preparation for biochemical assays.
Preparation of brain homogenate
The whole brain of each rat was removed from the ice cold phosphate buffer saline and thoroughly homogenized in 5 mL phosphate buffer of pH 7.4,using a laboratory mortar and pestle.This homogenized tissue was centrifuged at 57×gfor 30 minutes.After centrifugation,the supernatant was withdrawn into a plain tube and used for biochemical analysis.
Glutamate dehydrogenase activity assay
The glutamate dehydrogenase activity assay was analyzed using the method described by Copenhaver et al.(1950).In the method,10 μL nicotin adenine dinucleotide solution was pipetted into a test tube and 20 μL phosphate buffer pH 7.4 and 0.03 mL ammonia acetate buffer pH 7.4 were added and shaken properly.To this reaction mixture,0.2 mL of supernatant was added followed by 0.02 m lactate dehydrogenase solution and 0.08 mL oxoglutarate solution.The mixture was allowed to stand for 5 minutes,thereafter absorbance was read at 1 minute interval for 10 minutes at 340 nm absorbance read at 540 nm using Jasco V-730 double beam UV-Visible spectrophotometer(Jasco International Co.,Ltd.,Tokyo,Japan).
Glutamate decarboxylase activity assay
Glutamate decarboxylase catalyzed the conversion of glutamate to GABA.The enzyme activity was measured using the method described by Meister(1985).The supernatant(20 μL)was added into a test tube followed by 100 μL reagent of 0.01 M imidazole-HCl,25 mM β-mecarptoethanol,50 mM-Sodium L-glutamate,10 mM Na-ATP,and 125 mM hydroxylamine.Thereafter,the mixture was shaken thoroughly and the pH adjusted to 6.8 using NaOH,and incubated for 15 minutes at 37°C and allowed to cool.Then,0.75 mL of cold solution mixture of 0.37 M FeCl2,0.67 M HCl and 0.20 M trichloroacetic acid was added.The reaction mixture was then centrifuged for 10 minutes at 128 ×g.The absorbance values were measured at 535 nm and 540 nm using Jasco V-730 double beam UV-Visible spectrophotometer(Jasco International Co.,Ltd.).
Glutamate synthetase activity assay
Glutamate synthetase catalyzes the conversion of glutamate to glutamine in the glutamate-glutamine cycle.The activity of this enzyme was analyzed using the method described by Konrad et al.(2012).The supernatant(50 μL)was added into a test tube,and then 100 μL of 50 mM imidazole-HCl(pH 6.8),100 μL of 0.5 mM EDTA,50 μL of 1 mM dithiothreitol were added and supplemented with protein inhibitor and protein concentration of the supernatant was adjusted to 1 mg/mL.An aliquot of 150 mL was used,in a solution containing 1 mL 50 mM imidazole-HCl(pH 6.8),10 mL 50 mM Gin,2 mL of 25 mM hydroxylamine,2 mL 25 mM sodium arsenate,2 mL of 2 mM MnCl2and 0.16 mL ADP and incubated for 30 minutes at 37°C.The reaction mixture was then stopped by adding 2 mL solution of 2.42% ferric chloride(FeCl3)and 1.45% trichloroacetic acid in 1.88% HCl.To remove the precipitate,the tube was centrifuged for 5 minutes at 57 ×gand absorbance was read at 540 nm using Jasco V-730 double beam UV-Visible spectrophotometer(Jasco International Co.,Ltd.).
Aminotransferase activity assay
Alanine aminotransferase(ALT)and aspartate aminotransferase(AST)activities were assayed following the method described in the analytical kit obtained from Randox(Randox Laboratories,United Kingdom).
Procedure
Exactly 0.1 mL of supernatant(from brain homogenate)was pipetted into a test tube and 0.1 mL of distilled water(blank)was pipetted into another test tube.In the two test tubes,500 μL each of R1 the ALT substrate buffer solution(containing phosphate buffer,L-alanine and α-oxoglutarate)was added.The reaction tubes were properly mixed and incubated for 30 minutes in a water bath at 37°C.Immediately following incubation,0.5 mL of Reagent R2(containing 2,4-dinitrophenylhydrazine)was added to both the sample test and tube blank,which were again properly mixed and allowed to stand for exactly 20 minutes.Lastly,0.5 mL of sodium hydroxide pH 7.0 solution was added into samples test tubes as well as the blank mixed thoroughly and,the absorbance was read at 564 nm initially and subsequently after 3 minutes.ALT activity was calculated according to the formula ALT(IUL)=2742 ×ΔA 546 nm/min,where 2742=extinction coefficient;ΔA 564 nm/min=change in absorbance per minute for the homogenate sample.The same method was used for AST assay,but ALT reagent was replaced with AST reagent accordingly.
Statistical analysis
Data obtained were analyzed by one-way analysis of variance followed by least significant differencepost hoctest.The Statistical Package for the Social Science version 21.0(IBM,Armonk,NY,USA)was used for the analysis.Differences were considered significant atP<0.05 and expressed as mean ± SEM.

Figure 1:Effect of monosodium glutamate administration on glutamate dehydrogenase activity in the brain of neonatal and adult rats.
RESULTS
Effect on glutamate dehydrogenase activity
MSG administration caused a significant increase in glutamate dehydrogenase activity in all MSG administered groups compared with the control group(P<0.05),with the exception of the group administered 4 mg/g-neonate plus 5 mg/g-adult which showed a reduced activity compared with the control.The 4 mg/g-neonate plus 10 mg/g-adult group showed a higher increase in activity,while no significant effects were obtained in 4 mg/g-neonate only compared with 5 mg/g-adult(P>0.05),followed by 10 mg/g-adult,which indicated a lower enzyme activity.Glutamate dehydrogenase activity was similarly affected in neonate,neonate plus adult and adult only groups following MSG exposure(Figure 1).
Effect on glutamate decarboxylase activity
Administration of MSG caused a dose-dependent increase in glutamate decarboxylase activity significantly compared with the control(P<0.05).There was no significant difference in glutamate decarboxylase activity between 4 mg/g-neonate plus 10 mg/g-adult and 10 mg/g-adult(P>0.05).A similar effect was also recorded in 5 mg/g-adult compared with 4 mg/g-neonate plus 5 mg/g-adult,which showed on significant difference in activity.A dose increase was recorded in glutamate dehydrogenase activity similarly in neonate,neonate plus adult and adult only groups following MSG exposure(Figure 2).
Effect on glutamate synthetase activity
A significant increase in glutamate synthetase activity was recorded in all the MSG administered groups except in 4 mg/gneonate plus 5 mg/g-adult(P<0.05),which indicated a reduction in enzyme activity compared with the control group.The group administered 10 mg/g-adult showed a higher increase in activity followed by 4 mg/g-neonate group while 5 mg/g-adult indicated a lower activity but significantly increased compared with 4 mg/g-neonate plus 5 mg/g-adult(P<0.05).No difference in activity was recorded in 4 mg/g-neonate compared with 4 mg/g-neonate plus 10 mg/g-adult.Glutamate synthetase activity was similarly affected in neonate,neonate plus adult and adult only groups following MSG exposure(Figure 3).
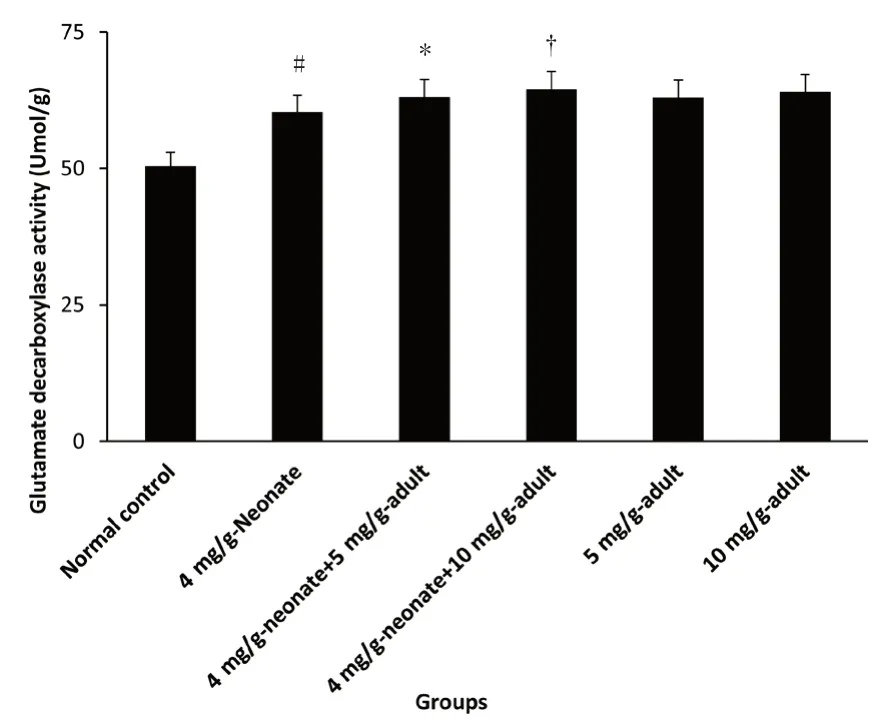
Figure 2:Effect of monosodium glutamate administration on glutamate decarboxylase activity in the brain of neonatal and adult rats.
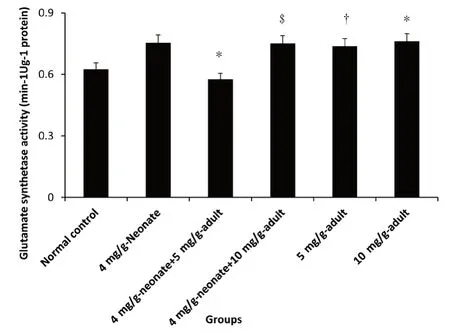
Figure 3:Effect of monosodium glutamate administration on glutamate synthetase activity in the brain of neonatal and adult rats.
Effect on ALT activity
Administration of MSG caused a significant difference in alanine aminotransfaraes activity in all the MSG groups compared with the control group(P<0.05).10 mg/g-adult showed the highest activity followed by 4 mg/g-neonate and 4 mg/g-neonate plus 10 mg/g-adult.No difference in enzyme activity was recorded between 4 mg/g-neonate plus 5 mg/g-adult and 5 mg/g-adult,though the activity was significantly lower compared with 10 mg/g-adult(P<0.05;Figure 4).
Effect on AST activity
MSG administration caused a dose-dependent increase in AST activity.AST activity was significantly higher in 5 mg/g-adult compared with control(P<0.05).AST activity was significantly increased in 10 mg/g-adult compared with 4 mg/g-neonate plus 10 mg/g-adult(P<0.05).A similar effect was also recorded between 4 mg/g-neonate plus 5 mg/g-adult and 4 mg/g-neonate(Figure 5).
DISCUSSION
The blood-brain barrier serves the purpose selecting/restricting the entry of certain substances from the blood(Kouzuki et al.,2019).This function of the blood-brain barrier has been linked to the role of the liver in reducing or completely removing toxic substances from the blood(Blaylock et al.,1997).In such condition as hepatic encephalopathy,a decline in brain function occurs as a result of severe liver disease which maybe as a result of exposure to toxic substances such as MSG administered in this study that may prove to be harmful to the liver(Hohnholt et al.,2018).In this condition,the liver cannot adequately reduce,maybe completely remove toxins from the blood causing a buildup of these toxins in the bloodstream(Cynober et al.,2018),which finds its way across the bloodbrain barrier into the central nervous system and promotes increases of respective enzyme activities.
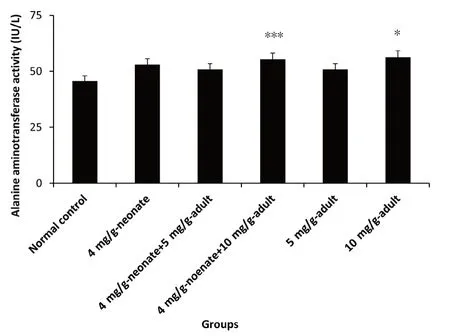
Figure 4:Effect of monosodium glutamate administration on alanine aminotransferase activity in the brain of neonatal and adult rats.
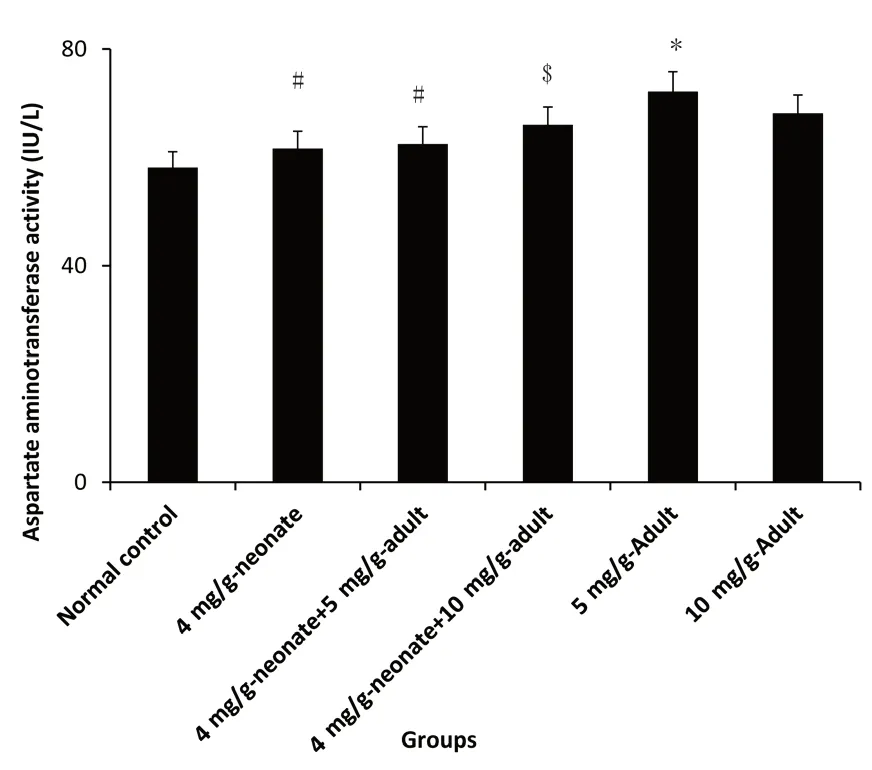
Figure 5:Effect of monosodium glutamate administration on aspartate aminotransferase activityin the brain of neonatal and adult rats.
Glutamate metabolism in the brain is carried out by a number of important enzymes that catalyze the conversion of glutamate to glutamine and α-ketoglutarate in a reversible reaction and GABA catalyzed by glutamate decarboxylase in a non-reversible reaction(Gudiño-Cabrera et al.,2014).Data obtained in this study showed that MSG administration increased the level of glutamate in the brain and the activities of key metabolic enzymes were elevated when compared to the control group.This observation indicated that MSG administration affected liver function and caused a buildup of glutamate in the blood,which crossed the blood brain barrier into the CNS in high concentration both in neonatal and adult administered rats similarly(Gudiño-Cabrera et al.,2014).The increase in concentration of glutamate promoted the increase in activities of metabolic enzymes for its breakdown and clearance from the whole brain,in line with the report,of Konrad et al.(2012)who reported increased catabolic enzymes of glutamate(glutamate synthetase and glutamate decarboxylase)in MSG administered Wistar rats.Moreover,Blaylock(1997)reported that consumption of MSG foods causes elevation of glutamate in the CNS of both young and adult humans similarly,and suggested that even after development,the brain contains regions that are not protected by the brain barrier,which means that the blood-brain barrier though an active mechanism that prevent peripheral glutamate and other neurotoxic agents entry into the brain may not be the key determinant for the transport and metabolism of glutamate following MSG exposure in the rat brain.This is an indication that age of exposure did not determine the effect of MSG following its exposure to the rats.In this study,following MSG administration,glutamate from the blood was able to cross the blood-brain barrier into the brain even after been fully developed(in adult administered group)in a similar manner it did during brain development(in neonatal administered group).The report that there exists certain region of the brain not protected by the blood-brain barrier is accepted(Rojas-Castañeda et al.,2016).
Glutamate dehydrgenase(E.C.1.4.2.)carries out an important function in the conversion of glutamate to α-ketoglutarate reducing NAD to nicotin adenine dinucleotide +HH4+(Kanamori et al.,2017).In whole brain of MSG exposed rats,this reaction is important in the metabolism of glutamate the primary excitatory neurotransmitter and necessary for the complete function of the brain(Gudiño-Cabrera et al.,2014).Thus,play an important role in the regulation and disposal of glutamate and related metabolites from the brain.Glutamate dehydrogenase catalyzes the deamination of glutamate to form α-ketoglutarate releasing NH3 and form NAD(P)H(Gudiño-Cabrera et al.,2014).Glutamate dehydrogenase also catalyzes the opposite reaction,which requires energy(e.g.,convert NAD(P)H to NAD(P+)),the reductive amination of α-ketoglutarate to form glutamate;it has been shown that in rat brain,glutamate reaction operates primarily in the direction of ammonia and acts in the generation of energy for the oxidative metabolism through krebs cycle(Gudiño-Cabrera et al.,2014).In this study,increased level of glutamate dehydrogenase activity points to an increase in the concentration of glutamate following MSG administration and its breakdown and utilization for different purposes similarly in both neonate and adult administered rats.
Glutamate decarboxylase(E.C.4.1.1.15),is another important enzyme in the metabolism of glutamate in the brain for the synthesis of GABA,using H2O for the production of CO2in a non-reversible reaction(Konrad et al.,2012).Reducing the concentration of glutamate in the brain which is harmful in accumulated level(Zanfirescu et al.,2019).Result of this study indicated a significant increase in the enzyme activity in MSG administered groups compared with control,pointing to its role in the regulation of glutamate concentration in the brain(Akataobi,2020).Studies have shown that glutamate decarboxylase is a rate-limiting enzyme in the breakdown of glutamate to GABA the main inhibitory neurotransmitter of the CNS where it primarily coordinates with the control as well as regulating GABA level(Rotimi et al.,2012).According to the study of Hawkins and Viña(2016),glutamate decarboxylase is present in the brain as an apoenzyme,which is activated by the availability of substrate(Zanfirescu et al.,2019).In this current study,the difference in activity of the enzyme between the administered groups and the control group indicated that glutamate entry into the brain activated the enzyme and increased its activity dose dependently.
Glutamate synthetase(E.C.6.3.1.2)located in the astrocytes synthesizes glutamine from glutamate in the brain in an energy-dependent reaction,where adenosine triphosphate is reduced to ADP + Pi in the presence of NH4+in a reversible reaction,resulting in the breakdown and utilization of glutamate,which may be elevated in the brain.Studies have shown that this enzyme regulates the main mechanism through which ammonia both derived and metabolically generated is eliminated in the brain(Inuwa et al.,2011;Gudiño-Cabrera et al.,2014).Furthermore,this enzyme plays an important role in the brain to detoxify ammonia and regulate the concentration and compartmentalization of neurotransmitter pool of glutamate and GABA(Gudiño-Cabrera et al.,2014).It has also been reported to function in the termination of neurotransmitter signals;generally all activities of glutamate synthetase require the breakdown/conversion of glutamate in the brain to other intermediates,in turn reduce the concentration of glutamate in the brain.This current study shows that administration of MSG increased the activity of this enzyme,due to entry and increased level of glutamate from the blood into the CNS similarly in both neonate,neonate plus adult and adult administered groups
Furthermore,aminotransferase enzymes transfer amino group from glutamate in the formation of α-ketoglutarate while metabolizing glutamate(Cooper,2012).AST has also been shown to catalyze the synthesis and breakdown of glutamate in a reversible transamination of oxaloacetate to aspartate in conjunction with the conversion of glutamate to α-ketoglutarate(Gasem,2016).This study indicated the increased activity levels of AST and ALT in MSG exposed groups,which was significantly higher compared with the control.This result further showed that the difference in the blood-brain barrier did not affect nor determine the entry of glutamate into the brain and its metabolism and breakdown as well as utilization,which occurred similarly.
Study limitation
This study is limited to the effect of MSG exposure to neonatal and adult rats,and covers only the measurement of glutamate metabolic enzyme activities in the whole brain of rats exposed to MSG either as neonate only,neonate plus adult and adult only at different concentrations.
Conclusion
MSG administration increased the concentrations of glutamate in the brain of exposed rats.The metabolism of the increased glutamate occurred similarly in neonate,neonate plus adult and adult groups in the same manner,which indicates that the level of blood brain barrier or age of exposure did not determine the effect of MSG in whole brain of exposed rats.
Author contributions
The author contributed to the study concept,experiment implementation,data analysis,and paper writing,and approved the final version of the paper.
Conflicts of interest
The author declares that there are no conflicts of interest.
Financial support
None.
Institutional review board statement
All authors hereby declare that Principles of Laboratory Animal Care(NIH publication No:83-23 reversed 1985)were followed as well as specific national laws where applicable.All experiments were approved by the Faculty of Basic Medical Sciences University of Calabar and ethics committee-04/11/2018.
Copyright license agreement
The Copyright License Agreement has been signed by the author before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
 Asia Pacific Journal of Clinical Trials:Nervous System Diseases2020年4期
Asia Pacific Journal of Clinical Trials:Nervous System Diseases2020年4期
- Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Effect of Ayurveda gut therapy protocol in managing dysbiosis of children with autism:study protocol for a randomized controlled trial
- Comparison of factor structure and psychometric properties of original and abbreviated version of the Penn State Worry Questionnaire in a nonclinical sample:a cross-sectional psychometric study
