Small balloon strategy associated with low pacemaker implantation rate after self-expanding transcatheter valve implantation
Yuan Zhang, Wen-zhi Pan, Li-hua Guan, Xiao-chun Zhang, Sha-sha Chen, Li-fan Yang, Lei Zhang, Ming-fei Li,Dan-dan Chen, Da-xin Zhou, Jun-bo Ge
Department of Cardiology, Shanghai Institute of Cardiovascular Disease, Zhongshan Hospital, Fudan University, Shanghai 200032, China
Corresponding Author: Da-xin Zhou, Email: 1194180219@qq.com; Jun-bo Ge, Email: ge.junbo2@zs-hospital.sh.cn
KEYWORDS: Transcatheter aortic valve implantation; Balloon aortic valvuloplasty; Balloon size;Permanent pacemaker implantation
INTRODUCTION
Transcatheter aortic valve impla ntation (TAVI) is increasingly applied for treating patients with severe symptomatic aortic stenosis.[1-3]One of the most common complications after TAVI is the need for new permanent pacemaker implantation (PPMI), especially in patients with self-expanding prostheses.[4]New PPMI is related to a longer hospitalization duration, reduced survival,and higher rates of repeated hospitalization.[5]Thus, the reduction of PPMI incidence after TAVI is a worthwhile goal.
Multiple reports state that balloon aortic valvuloplasty (BAV) is associated with the development of conduction disorders.[6-8]However, few studies have investigated the relationship between balloon size in BAV and the rates of PPMI. We hypothesize that the small balloon size in BAV is associated with a decrease in the PPMI rate. Therefore, this study aims to investigate whether a small BAV strategy reduces the need for permanent pacemakers after TAVI with a self-expanding transcatheter valve.
METHODS
Study design
This was a retrospective analysis using data from our local TAVI database that included 99 consecutive high-to-prohibitive surgical risk patients with severe aortic stenosis undergoing TAVI between 2015 and 2018. Baseline demographics, procedural data, and clinical outcomes were prospectively collected, while the analysis was performed retrospectively. All patients received pre-dilation using a Z-Med balloon (NuMED,Hopkinton, New York, USA) under rapid pacing routinely. The choice of balloon size in BAV was based on the operator preference in all cases. The size of the balloon was 18-23 mm in the f irst 65 patients and 18 mm in the last 34 patients after noticing that PPMI occurred less after the utilization of a smaller valvuloplasty balloon. The 18-mm balloon was the smallest available balloon. Thus, patients were divided into two groups based on the balloon size of BAV: the small BAV group(balloon size=18 mm) and the normal BAV group(balloon size >18 mm). If BAV was performed for two times, the bigger balloon size was used for classif ication.Baseline characteristics and clinical outcomes were compared between the two groups. The primary endpoint was the incidence of new PPMI. The indications for new PPMI were unrecovered high degree or III°atrioventricular block at any time during or after TAVI or by symptomatic bradycardia after TAVI. However,asymptomatic left bundle branch block (LBBB), I°and II° atrioventricular block, was not viewed as an indication for PPMI.
Patients and procedures
All potential TAVI candidates were assessed by a local heart team, including interventional cardiologists and cardiac surgeons. Severe aortic valve stenosis was defined by echocardiographic criteria including a mean gradient >40 mmHg (1 mmHg=0.133 kPa) or peak jet velocity >4.0 m/s and aortic valve area <0.8 cm2or aortic valve area index <0.5 cm2/m2. Baseline surgical risk was estimated using the European logistic system for cardiac operative risk evaluation (EuroSCORE) and Society of Thoracic Surgeons (STS) score. Multi-slice computed tomography (MSCT) was performed to assess aortic valve anatomy and vascular access. The transfemoralf irst approach rule was preferred.
General anesthesia was generally used in our TAVI procedure. Transesophageal echocardiography (TEE)and angiography were used for procedural guidance during the index procedure. Self-expanding prostheses were used in all patients, including two domestic selfexpanding transcatheter heart valves (THVs): Venus A(Venus Medtech Inc., Hangzhou, China) and VitaFlow valve (Shanghai MicroPort CardioFlow Medtech Co.,Ltd., Shanghai, China). The design of both domestic devices is similar to that of the Medtronic CoreValve(Medtronic Inc., Minneapolis, USA). An aorta angiogram and echocardiography were used to evaluate regurgitation. The implantation depth was def ined as the distance from the native aortic annulus plane to the left ventricular edge of the THV by fluoroscopy.[9,10]A postballoon dilation was performed if a more severe than moderate aortic valve regurgitation (AVR) and high transvalvular gradient existed. The post-dilation balloon size was generally 3-4 mm larger than the diameter of the narrowest part of the stent after implantation, as measured by fluoroscopy. The site-specific institutional review boards approved the protocol and consent forms.Written informed consent was obtained from all patients.
Statistical analysis
All statistical analyses were performed using SPSS version 19.0. Continuous variables were expressed as mean±standard deviation. Categorical variables were presented as numbers and proportions (%). Comparisons between the two groups (small BAV group and normal BAV group) were performed using the Chi-square test or Fisher exact test for categorical variables, and independentt-test for continuous covariates. To identify independent predictors of PPMI, candidate variables for the multivariable model were required to have clinical relevance and aP-value <0.15 in the univariate analysis, which included all available baseline clinical, echocardiographic, electrocardiogram (ECG),and procedural data. A value ofP<0.05 was considered signif icant.
RESULTS
Patient population
From the initial cohort of 99 patients, five had to be excluded because of a previous PPMI. Of the remaining 94 patients, small balloon pre-dilatation (=18 mm) was performed in 57 patients, regarded as the small BAV group, whereas 37 patients received balloon with pre-dilatation >18 mm, which were considered as the normal BAV group. Baseline characteristics are displayed, stratified by small BAV and normal BAV groups, as shown in Table 1. There were no significant differences between groups with respect to demographic characteristics, ECG characteristics, annulus diameter,percentage of the bicuspid valve, and echocardiographic variables, including mean aortic gradient, aortic valve area, left ventricular end-diameter, left ventricular ejection fraction (LVEF) (Table 1).
Procedural and clinical outcomes
Procedural characteristics are presented in Table 2.The prosthesis size was signif icantly larger in the normal BAV group than in the small BAV group (27.6±1.9 mm vs. 25.7±1.8 mm,P<0.001). There were no significant differences in the implantation depth, prosthesis diameter ratio to annulus diameter, or post-dilation rate between the two groups (allP>0.05). Procedural and clinical outcomes are shown in Table 3. There were no signif icant differences in severe or moderate AVR, post-procedural mean aortic gradient, or post-procedural aortic valve area (allP>0.05). Only one patient experienced severe AVR and conversion to surgery (normal BAV group)due to migration of the prosthesis, while no statistically significant difference was detected in the rate of device success (94.7% vs. 91.8%,P=0.680). Notably,the proportion of patients requiring new PPMI was significantly lower in the small BAV group than in the normal BAV group (3.5% vs. 18.9%,P=0.026).
Multivariable analysis
All 94 patients completed a 30-day follow-up, and there were no new pacemaker implantations observed after discharge. The rate of PPMI in the entire population was 9.6%. The candidate variables for the univariate logistic regression analysis for predictors of PPMI are shown in Table 3. Candidate variables for the multivariable model were required to have clinical relevance and aP-value<0.15 in the univariate analysis. Interestingly, the mean implantation depth was a predictor of new PPMI on univariate analysis. However, on multivariate analysis,where other parameters were selected as confounders, the mean implantation depth was not significantly different(Table 3). By multivariate analysis, predictors of PPMI included small BAV (odds ratio [OR] 21.30, 95% conf idence interval [CI] 1.41-321.18,P=0.027), prosthesis diameter/annulus diameter ( for each 0.1 increment,OR2.70, 95%CI1.00-7.31,P=0.048), and mean aortic gradient by echo at basement (for each 1 mmHg increment,OR-1.08, 95%CI-1.01 to -1.16,P=0.021), as shown in Table 3.
DISCUSSION
This study’s objective is to test our hypothesis that a small balloon strategy in BAV is associated with low PPMI after TAVI. The primary results of our study were as follows: (1) the rate of new PPMI after TAVI in the whole cohort was low (9.6%); (2) the rate of PPMI in the s mall BAV group was signif icantly lower than that in the normal BAV group, while the hemodynamic parameter,procedure success rate, and clinical outcomes were not different between the two groups; (3) small balloon strategy and prosthesis diameter/annulus diameter were independent predictors of PPMI after TAVI.
Current evidence shows that the PPMI rate is more frequent after TAVI with a self-expanding valve than the balloon-expandable valve.[11]Regarding self-expanding prostheses, the PPMI rates were higher with the early firstgeneration CoreValve device (16.3%-37.7%),[12-14]and despite a reduction in PPMI rates with the new Evolut R,the rates remained relatively higher (14.7%-26.7%).[15,16]The new PPMI rate in this study was lower (9.6%), which was also lower than in the Chinese Venus A analysis(18.8%).[17]In this study, the 18-mm balloon during BAV was used in 60.6% of patients. This indicates that the balloon size during BAV in our cohort was generally smaller than that in other studies.
In a previous study[13]using the CoreValve prosthesis, pre-dilatation with a smaller valvuloplasty balloon resulted in significantly lower PPMI rates.Notably, in that study, only 1.2% of patients were treated with an 18-mm balloon during BAV, and most balloon sizes were 23-25 mm. However, in this study, a balloon of 18-mm diameter or no balloon valvuloplasty was performed in more patients (60.6%). This might partially explain the lower rate of PPMI after TAVI in the present study (9.6%). This was also consistent with the logistic regression analysis result: a valvuloplasty balloon of greater diameter was found to be an independent predictor of PPMI.[13]These f indings support our results,stating that a small BAV strategy may be more effective in reducing the need for new PPMI after TAVI.
There are several potential explanations for the association of small balloon strategy with low PPMI after TAVI. First, a small BAV results in a small supraannular structure orifice before the final deployment;thus, small-sized prostheses could be anchored. A prosthesis to left ventricular outflow tract diameter and left ventricular end-diastolic dimension ratio is identif ied as a novel predictor of PPMI.[18]Moreover, in this study, the multivariable analysis demonstrated that the prosthesis diameter/annulus diameter was a predictor of new PPMI (for every 0.1 increments,OR2.70, 95%CI1.00-7.31,P=0.048). With the small BAV strategy, the small BAV group had a significantly smaller protheses
deployment (27.6±1.9 mm vs. 25.7±1.8 mm,P<0.001),thus attributing to a low rate of new PPMI. Second, a small BAV causes less damage to the conduction system than a balloon with a large diameter. Furthermore, after a small BAV, prostheses are easily expanded, exerting less force on the annular.
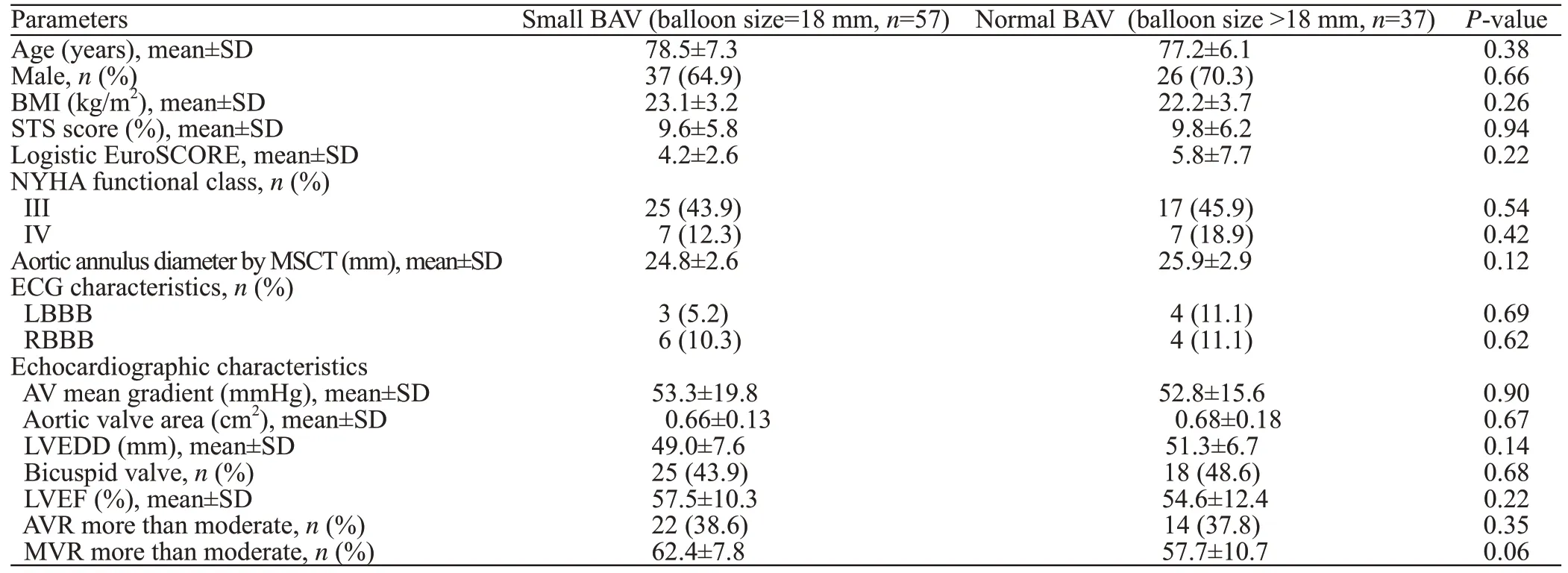
Table 1. Baseline characteristics

Table 2. Procedural characteristics and clinical outcomes
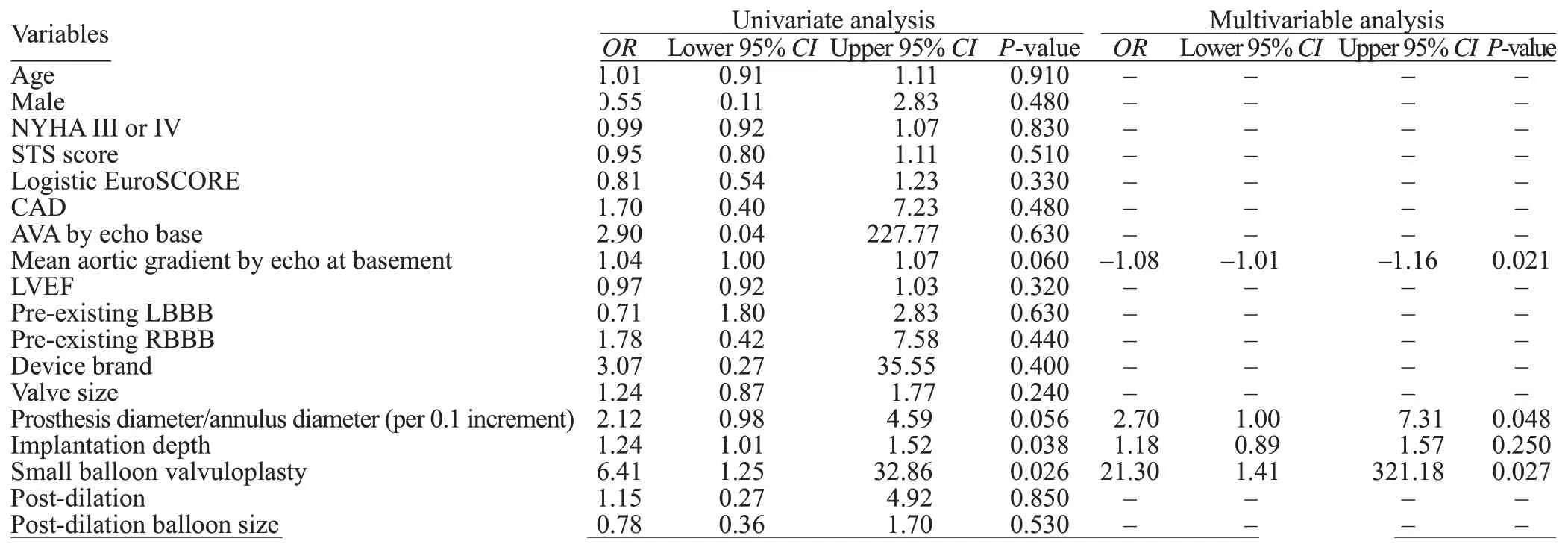
Table 3. Univariate and multivariable predictors of the implantation of permanent pacemaker after TAVI
A small balloon size strategy may cause concerns about the incomplete expansion of prostheses. Also, it may increase the rate of AVR and aortic gradient after the procedure. Another concern regarding impaired selfexpansion is the migration of prostheses due to leaflet incompetence, signif icant AVR, or further need for postdilation.[19]In this study, we did not f ind any differences in aortic regurgitation grades and AVR rates between the two groups. Indeed, these results are in line with previously reported evidence. Grube et al[20]reported no difference in aortic regurgitation with or without balloon pre-dilation in 60 CoreValve implantations. Fiorina et al[21]found a lower incidence of moderate to severe AVR without pre-implantation BAV.
A previous study[22]identif ied implantation depth as a risk factor for PPMI. In our series, the mean implantation depth was a predictor of new PPMI on univariate analysis. However, on multivariate analysis, implantation depth was not an independent predictor. The reason might be that the small BAV strategy has adjusted the effect of PPMI. Notably, there were 19 cases in our series with the implantation depth >6 mm, and we did not f ind any conduction disorder.
Interestingly, the mean aortic gradient by echo in the basement is found to be another independent predictor of new pacemaker implantation after TAVI. New PPMI may be less frequent in patients with a higher mean aortic gradient in the basement. This may be partially explained by the fact that a high aortic gradient is normally related to a small aortic valve orifice area and high calcium load; therefore, it is easy to under-expand for the device, resulting in less pressure in the landing zone.Unfortunately, the calcification load was not analyzed in this study due to the absence of data. More research is warranted. In addition, most predictive methods for PPMI are pre-existing RBBB in other studies.Interestingly, pre-existing RBBB was not a predictor in our study. In our patient cohort, pre-existing RBBB rate was 10.6%, which was lower than that reported in other studies.[23]This might be a partial reason for this.
This study had several limitations. First, the study was a retrospective analysis with a small number of patients within each group. Second, this study was a non-randomized trial. Importantly, a selection bias was possible. Whether or not to use a small BAV was made by the TAVI team operator, who had a perception of successful valve delivery. Further studies, including many patients, are needed to ascertain small BAV strategy as a standard practice.
CONCLUSIONS
With a small BAV strategy, a very low rate of new PPMI is achieved without increasing the amount of AVR, similar hemodynamic performance, and similar device success rate. A small BAV strategy is feasible and beneficial in our single-center experience. Further studies, including a large number of patients, are needed to ascertain small BAV strategy as a standard practice.
Funding:This work was supported by National Key R & D Plan(2017YFC1104202).
Ethical approval:The protocol was approved by the Site-specif ic Institutional Review Boards.
Conf licts of interests:The authors declare no conf licts of interest.
Contributors:YZ and WZP contributed equally to this work. All authors revised and approved the f inal version of the manuscript.
 World journal of emergency medicine2021年1期
World journal of emergency medicine2021年1期
- World journal of emergency medicine的其它文章
- Trends and challenges of emergency and acute care in Chinese mainland: 2005-2017
- Factors associated with refractory pain in emergency patients admitted to emergency general surgery
- Identifying critically ill patients at risk of death from coronavirus disease
- Clinical correlates of hypotension in patients with acute organophosphorus poisoning
- Effects of viral infection and microbial diversity on patients with sepsis: A retrospective study based on metagenomic next-generation sequencing
- Effects of metabolic syndrome on onset age and long-term outcomes in patients with acute coronary syndrome
