Meta-analysis of randomized controlled trials of traditional Chinese medicine in the treatment of obstructive sleep apnea syndrome
Jun-Li Bao, Yu-Bo Han, Ke Zhang, Xin-Yuan Gao, Kun-Ming Peng, Li Liu✉
1.Heilongjiang University of traditional Chinese Medicine. No.24 Heping Road, Xiangfang District, Harbin, Heilongjiang 150036,China 2.Fist Affiliated Hospital, Heilongjiang University of traditional Chinese Medicine Harbin, Heilongjiang 150036,China
3.University of Chinese Academy of Sciences, Shenzhen Hospital
Keywords:Obstructive sleep apnea syndrome (OSAS)Traditional Chinese medicine Randomized controlled trial Meta analysis
ABSTRACT Objective: Though Chinese medicine (CM) has showed its clinical efficacy for the treatment of obstructive sleep apnea (OSA) in China, no systematic reviews or meta-analyses provide evidences for its therapeutic effects on OSA and the long-term safety. The aim of the present study is to evaluate the effectiveness and safety of CM on OSA using meta-analysis. Methods: We used search items of “Chinese Medicine” AND “obstructive sleep apnea” to retrieve the randomized control trials (RCTs) of CM treatments for OSA in PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP database, and Wanfang database from their respective inception dates to December 2019. Only RCTs of CM therapy versus no treatment which could be quantitatively synthesized were included. Finally, 20 studies representing 1,297 participants were included after extraction. Two investigators independently extracted and analyzed the data using RevMan5.3 software. Results: The treatment group using CM decoctions or CM granules presented significantly ameliorative effects on apnoea-hypopnoea index (AHI) compared with the control group (MD: -2.58, 95% CI: -3.59 to -1.56, P < 0.00001 and MD: -5.47, 95% CI: -6.75 to -4.19, P < 0.00001, respectively) in the sensitivity analysis. However, there were non significant differences in the duration of treatment between subgroups, indicating that the duration of treatment has no impacts on the therapeutic effects on AHI. CM granules also showed significantly ameliorative effects on the lowest nocturnal oxygen saturation (LSaO2) (MD: 2.76, 95% CI: 1.85 to 3.68, P < 0.00001). CM decoctions exhibited significantly improved Epworth Sleepiness Scale (ESS) scores compared with the control group in a sensitivity analysis (MD: -1.50, 95% CI: -2.13 to -0.88, P < 0.00001). CM granules showed a better improvement of ESS than the control group (MD: -1.35, 95% CI: -1.92 to -0.78, P < 0.00001). Mild adverse reactions occurred only in five patients and disappeared without special treatment. Conclusion: This study showed favorable therapeutic efficacy of CM on OSA. However, in consideration of the low methodological quality of the included RCTs, more rigorous designed, large sample size RCTs are recommended for providing more high-quality evidences.
1. Introduction
OSA mainly features repetitive episodes of cessation (apnea) or reduction (hypopnea) in airflow during sleep caused by upper airway obstruction [1]. About 22% of men and 17% of women have been diagnosed as OSA with AHI 5/h according to the epidemiological data reported from 1993 to 2013 [2]. Epidemiological surveys from many hospitals showed that the prevalence of OSA ranges from 3.5% to 4.8% in China [3].
OSA as a systemic disorder can result in multiple problems, encompassing hypertension, cardiovascular disease with an increased rate of cardiovascular events (e.g.,myocardial infarction and stroke atrialfibrillation), insulin resistance, the increased morbidity and mortality of cancers, and neurodegeneration. All this has made this malady escalate into a very significant public health issue [4].
Though the exact etiology of OSA still remains unclear, multiple western medications and management options have been developed so far [5]. Continuous airway positive pressure (CPAP) therapy is the most common treatment for OSA with the best clinical efficacy. It is a type of positive airway pressure, where the air flow is introduced into the airways to maintain a continuous pressure to constantly keep the airways open, in people who are breathing spontaneously. Side effects of CPAP treatment may include congestion, runny nose, dry mouth, and nosebleeds [6]. Moreover, its poor compliance restricts its clinical use and effectiveness [7]. Current evidences are still not strong enough to confirm the superiority of oral appliance therapy and surgical treatment that reveal high risks of serious adverse reactions over CPAP treatment [8]. For another thing, they also bring financial burdens to the patients. Therefore, some researchers have turned to complementary and alternative medicine (CAM) for averting these side effects as much as possible.
Acupuncture and Chinese herbal medications as the mainstream of CAM, have been popular in China for thousands of years for treatments of various diseases [9], but have not been widely accepted by the western world. Only one meta-analysis has reported the efficacy of acupuncture in OSA in 2016 [10].
Recently, increasing clinical studies have supported the advantages of CM for OSA, which are affordable with less side effects. However, details of these results have not been synthesized, and there has been no sufficient scientific evidence supporting the therapeutic efficacy of CM in OSA for clinical practices. For this reason, CM still cannot be recommended as a primary treatment of OSA.
In this systematic review, we investigated the clinical efficacy and safety of CM in OSA using meta-analysis, for providing scientific evidence for its clinical use.
2. Methods
This study complied with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (the PRISMA statement). The protocol of this review has been registered on PROSPERO (http://www.crd.york.ac.uk/ PROSPERO); the ID is CRD42020154864
2.1. Search strategy
We conducted a comprehensive literature search in PubMed, Embase, Cochrane Library, VIP, Wanfang and CNKI databases from their respective inception dates to December 2019. There was no limit to the language or publication status. A combination of medical subject headings (MeSH) and free terms was applied to retrieve the potentially eligible studies. MeSH was slightly modified based on the specification of each database. The search terms of English language databases were as follows: (“Sleep Apnea, Obstructive” OR “osahs” OR “obstructive sleep apnea” OR “sleep apnea” OR “sleep hypopnea” OR “upper airway resistance sleep apnea syndrome”) AND (“Medicine, Chinese Traditional” OR “Traditional Chinese Medicine” OR “Chinese Medicine, Traditional” OR “Chung I Hsueh” OR “R Hsueh, Chung I” OR “Zhong Yi Xue” OR “Chinese Traditional Medicine” OR “Traditional Medicine, Chinese”). we used search terms of Chinese language databases as follows: (“zhongyao” OR “zhongyi”) AND (“shuimian” AND (“huxizanting” OR “ditongqi” OR “zusexing”). The detailed procedure of the literature search in PubMed and Embase was presented in Appendix.
2.2 Literature search and inclusion-exclusion criteria
The PICOS (participants, interventions, comparisons, outcomes, and study design) principle was utilized for our inclusion and exclusion criteria.
2.2.1 Inclusion criteria
Studies were considered eligible when they met the following criteria. (i) Participants included in our study must be diagnosed with OSA according to the results of PSG (AHI > 5/h). No restrictions on age, sex and race were imposed. (ii) Patients with OSA in the experimental groups mainly received CM treatments without regard to dosage forms. But the dosage forms in the same trial must be consistent. (iii) Subjects allocated in the control groups received no treatment. (iv) The primary outcome was AHI, and the second outcomes included LSaO2, ESS and adverse actions. (v) When the recruitment subjects and the duration of treatment overlapped by more than 60% in two or more papers by the same authors, we only included the most recent study. (vi) All studies must be RCTs.
2.2.2 Exclusion criteria
Studies met the following criteria would be rolled out: (i) animal experiments, reviews, case reports, and repetitive publications or search outcomes; (ii) an article containing less than ten cases; (iii) incomplete data in trials.
2.3. Study selection
Two authors independently read titles, abstracts, and key words and initially screened potential studies based on the inclusion and exclusion criteria. Full texts of the potential studies were obtained for a final determination.Disagreements were resolved by discussion between the two researchers after rereading the full text.The remaining disagreements were resolved by a third researcher. Disagreements were resolved by discussion between the two researchers after rereading the full text. The remaining disagreements were resolved by a third researcher.
2.4 Quality assessment
Two authors assessed the quality of all included studies. The Cochrane risk of bias assessment tool was used, including: adequate sequence generation, allocation concealment, patient blinding, investigator blinding, assessor blinding, incomplete outcome data, and selective outcome reporting. The baseline data of participants were compared and clinical trial registration was evaluated. The items were scored with three levels: low, unclear and high risk of bias. Jadad scale as an easy-to-perform assessment was used to evaluate the quality of all included studies. The score ranged from 0 to 7. Research papers scored 1 to 3 was considered to be lowquality, and those scored 4 to 7 high-quality. Discrepancy was resolved by discussion between the two reviewers. Judgments were independently performed by two investigators and disagreements were remedied after discussion with a third investigator.
2.5 Data management
Two authors independently extracted the following variables, including: article title, author(s), journal title, year of publication, sample size, sex, age, the duration of treatment, outcome, CM formula, CM preparation and dosage form. A third reviewer reviewed the extracted data and stored the original data in a secure computer to avoid changes.
2.6 Statistical analysis
We performed a meta-analysis using RevMan 5.3 only when sufficient and suitable data were obtained. A random-effect model was adopted for overall and subgroup analysis if obvious heterogeneity had existed, otherwise a fixed-effect model metaanalysis was performed. Furthermore, they were adopted to test the difference of the two models by sensitivity analysis. To illustrate, sensitivity analysis was performed by removing any single trial in each group. Statistical heterogeneity was evaluated by the Cochran's Chi-squared test (with a P value of < 0.10 indicating statistically significant heterogeneity) and the statistic I2. The heterogeneity might not be important with an I2value ranging from 0% to 40%. The statistical heterogeneity was moderate with an I2value from 30% to 60%, substantial with an I2from 50% to 90%, and considerable with an I2from 75% to 100%. Publication bias was assessed by funnel plot and Egger's test. Egger's test was conducted when the number of studies was no less than 10, whereas trim-andfill analysis was performed when publication bias existed.
3. Results
3.1 Search results
After primary searches in the databases, 1,418 articles were initially screened. After removal of duplicates, 1,040 ones remained. After reading the titles and abstracts, 916 were excluded and 113 full texts remained. By reading the full texts, 55 of them were excluded because the types of intervention did not meet our inclusion criteria. Besides, 30 non-RCT studies, 1 without any indice of AHI, LSaO2or ESS, and 6 repetitive publications were rolled out; so did another 2 because the dosage forms did not meet the inclusion criteria. Finally, 20 studies representing 1,297 participants, which met the inclusion criteria, were included in the meta-analysis [11-30]. The screening process was summarized in the Fig.1.
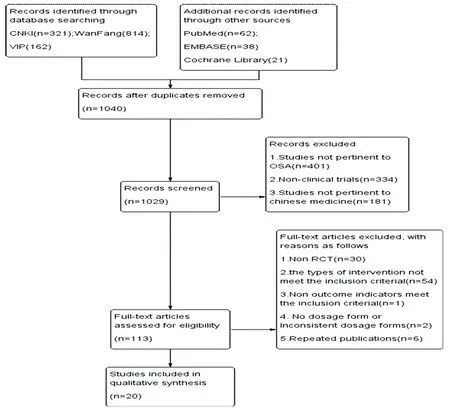
Figure1.Flow chart of the process for literature retrieval
3.2 Characteristics of included trials
All included RCTs were conducted in China, of which 19 were published in Chinese and 1 in English. A total of 1,297 patients with OSA were involved in the studies. All clinical characteristics, such as study size, sex, age, the duration of treatment, outcomes, and other basic information, were listed in Table 1. In addition, all studies exhibited comparable baseline patient characteristics, including age and sex. And there were nonsignificant differences in the baseline indices between groups.
Of the 20 studies, 2 used the same prescription with the same herb composition, and another 2 adopted the same prescription with slight differences in the drug composition. The detailed formula composition used in the 20 studies was summarized in Table 2
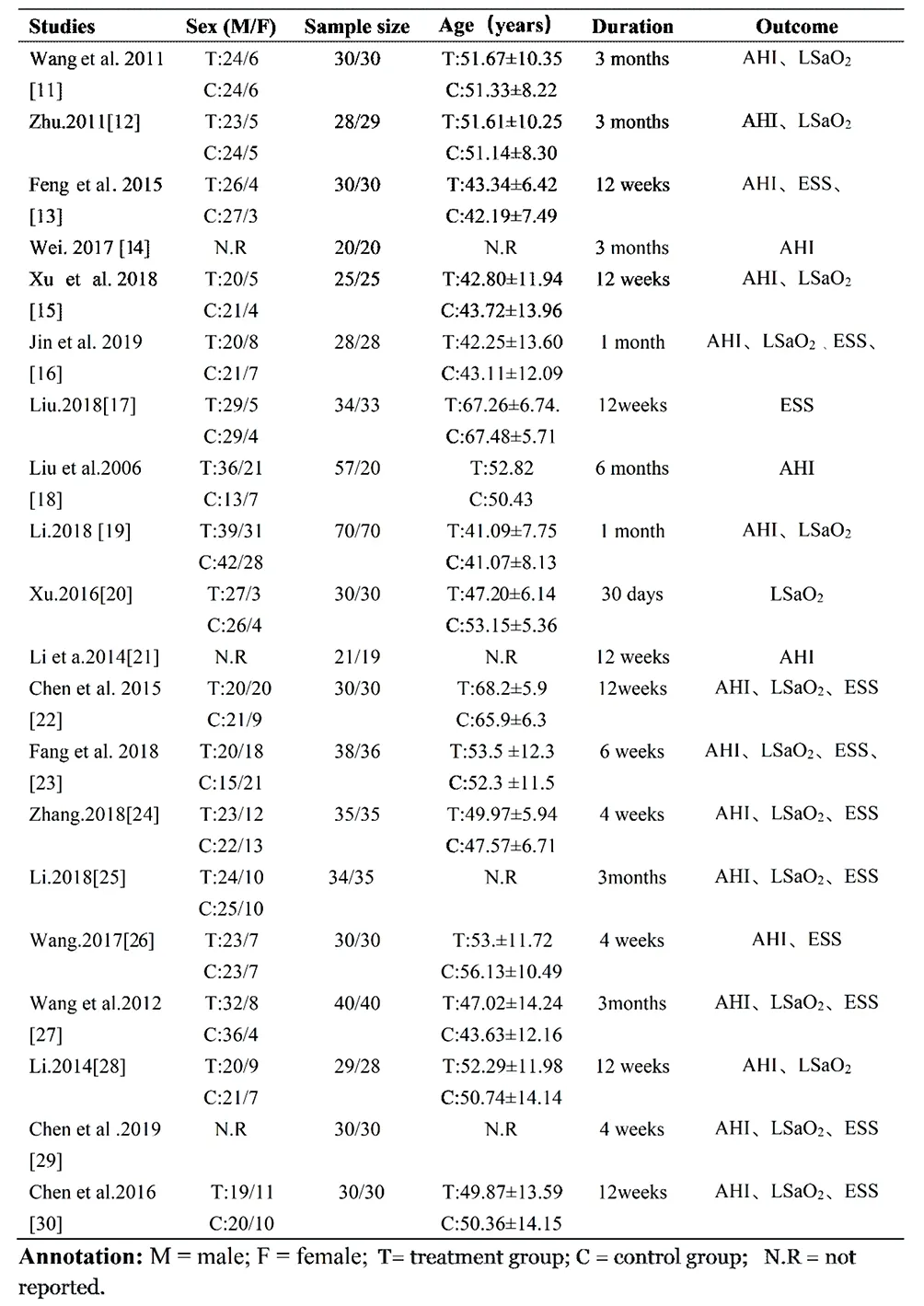
Table 1 Characteristics of included trials.
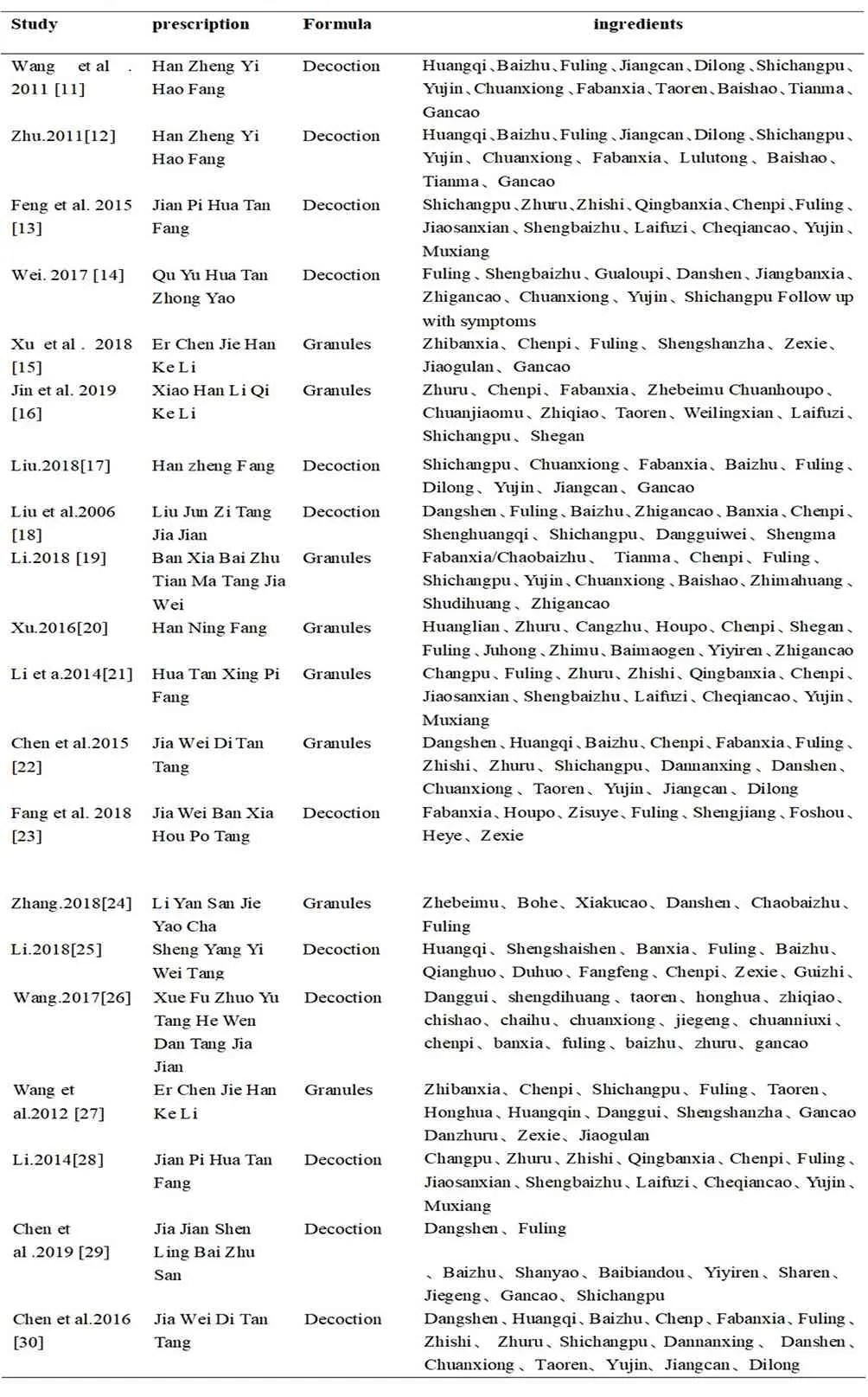
Table 2 Ingredients of each prescription.
3.3 Evaluation of risk of bias
The methodological quality of the included studies was shown in Table 3. All data of clinical characteristics were comparable [11-30]. With exception of 8 studies using a random number table [13,14,15,17,22,24,26,30], 2 following the random principle of RCTs [16,23], 1 following the patient's wishes[12] and 2 depending on the visiting order [25,29], the remaining 9 used the word “randomization” without any explanation of a random allocation process [11,18,19,20,21,27,28]. Only one study mentioned allocation concealment [13].However, blinding was not mentioned in all trials [11-30]. Details of the risk of bias were presented in Fig. 2.

Table 3 Assessment of methodological quality.
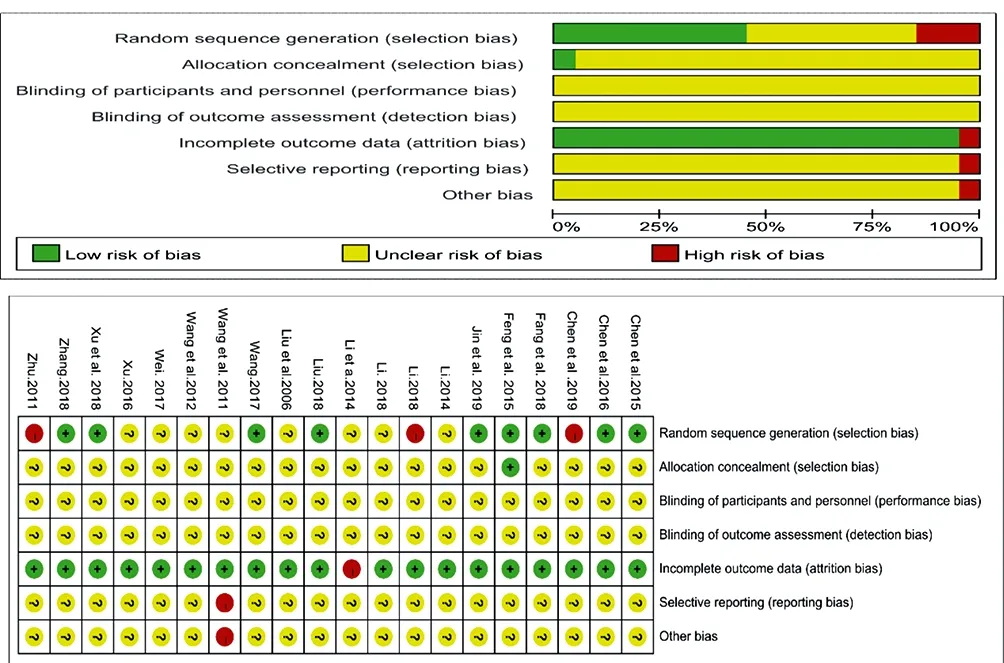
Figure2. Risk of bias summary and graph.
3.4. Primary outcomes
3.4.1 AHI
Eighteen trials including 1,170 patients reported the improvement of AHI [11-16,18-19,21-30]. The main dosage forms of CM prescriptions were decoctions used in the treatment group of 11 studies [11-14,18,23,25,26,28-30], and granules in the treatment group of 7 studies [15,16,19,21,22,24,27]. Therefore, two meta-analyses were performed according to the dosage forms.
As shown in Fig.3., a fixed-effect model was adopted with an I2= 0 (Chi2= 4.18, P = 0.65). Compared with the control group, the treatment group (with CM granules) showed a significant improvement of AHI (MD: -5.47, 95% CI: -6.75 to -4.19, P < 0.00001).
As shown in Fig. 4, a random-effect model was adopted with an I2= 89% (Chi2= 87.48, P < 0.00001). As a result, the data was not convincing, so the following sensitivity analysis was performed.
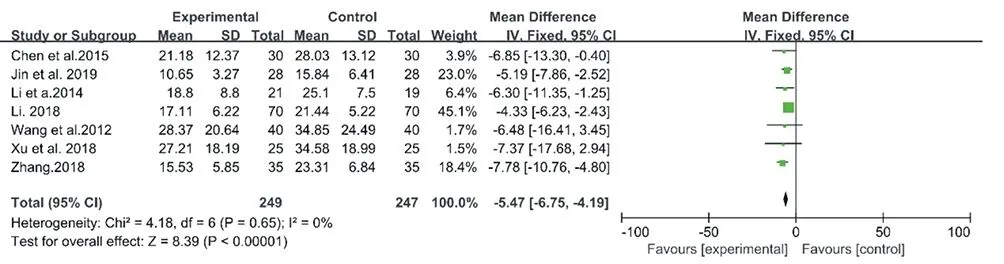
Figure3. Forest plot of CM granules versus control group:AHI
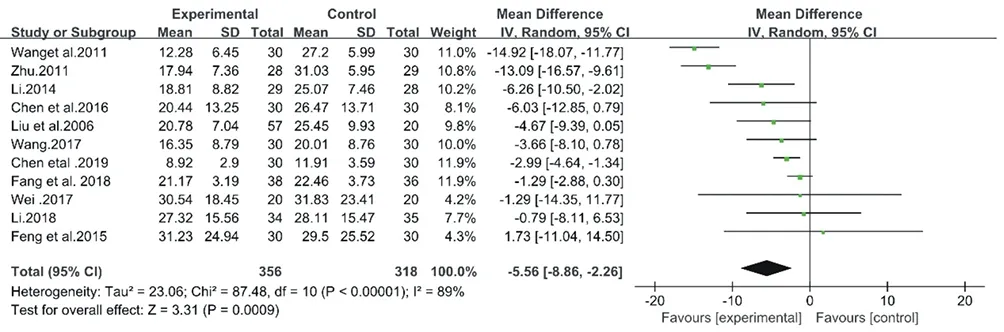
Figure4. Forest plot of CM decoctions versus control group:AHI
3.4.2 Sensitivity analysis
As we reviewed the 11 studies on CM decoctions, it was found that 2 studies scored 3 points in JADAD scale. Wang et al.[11] conducted a pseudorandom test in 2011. However, its stochastic method did not follow the random principle of RCT design. In the study of Zhu et al. in 2011[12], subjects were divided into groups according to their wishes. However, there was a high risk of non-normal random processes using this method according to the Cochrane Collaboration's tool. This could explain the high heterogeneity. After removing the 2 studies, the I2reduced from 89% to 4%, indicating that the results of the meta-analysis after removal were relatively stable. There are 9 trials including 557 patients included [13,14,18,23,25,26,28-30]. As shown in Fig. 5, a fixed-effect model was selected with an I2= 4% (Chi2= 8.35, P = 0.4). Compared with the control group, the treatment group (using CM decoctions) presented a significant improvement of AHI (MD: -2.58, 95% CI: -3.59 to -1.56, P < 0.00001).
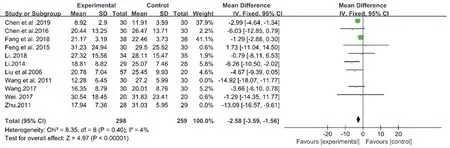
Figure5. Forest plot of CM decoctions versus control group in the sensitivity analysis:AHI
3.4.3. Publication bias
To assess publication bias, funnel plots were conducted for primary outcomes (Fig.6, Fig. 7). This symmetrical funnel plot did not reflect the possibility of potential publication bias.
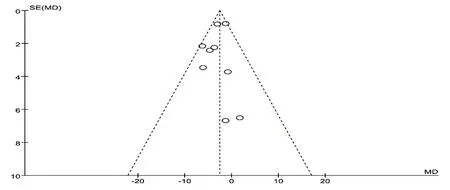
Figure6. Funnel plot of CM degpctions versus control group:AHI.
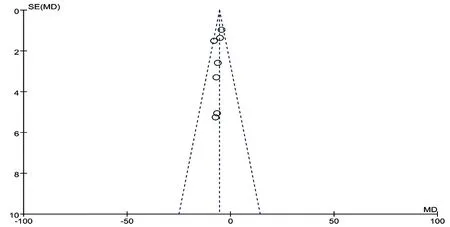
Figure7. Funnel plot of CM granules versus control group:AHI.
3.4.4. Subgroup analysis
Different the duration of CM treatments could impact their therapeutic efficacy, so we carried out two subgroup meta-analyses. A pooled estimate of cases of OSA who received CM decoctions showed that there was a significant difference between the > 6 weeks group and the control group in 6 studies (MD: -4.58, 95% CI: -7.14 to -2.02, I2= 0, P = 0.0004). The meta-analysis of another 3 studies also showed that there was a significant difference between the 6 weeks group and the control group (MD: -2.20, 95% CI: -3.31 to -1.09, I2= 22, P < 0.0001) (Fig.8). However, there was a nonsignificant difference between the > 6 weeks group and the 6weeks group (P for interaction = 0.09) (Table 4). This indicated that the duration of CM decoctions presented no impact on the therapeutic effects.
A pooled estimate of cases of OSA who accepted CM granules showed that there was a significant difference between the > 6 weeks group and the conventional treatment group in 4 studies (MD: -6.6, 95% CI: -10.08 to -3.03, I2= 0, P = 0.0002). The meta-analysis of another 3 studies also showed that there was a significant difference between the 6weeks group and the control group (MD: -5.29, 95% CI: -6.67 to -3.92, I2= 45, P < 0.00001) (Fig.9). However, nonsignificant differences between the > 6 weeks group and the 6 weeks group were found (P for interaction = 0.49) (Table 5). This suggested that the duration of CM granules showed no influence on its therapeutic effects.
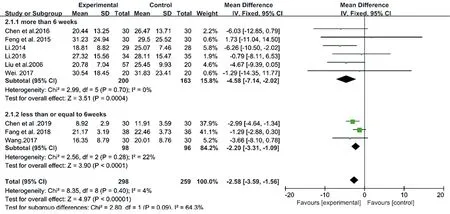
Figure8. Forest plots of subgroup of CM decoctions
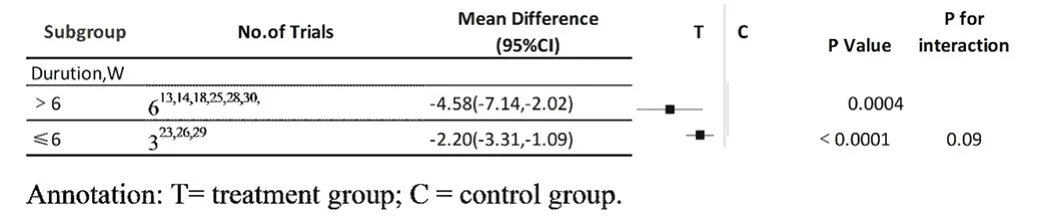
Table 4 outcome of subgroup analysis:Decotion
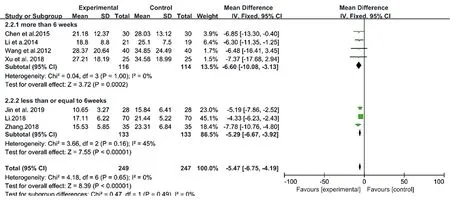
Figure9. Forest plots of subgroup of CM granules.
3.5. Secondary outcomes
3.5.1 LSaO2
Fourteen trials representing 953 patients reported the recovery of LSaO2 after CM treatment [11,12,15,16,19,20,22,23,25,24,27-30]. These studies used different dosage forms of CM prescriptions, of which 7 studies adopted CM decoctions [11,12,23,25,28-30] and 7 CM granules [15,16,19,20,22,24,27]. Therefore, two meta-analyses were performed according to the dosage forms.
The meta-analyses for CM decoctions and granules showed high inconsistency with I2= 86% (Chi2= 41.85, P < 0.00001) and 69% (Chi2= 19.33, P = 0.004), respectively (Fig.10& Fig.11). However, the high heterogeneity of two meta-analysis made the data unconvincing, so the further sensitivity analyses were needed.
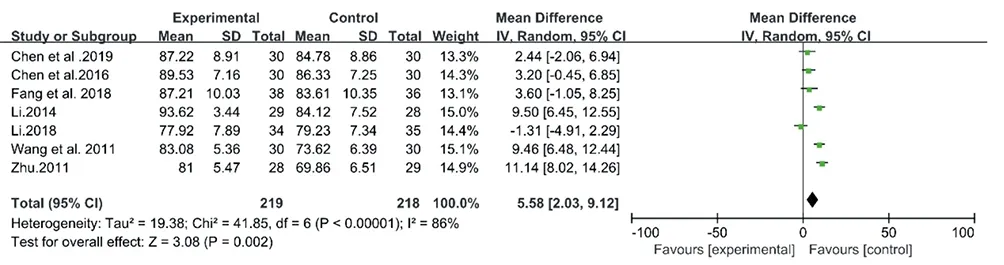
Figure10. Forest plot of CM decoctions versus control group: LSa02
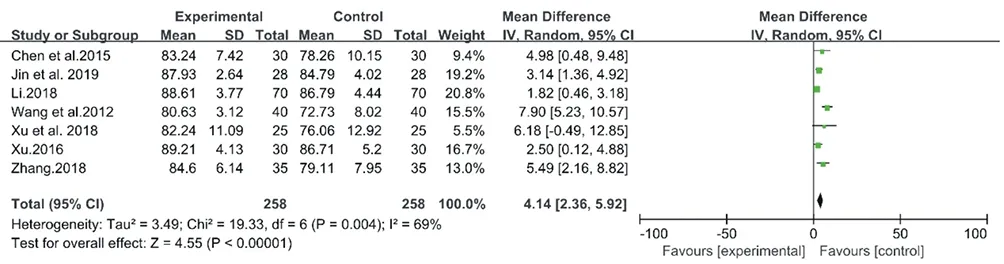
Figure.11. Forest plot of CM granules versus control group: LSa02
3.5.2 Sensitivity analysis
In the sensitivity analysis for the above 7 studies on CM decoctions, I2values did not significantly decrease after eliminating any one study. This indicated that the results of the meta-analysis for CM decoctions were still unconvincing. So the meta-analysis was suspended.
In the sensitivity analysis for the above 7 studies on CM granules, Wang et al. 2012 [27] provided unclear diagnostic criteria of OSA, which could result in high heterogeneity. After eliminating the study, I2reduced from 69% to 24%, which indicated that the results of the meta-analysis were relatively stable after removal.
Therefore, 6 trials representing 436 patients were included in the meta-analysis for CM granules [15,16,19,20,22,24], and a fixedeffect model was selected with an I2= 24% (Chi2= 6.57, P = 0.25) (Fig.12). As a result, the treatment group (using CM granules) showed better recovery of LSaO2 than the control group (MD: 2.76, 95% CI: 1.85 to 3.68, P < 0.00001).
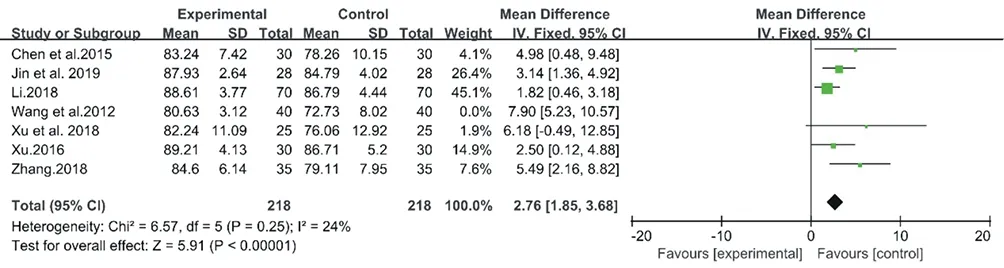
Figure12. Forest plot of CM decoctions versus control group in the sensitivity analysis: . LSaO2
3.5.3. Publication bias
For assessing publication bias, funnel plot was conducted for primary outcomes (Fig.13), which was symmetrical, indicating a nonsignificant possibility of potential publication bias.
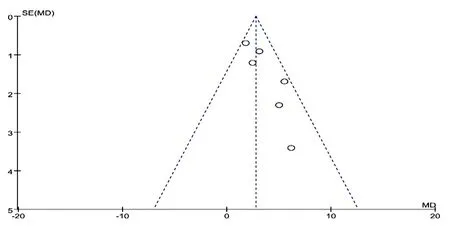
Figure13. Funnel plot of CM granules versus control group: LSaO2
3.5.4 ESS
Eleven trials including 716 patients reported ameliorated ESS scores [13,16,17,22-27,29,30]. Among the included studies, 7 used CM decoctions [13,17,23,25,26,29,30] and 4 CM granules [16,22,24,27], so the following two meta-analyses for the two dosage forms were carried out.
The inconsistency of the 7 studies on CM decoctions was high with an I2= 86% (Chi2= 44.05, P < 0.00001), so a random-effect model meta-analysis was conducted (Fig. 14.). However, as the heterogeneity was high, the data was unconvincing. So the following sensitivity analysis was performed. Then the inconsistency index I2was 49% (Chi2= 5.92, P = 0.12), a fixed-effect model was selected for the meta-analysis for CM granules. As a result, CM granules achieved better outcome than the no treatment (MD: -1.35, 95% CI: -1.92 to -0.78, P<0.00001) (Fig.15).
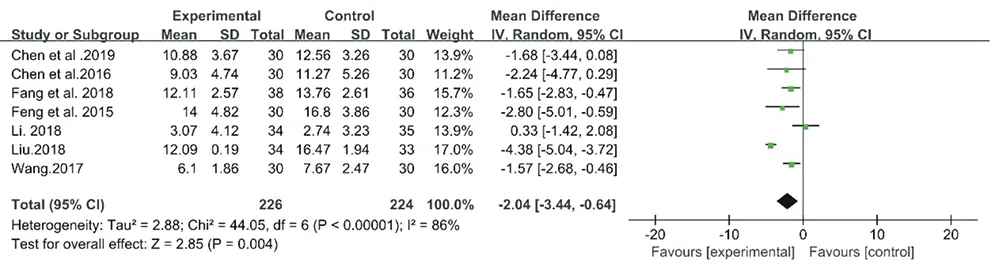
Figure14. Forest plot of CM decoctions versus control group: ESS
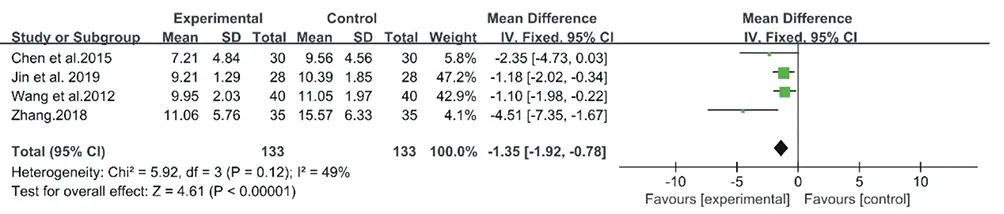
Figure15. Forest plôt of CM granules versus control group:ESS
3.5.5 Sensitivity analysis
The sensitivity analysis was performed for the aforesaid 7 studies on CM decoctions. After a careful reading, we agreed that the bias was most obvious in the study by Liu et al. 2018. After eliminating the study, I2reduced from 86% to 16%, which indicated stable results of the current meta-analysis. It was found that CM decoctions significantly promoted the outcome compared with no treatment (MD: -1.50, 95% CI: -2.13 to -0.88, P < 0.00001) (Fig. 16).

Figure16. Forest plot of analysis of ESS taking decotion in the sensitivity analysis
3.5.6 Publication bias
Funnel plot was performed for secondary outcomes (Fig.17). However, studies on CM granules were too few to conduct the funnel plot, we only did publication bias of those on CM decoctions. A nonsignificant possibility of potential publication bias was found.
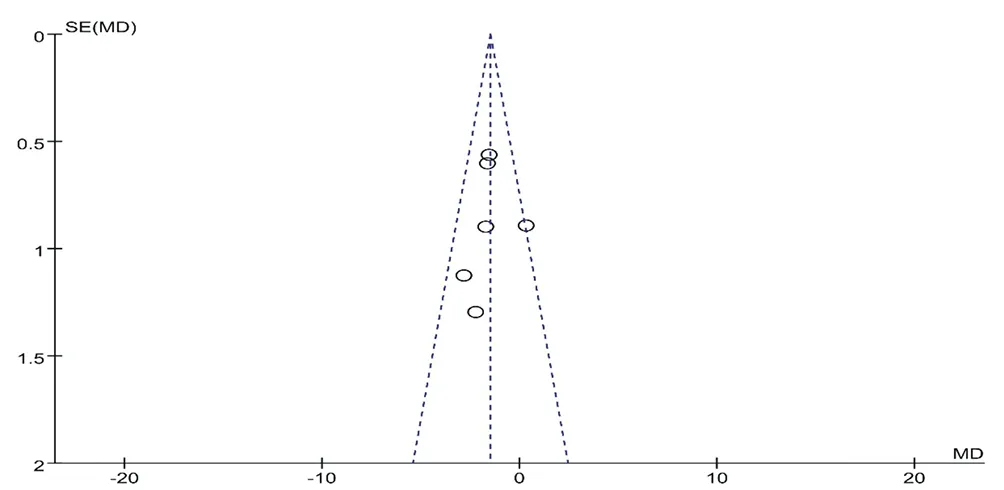
Figure17. Funnel plot of CM granules vensus catnd pu se
3.5.7. Adverse reactions
Of all included trials, adverse events including gastrointestinal reaction symptoms, mild headache, nausea and abdominal distention, were reported in 3 studies representing 5 patients who were treated with Han Ning Fang (anti-snoring decoction) and Jia Wei Di Tan Tang (modified Rehmannia decoction). However, these reactions were not severe and disappeared without special treatment (Table5).
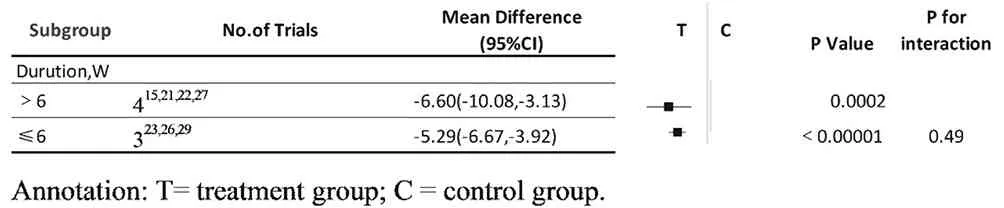
Table 5 outcome of subgroup analysis: granules
4. Discussion
Treatments for OSA incorporate CPAP therapy, surgical therapy, and implantable and wearable devices [31]. CPAP therapy is considered as the gold-standard treatment of OSA. Though CPAP achieves favorable therapeutic efficacy, the low patient compliance, ranging from 29% to 81%, poses a challenge to medical researchers [32]. Non-CPAP therapies (e.g., oral appliance therapy and upper airway surgery) also achieve positive results in many cases, but their therapeutic efficacy is unstable [33,34]. Mandibular advancement devices even have negative impacts on the oral structure, resulting in changes in the strength and speed of a bite [35]. According to the guideline 2013 issued by the American Medical Association [36], the current researches uncover insufficient evidence that surgical treatment is superior to CPAP treatment. Besides, surgeries even present high risks of serious adverse reactions. At present, drug therapy has not be used as the treatment of OSA according to the guideline 2011 issued by the Chinese Medical Association [37] and guideline 2013 issued by the American Medical Association [36]. Thus, new approaches to treat OSA are required. CM therapy is safe with less adverse reactions. Many studies have pointed out that CM can solve the problem of "difficulty in obtaining medical services and high medical cost". However, there is no strong evidence for the therapeutic effect of CM on OSA. So we have conducted this metaanalysis to find out evidence-based medicine (EBM) support of CM for the treatment of OSA.
The polysomnogram (PSG), first reported in 1974, remains as the gold-standard diagnostic test [38]. In our study, we have selected AHI and LSaO2as the outcome assessments because these data can be directly recorded using an overnight PSG. As the CM syndrome table varies in respective trials, which makes the results uncomparable, we are unable to evaluate CM symptom scores and judge the therapeutic efficacy.
To the best of our knowledge, this is the first meta-analysis aiming to assess the clinical efficacy of CM therapy for the treatment of OSA. The 20 studies involving 1,297 subjects have been enrolled in our study. The results indicate that CM therapy may be an effective CAM treatment for OSA with improved outcomes and it is safe for OSA. Both CM decoctions and granules are effective in the improvement of AHI and ESS scores, and CM granules are especially effective in the improvement of LSaO2. The low heterogeneity among the studies on CM decoctions and granules after sensitivity analysis indicates results of the meta-analysis data are convincing. Subgroup analyses suggest that the > 6 weeks group and the ≤ 6 weeks group present greater improvement in AHI than the control group after treatments of CM decoctions and granules. No serious adverse events have been reported in the included 20 trials, and mild adverse reactions have been reported by 5 patients, which have disappeared without special treatment. Accordingly, we propose that CM is able to provide a high-quality sleep at night and a clear mind in the day. Our results of the meta-analysis demonstrate that CM is an effective and safe therapy for OSA, which makes up for the shortcomings of western medications.
OSA is a disease name of western medicine, without a fully corresponding name in ancient CM books. However, according to the different pathogenesis and symptoms of OSA, it can be classified it into "prone to sleep", "multiple sleep", "snoring sleep" and other diseases in CM. CM physicians believe that the main pathogenesis of OSA is phlegm-dampness stagnation according to the thousandyear experience in CM clinical practices [39]. Several studies have reported that the phlegm-dampness syndrome phlegm-dampness syndrome is the most common CM syndrome observed in patients with OSA [40], and the CM prescriptions included in our study are mainly for removing dampness and phlegm, with the most common drugs of ban-xia (Pinellia ternata), chen-pi (tangerine peel), bai-zhu (Rhizoma atractylodis macrocephalae), tian-ma (Gastrodia elata), yu-jin (radix curcumae) and dan-nan-xing (Arisaema cum bile). This will turn our focus on CM treatments for PDS in OSA, and will inspire new findings in the therapeutic efficacy and mechanisms of the treatments. Many studies have found that inflammatory factors are involved in the pathogenesis of OSA[41]. In our previous reviews by Xu et al. 2018 [15] and Chen et al. 2016 [30], we have found that CM can reduce inflammation, regulate blood-levels of TNF-α, NF-κB, IL-1 and IL-6, inhibit inflammatory reaction, and exert its therapeutic effect on OSA. This will help us to further understand inflammatory mechanisms of CM treatments for obstructive sleep breathing syndrome.
However, this meta-analysis has the following limitations. Firstly, the majority of the included studies are small sample size trials, with mid- to low-quality designs. Secondly, all included studies have been conducted in China. According to our experience, only positive results can be published in Chinese medical journals. So we have cautiously drew the conclusion that publication bias may exist in this meta-analysis. Thirdly, most included studies have failed to address blinding assessments, which may influence the objectivity of OSA outcomes. In our study, we have selected AHI and LSaO2as the outcome assessments because these data can be directly recorded by an overnight PSG. Thus, the accuracy and the objectivity of outcomes can be impervious to the lack of blinding. Fourthly, due to the high heterogeneity of LSaO2in the studies on CM decoctions, the meta-analysis has to be suspended. Furthermore, high-quality, welldesigned, large sample trials focusing on the therapeutic efficacy and safety of CM therapy for OSA should be performed in the future.
In conclusion, this review of 20 RCTs shows that CM therapy can improve AHI, LSaO2and ESS in patients with OSA. CM therapy seems to be generally safe for clinical applications. However, the beneficial findings are inconclusive due to generally weak evidence, and further large sample size, rigorous trials are still warranted.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this article.
 Journal of Hainan Medical College2020年22期
Journal of Hainan Medical College2020年22期
- Journal of Hainan Medical College的其它文章
- Mechanism of therapeutic effects of Huangbo on ureteral stones based on network pharmacology
- Molecular mechanism of Turmeric in the treatment of liver cancer based on network pharmacology
- Therapeutic efficacy of traditional Chinese medicine compound in the treatment of membranous nephropathy: A systematic review and metaanalysis
- Correlation between CT dynamic enhanced scanning parameters and serum tumor markers before and after radiofrequency ablation in patients with lung cancer
- Effects of fumigation and washing with Shujin Huoluo decoction combined with Duhuo Parasitic decoction on TIMP-1 and PON1 levels in KOA patients
- The effect of Xuebijing on burn patients and its effect on pain and wound healing
