Bowel adhesion and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine in rats
Lidija Berkopic Cesar, Slaven Gojkovic, Ivan Krezic, Dominik Malekinusic, Helena Zizek, Lovorka Batelja Vuletic, Andreja Petrovic, Katarina Horvat Pavlov, Domagoj Drmic, Antonio Kokot, Josipa Vlainic, Sven Seiwerth, Predrag Sikiric
Abstract
Key Words: Abdominal wall defect; Adhesions; BPC 157; Vascular recruitment; Nitric oxide-agents; Rats
INTRODUCTION
We employed an excision of the parietal peritoneum with the underlying superficial layer of muscle tissue, and assessed the consequences thereof.There was a downhill course, from the very early events to massive bowel adhesion.However, recovery was achieved with the stable gastric pentadecapeptide BPC 157 and nitric oxide (NO)system involvement[1-12].This may be due to the rapid recovery of failed blood vessels after induction of the initial injury.Such therapy has had beneficial effects in previous studies on vascular occlusion[13-19].This recovery effect may be due to its effect on occluded vessels; it activated bypassing pathways, thereby counteracting occlusion consequences[13-19].This was described[13-17]in the treatment of deep vein thrombosis and colitis ischemia/reperfusion, duodenal venous congestion and cecum perforation[13-16], and bile duct ligation-induced liver cirrhosis and portal hypertension rat injuries[17].Recently, this recovery was also shown in the rescue of quite complex severe circumstances,i.e., Pringle manoeuvre ischemia and reperfusion (portal triad temporary occlusion) as well as Budd-Chiari syndrome (induced by the suprahepatic occlusion of the inferior vena cava)[18,19].The cytoprotective impact of BPC 157 on the endothelium[1-12]may have special importance.Namely, the peritoneal lining of the cavity serves as a conduit for blood vessels that are markedly disturbed by parietal peritoneum injury[20].This study assessed the normalisation of the level of malondialdehyde (MDA) and NO in tissue and the effect of the NO agents L-NAME and/or L-arginine, given alone and/or together, as confirmation[13-19].
There, BPC 157 (GEPPPGKPADDAGLV, M.W.1419, LD1 not achieved,implemented in inflammatory bowel disease trials and now multiple sclerosis[1-12]) as a prototype of the more potent cytoprotective agent (i.e., it also counteracts pre-existing lesions[1-12]), may be an agent to counteract adhesion formation, as a therapy.As an anti-ulcer peptide, stable in human gastric juice, it is designed to be a novel mediator of Robert’s cytoprotection[1-12](epithelium/endothelium protection[1-12]).Likely, due to this effect (epithelium/endothelium protection[1-12]as a shared class effect of cytoprotective agents), it is effective in the entire gastrointestinal tract[1-12], and counteracts the bowel adhesion formation, which is induced by different procedures[21-33].Thereby, with an apparent vessel-adhesions related effect, BPC 157 corroborates all these vascular effects, and enhances vascular rescue after injury in particular[13-19], by counteracting adhesion formation.Also, it interacts with the NO system in various species and models[1-12], and counteracts the adverse effect of both LNAME and L-arginine, given as individual agents or combined[1-12].
The NO system is implicated in cytoprotection[1-12]and adhesion formation[34].However, there has been no study of the effect of L-NAME (ulcerogenic) and/or Larginine (beneficial)[1-12]application on the immediate presentation of the blood vessels as a hallmark (vessel empty/disappeared; vessel filled/reappeared), during and after parietal peritoneum injury.
Therefore, it may be that formation of adhesion as an effect on the NO system suggests the beneficial effect related to the NO system.Thus, BPC 157, L-NAME and L-arginine were administered alone or in combination.The medication BPC 157 was applied either once, at 1 min after defect creation as an abdominal bath (1 mL/rat) to assess the initial post-injury course, blood vessel presentation, or to assess the subsequent course leading to adhesion formation intraperitoneally once daily, with the first application at 30 min after surgery and the last application 24 h before the assessment.Alternatively, from day 7, BPC 157 was given intraperitoneally once daily as a postponed therapy for a pre-existing adhesion.At these points in the injured tissue, levels of MDA and NO were assessed, and the results showed the NO levels and oxidative stress (MDA) were normalised in injured tissue.
MATERIALS AND METHODS
Animals
Male Albino Wistar rats, 200 g, were randomly assigned (7 rats per group) and used for the experiments, which were approved by the local ethics committee.The perforation procedure was performed in rats that had food and water ad libitum before the procedure and until the end of the experiment.The animal protocol was designed to minimise pain or discomfort to the animals.The animals were acclimatised to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation.All animals were euthanised by a barbiturate overdose (intraperitoneal injection, 500 mg/kg thiopental sodium) prior to tissue collection.
Drugs
The pentadecapeptide Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val, M.W.1419, called stable gastric pentadecapeptide BPC 157, part of the sequence of the human gastric juice protein BPC, freely soluble in water and 0.9% NaCl at pH 7.0(Diagen, Ljubljana, Slovenia), prepared as described previously[1-12].The peptide had 99% purity by high-pressure liquid chromatography, with the 1-des-Gly peptide as a biologically inactive impurity[1-12].L-NAME and L-arginine were commercially purchased (Sigma, United States).
Procedure and adhesion assessment
Under deep anaesthesia (thiopental (Rotexmedica, Germany) 40 mg/kg, diazepam(Apaurin, Krka, Slovenia; 10 mg/kg), excision of the parietal peritoneum (1 cm × 2 cm,2 cm right of the median, 3 cm laparotomy) with an underlying superficial layer of muscle tissue was performed in rats.BPC 157 (10 µg/kg, 10 ng/kg, 1 mL/rat), or an equivalent volume of 0.9% NaCl were applied, with L-NAME (5 mg/kg) or L-arginine(200 mg/kg) given alone or in combination with BPC 157 at both concentrations.The medication was applied either once at 1 min after defect creation as an abdominal bath(1 mL/rat), or intraperitoneally once daily, with the first application at 30 min after surgery and the last application 24 h before the assessment.Alternatively, starting from d 7, BPC 157 (10 µg/kg, 10 ng/kg intraperitoneally, 1 mL/rat) was given once daily as a postponed therapy for a pre-existing adhesion.Immediately after defect creation, using a USB microscope camera (Veho discovery VMS-004D-400x USB microscope; Veho®, United Kingdom), we recorded the presentation of blood vessels,i.e.augmentation/reduction[13-19](emptied/disappeared; refilled/reappeared) and assessed at the end of the next 10-min period as total% of vascular vessels presentation in and close to the defect concerning the point immediately before therapy.Also, using a USB microscope camera (Veho discovery VMS-004D-400x USB microscope; Veho®,United Kingdom) at d 7 or d 14, adhesions were scored using Mazuji’s classification[no adhesion (score 0); very small, irregular adhesion (score 1); easily separable medium intensity adhesion (score 2); intense, not easily separable regular adhesion(score 3); very intense, not easily separable, homogenous adhesion][35].
Immediately after culling the animals, tissue specimens were fixed in buffered formalin (pH 7.4), for 24 h, dehydrated and embedded in paraffin wax.The samples were stained with haematoxylin–eosin.Tissue injury was evaluated microscopically by a blinded examiner.
Oxidative stress in adhesion tissue
At 7 d or 14 d post-injury, oxidative stress in the tissue samples (1 cm2around the defect) was assessed by quantifying thiobarbituric acid reactivity as MDA equivalents.Trichloroacetic acid was added to homogenise the tissue samples, which were then centrifuged (3000 rpm, 5 min), and the supernatant was collected.Thereafter, 1%thiobarbituric acid was added, and the samples were boiled (95 °C, 60 min).The tubes were kept on ice for 10 min, and the absorbance was determined at the wavelengths of 532 and 570 nm.The concentration of MDA was read from a standard calibration curve plotted using 1,1,3,3’-tetra-ethoxy propane.The extent of lipid peroxidation was expressed as MDA using a molar extinction coefficient for MDA of 1.56 × 105mol/L/cm.The results are expressed in nmol/mg of protein[13-19].
Nitric oxide determination in adhesion tissue
At 7 d or 14 d post-injury, we determined the NO levels in cecum tissue samples using the Griess reaction (Griess Reagent System, Promega, United States).Sulphanilamide was then incubated with the homogenised tissue, and then, N-1-naphthyl ethylenediamine dihydrochloride was added.The Griess reaction is based on a diazotisation reaction in which acidified nitrite reacts with diazonium ions and, in a further step, are coupled to N-1-naphthyl ethylenediamine dihydrochloride, forming a chromophoric azo derivate.The absorbance was measured at 540 nm using a sodium nitrite solution as the standard.NO levels are reported in µmol/mg protein.The protein concentrations were determined using a commercial kit (BioRad Protein DR Assay Reagent Kit, United States)[13-19].
Statistical analyses
Statistical analysis was performed by parametric one-way ANOVA with the post-hoc Newman-Keuls test and non-parametric Kruskal-Wallis and subsequent Mann-WhitneyU-tests to compare between groups.The values are represented as mean ± SD and minimum/median/maximum.Results withP< 0.05 were considered significant.The statistical methods of this study were reviewed by Bozo Radic, MD, PhD at Clinical Hospital Dubrava.
RESULTS
In this study, the focus was on the possible therapeutic effect of the application of the stable gastric pentadecapeptide BPC 157, and NO agents,i.e.L-NAME and L-arginine,after the creation of a defect by the excision of the parietal peritoneum with an underlying superficial layer of muscle tissue in rats.Therefore, this study reveals the formation of adhesions which could extensively occupy and obstruct the abdominal wall and intestine and pelvic organs (Figures 1-5 with respect to the initial defect creation, and the early presentation of blood vessels in and close to the peritoneal defect (Figures 6 and 7).Support was obtained from the presentation of MDA- and NO tissue levels in adhesion tissues (Figure 8).

Figure 1 At 7 d (7) or 14 d (14) post-injury, we assessed the adhesion score (0-4), min/med/max.Medication (/kg), was BPC 157 [10 µg (µ) or 10 ng (n)] (b - 1 mL bath/rat at abdominal cavity, B – once daily intraperitoneally, first application at 30 min following surgery, last 24 h before sacrifice; B once daily intraperitoneally, first application at day 7 following surgery, last 24 h before sacrifice) or saline(controls)(C) (bath equal volume, or once daily intraperitoneally (since not different results are shown together).aP < 0.05 at least vs control.
Adhesions
Controls:The induction of particular defect resulted in the formation of a large adhesion, which occupied and obstructed the abdominal wall, intestine and pelvic organs, as observed on d 7 and d 14 in all controls (Figures 1-5).
BPC 157:On d 7 and 14, after treatment with BPC 157, the degree of adhesion was minimal or absent.To emphasise the conclusive point (less or no adhesion formation),medication (/kg), BPC 157 (10 µg or 10 ng) was applied once as a 1 mL bath/rat in the abdominal cavity immediately after the creation of the defect, or once daily intraperitoneally, with the first application at 30 min after surgery and the last 24 h before culling.The final application was given once daily intraperitoneally, with the first application on d 7 following surgery and the last 24 h before culling (Figure 1,Figure 4, and Figure 5).
NO agents:The application of L-NAME resulted in severe worsening.On the contrary, the L-arginine caused the attenuation of adhesion formation.When used together, L-NAME and L-arginine antagonised each other, leading to adhesion formation similar to control animals (Figure 2).
BPC 157 and NO agents:Together, all these treatments (L-NAME + BPC 157, Larginine + BPC 157, L-arginine + L-NAME + BPC 157) produced a benefit similarly to the application of BPC 157 alone (Figure 2).
Microscopic images (Figure 5) are provided along with the gross presentation.After the first week, controls presented oedematous granulation tissue, covering large areas of the serosa, whereas in BPC 157-treated rats, far smaller areas were covered with denser and more mature granulation tissue with vessels appearing to be more mature and better formed.After two weeks, large areas of still poorly organised granulation tissue invading the bowel and abdominal wall were obvious in control animals.In BPC 157-treated rats, a young connective tissue scar had formed in small areas, with poor invasion into the bowel serosa/subserosa leading to very limited and nonstrangulating adhesions.
Vascular presentation
Controls: Along with defect creation, since the blood vessels quickly became empty,they rapidly disappeared close to the defect.
BPC 157:Recruitment of blood vessels appeared after or with BPC 157 treatment as an abundant vascular presentation within and close to the defect, which occurred rapidly.
NO agents:The application of L-NAME and L-arginine led to the slowing of blood vessel disappearance.When used together, L-NAME and L-arginine antagonised each other, showing similar vessel presentation as with the application of the saline in control animals.

Figure 2 At 7 d or 14 d post-injury, we assessed the adhesion score (0-4), min/med/max.Medication (/kg, 1 mL bath/rat) at abdominal cavity was BPC 157 (10 µg) (B), L-arginine (A) (100 mg), L-NAME (5 mg) alone or in combinations (L-NAME + L-arginine (NA), L-arginine + BPC 157 (AB), L-NAME + BPC 157(NB), L-arginine + L-NAME + BPC 157 (ANB) or saline bath equal volume (controls) (C).aP < 0.05 at least vs control; BPC 10 ng presented adhesion score 1/1/1 with L-arginine (L-arginine + BPC 157); Score 1/1/1 with L-NAME (L-NAME + BPC 157); Score 1/1/1 with L-arginine and L-NAME (L-arginine + L-NAME + BPC 157), aP <0.05 at least vs control.
BPC 157 and NO agents:Given together, these treatments (L-NAME + BPC 157, Larginine + BPC 157, L-arginine + L-NAME + BPC 157) produced a benefit in much the same way as the application of BPC 157 alone.
NO/oxidative stress
Controls:Along with adhesion formation, increased values appeared at day 7, while on day 14 the values, particularly NO were not as high.
BPC 157:With BPC 157 treatment, the values of NO and MDA appeared to be normalised (decreased) at both time points.
NO agents:The application of L-NAME reduced NO values, and L-arginine increased NO values at day 7 and d 14.When given together, L-NAME and L-arginine antagonised each other.Interestingly, compared with the controls at day 7 and day 14,both L-NAME and L-arginine increased MDA values, and when given together, LNAME and L-arginine antagonised each other.
BPC 157 and NO agents:Given together, all of these treatments (L-NAME + BPC 157,L-arginine + BPC 157, L-arginine + L-NAME + BPC 157) produced a counteracting effect on both increased NO and MD levels, much like the application of BPC 157 alone.

Figure 3 Adhesion formation (Control rats).Illustrative presentation of adhesion formation leading to subileus presentation at day 14 post injury.
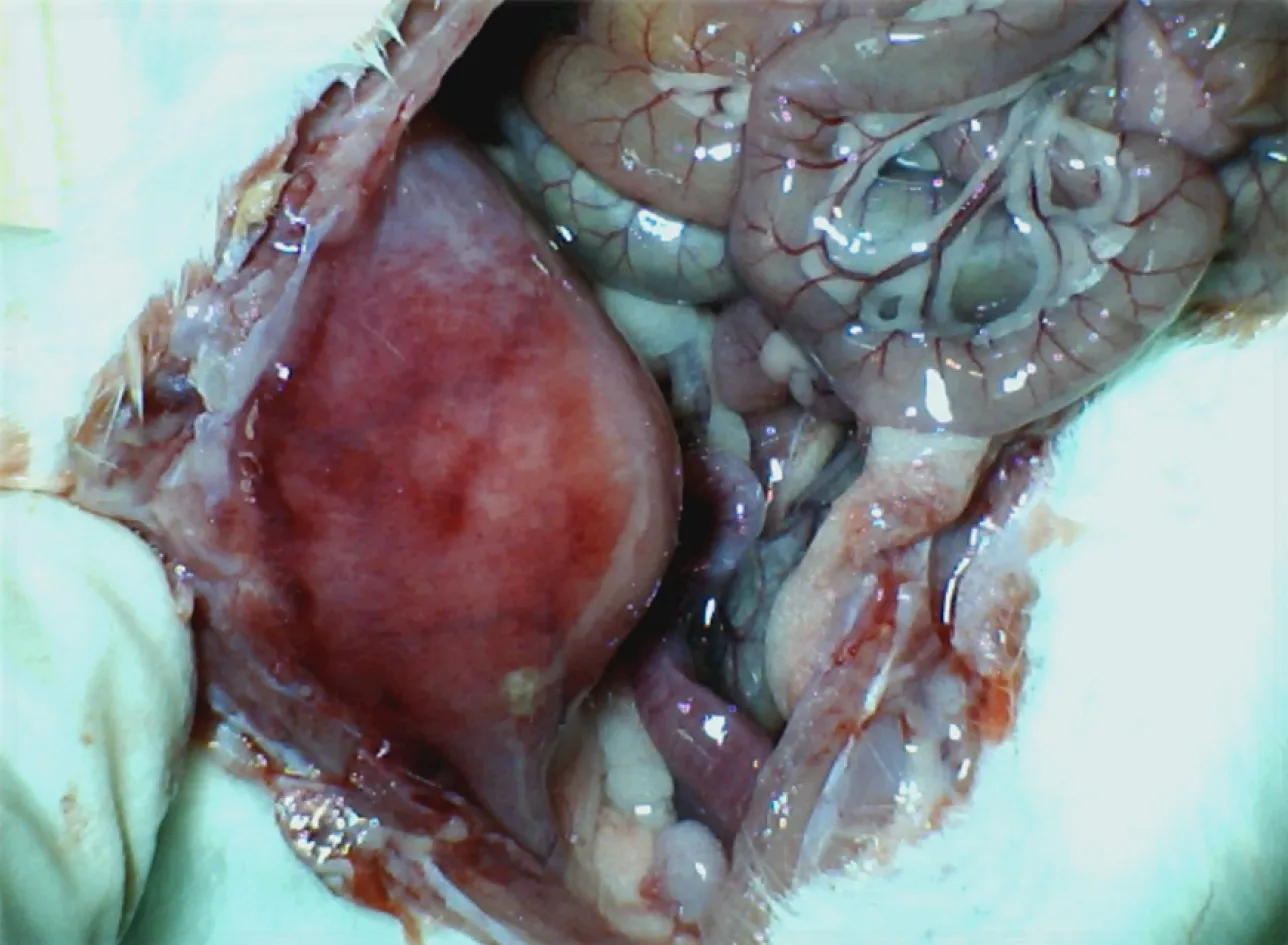
Figure 4 Adhesion formation.BPC 157 rats.Illustrative presentation of the abdominal wall healing without adhesion formation at d 14 post injury.
Thus, in rats with the parietal peritoneum and adjacent muscle removed, we demonstrated the particular combination of early to final response, which was affected by the application of the stable gastric pentadecapeptide BPC 157, L-arginine and LNAME, administered alone or together.Of note, only with the BPC 157 application was a beneficial outcome observed,i.e.less adhesion formation and suppressed NO and MDA values.
DISCUSSION
In this study, we performed an excision of the parietal peritoneum with the underlying superficial layer of muscle tissue, and assessed the downhill consequences thereof with the rapid recovery of the failed blood vessels after induction of the initial injury.Recovery with the stable gastric pentadecapeptide BPC 157 involved the NO system[1-12], as shown with the related effects of L-arginine and/or L-NAME.
This study on abdominal wall injury has basic relationships, such as vascular failure(vessels, one empty, “disappear”) at a very early post-injury time point, followed by a failure outcome (i.e., the formation of extensive adhesions).MDA values and NO levels increased after the initial abdominal wall defect was made[36,37], and increase during subsequent adhesion formation[36,37].On the contrary, the possible recovery effect is that the filled vessels “reappeared” and adhesion formation was attenuated.Suppression of NO and MDA levels matched with the beneficial outcome (less adhesion formation.Only BPC 157 therapy in the damaged abdominal wall led to this combined beneficial effect.The very rapid activation of the blood vessels in the initial defect likely circumvented the subsequent negative chain of events and resulted in the resolution of adhesion and obstruction.Similar effectiveness was observed after postponing therapy, as this counteracting effect occurred even with pre-existing adhesions (adhesion reduction from day 7 to d 14).
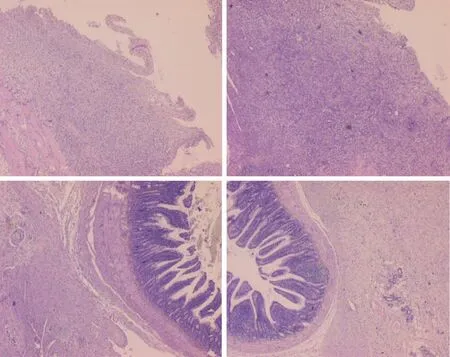
Figure 5 Histological assessment after 7 d (upper) and 14 d (low), in rats subjected to the excision of the parietal peritoneum with underlying superficial layer of muscle tissue, controls [upper left (7 d), low left (14 )] and BPC 157-treated rats [upper right (7 d), low right(14 d)]. 7 d.Upper, left, controls.Edematous, relatively poorly formed granulation tissue, covering large areas of serosa.Upper, right, BPC 157.By far smaller areas covered with more dense and mature granulation tissue with vessels appearing more mature and better formed.14 d.Low, left, controls.After two weeks large areas of still poorly organized granulation tissue invading the bowel and abdominal wall.Low, right, BPC 157.Young connective tissue scar is formed in smaller areas, with poor invasion into the bowel serosa/subserosa leading to very limited and non-strangulating adhesions.HE stain, objective × 10.
The effect of BPC 157 is quite persistent, since BPC 157 + L-NAME, BPC 157 + Larginine and BPC 157 + L-NAME + L-arginine rats showed similar outcomes as BPC 157-rats.Thus, this counteraction occurred when challenged with nitric oxide synthases (NOS) blockade (L-NAME; less vessel presentation within and close to the defect, more adhesions and higher NO and MDA values) as well as NOS overstimulation (L-arginine; less vessel presentation within and close to the defect,fewer adhesions but higher NO and MDA values).Thus, the NO system was immobilised when L-NAME and L-arginine counteracted each other’s effect.In these rats, which exhibited a disease course similar to the controls, in any circumstance,early or late, additional BPC 157 co-application (L-NAME + L-arginine + BPC 157) reestablished the counteraction, as did BPC 157 application (BPC 157 + L-NAME-, BPC 157 + L-arginine-; BPC 157 + L-NAME + L-arginine-rats ≈ BPC 157-rats).Important for the establishing BPC 157-NO relationships (for review see, see[1-12]), L-NAME (NOS blockade)vsL-arginine (NOS over-stimulation)vsthe combination (NO system immobilisation) were able to fully highlight the particularities of the NO system[1-12].Thus, with supportive evidence, it is conceivable that BPC 157 therapy mitigates the disturbances caused by parietal peritoneum and adjacent muscle removal and NO agent-induced effects (thereby, particular relations BPC 157 with the healing and NO system).It appears much like vessel recruitment to bypass occlusion and re-establish blood flow in previous occlusion- and ischemia/reperfusion studies[13-19].BPC 157 therapy may be useful as a combined therapeutic (cytoprotective), while NO agents,i.e., L-NAME and/or L-arginine, are used as an additional control.
Finally, the suggested vessels/adhesions/BPC 157/NO system relationship supports the evidence that the stable gastric pentadecapeptide BPC 157 counteracts bowel adhesion formation related to different procedures[21-28].Likewise, this may resolve adhesions as an aberrant peritoneal wound healing process[38].Namely, BPC 157 accelerates the healing of various wounds (i.e., skin[39-42], muscle[43-46], tendon[47-49],ligament[50], bone[51]; ulcers in the entire gastrointestinal tract[14-16,27,28,52-54], corneal ulcers[55], gastrointestinal fistulas[21-25,56]).Consistently, it appears to synchronise healing in various tissues.Also, once the peritoneum is damaged, the coagulation cascade is set in motion[57]; BPC 157 counteracts the whole Virchow triad[13], venous[13,18,19]and arterial thrombosis[18,19,58]and attenuates prolonged bleeding and thrombocytopenia after amputation and anticoagulant use[13,59,60].Therefore, BPC 157 is likely to interfere when two damaged peritoneal surfaces come into contact with each other.Therefore,by counteracting adhesion formation and reversing existing adhesions, BPC 157 is likely to interfere with and reverse the healing that would result in fusion to form a connection,e.g., an adhesion.Likely, this beneficial effect could be related to the temporary role of fibrin in healing without adhesions that must be degraded by the fibrinolytic system for the restoration of normal tissue structure and function[36,37].
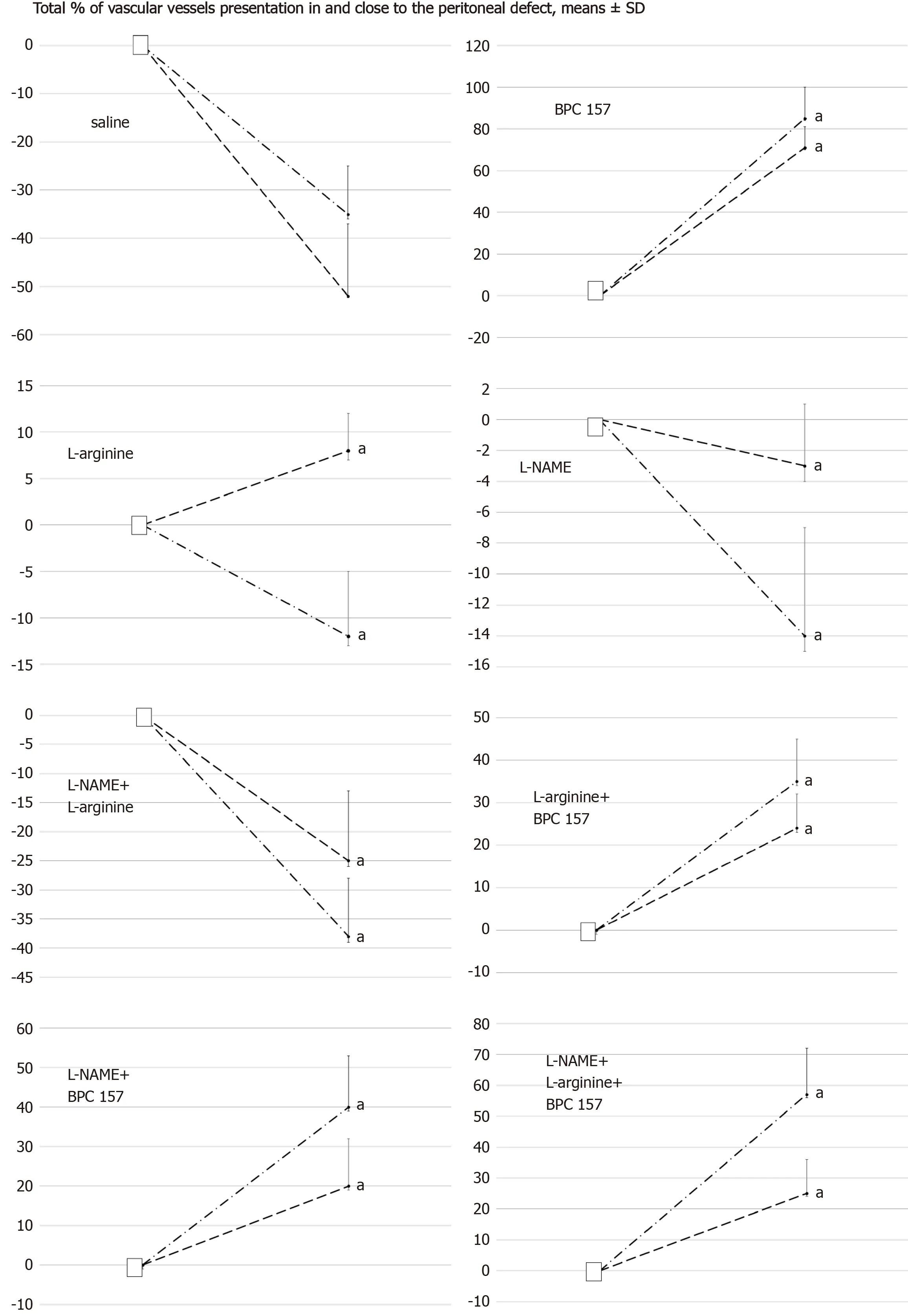
Figure 6 Total % of vascular presentation in (dash) and close to (dash dot) the defect.White squere indicates the values immediately before therapy and full oval the values at the end of the next 10 min.Medication (/kg, 1 mL bath/rat) at abdominal cavity was BPC 157 (10 µg) (B), L-arginine (A) (100 mg), L-NAME(5 mg) alone or in combinations (L-NAME + L-arginine (NA), L-arginine + BPC 157 (AB), L-NAME + BPC 157 (NB), L-arginine + L-NAME + BPC 157 (ANB) or saline bath equal volume (controls) (C).aP < 0.05 at least vs control; BPC 10 ng presented values 20 ± 8 (in) and 35 ± 8 (close to) with L-arginine (L-arginine + BPC 157);25 ± 9 (in) and 45 ± 8 (close to) with L-NAME (L-NAME + BPC 157); 20 ± 7 (in) and 55 ± 15 with L-arginine and L-NAME (L-arginine + L-NAME + BPC 157), aP <0.05 at least vs control.
Interestingly, when given with aspirin, clopidogrel or cilostazol, BPC 157 largely rescues thrombocyte function in rats and does not affect coagulation factors[61].Additionally, these BPC 157 assets, in particular abundant vascular presentation within and close to the defect as an extension of the original cytoprotective endothelium protection effect[1-12], should be viewed with the stronger angiogenesis effect than the standard anti-ulcer agents[62], along with increased expression of vascular endothelial growth factor (VEGF), and subsequent pathways[3,13,63-67].
In particular, BPC 157 increases the expression and internalisation of VEGFR2, the activation of the VEGFR2-Akt-eNOS signalling pathway without the need for other known ligands or shear stress[63], and heals corneal ulcer and lesions while maintaining corneal transparency[55,68], much like it heals lesions in the entire gastrointestinal tract[14-16,27,28,52-54]and other tissues, gastrointestinal fistulas in particular[21-25,56].BPC 157 also counteracts tumour cachexia and muscle wasting, characterised by increased proinflammatory/pro-cachectic cytokines such as interleukin-6 and tumour necrosis factor-α, and significantly corrects deranged muscle proliferation and myogenesis through changes in the expression of FoxO3a, p-AKT, p-mTOR and P-GSK-3β(mitigating cancer cachexia)[3].Also, BPC 157 actually counteracts the tumourpromoting effect of VEGF[69].
Finally, as an additional clue, a very recent investigation showed that BPC 157 is a stabiliser of cellular junctions, leading to significantly mitigated indomethacin-induced leaky gut syndrome,viaincreased expression of the tight junction protein ZO-1(Zonula occludens-1) as well as improved transepithelial resistance[70].Likewise, the mRNAs of inflammatory mediators [iNOS, interleukin-6, IFN-γ (Interferon gamma)and tumour necrosis factor-α] were inhibited, along with increased expression of HSP(Heat shock proteins) 70 and HSP 90, and antioxidant proteins such as HO-1, NQO-1,glutathione reductase, glutathione peroxidase 2 and GST-pi[70].
CONCLUSION
In summary, adhesion-related obstruction accounts for a considerable number of emergency surgical presentations, and continued research for preventing adhesions is more than desirable[71,72].Therefore, BPC 157 therapy, establishing the vessels/adhesions/BPC 157/NO system relationship, may be suitable for peritoneal defect healing with minimal or no adhesion formation.Finally, BPC 157[1-12], with lethal dose not achieved, has been implemented in inflammatory bowel disease trials, with a particular effect on vessel presentation and the ability to counteract the harmful effects of NO agents,i.e.free radical formation and elevated NO[1-12].BPC 157 should be further assessed as a therapy to avoid adhesion formation.

Figure 7 Early vascular presentation in and close to defect, before and immediately after therapy application.A: Control rats.Illustrative presentation of the vessels in and close to the defect immediately before therapy (left), and immediately after therapy application at the abdominal cavity, under saline solution immersion (right).No particular vessel recruitment; B: BPC 157 rat.Illustrative presentation of the vessels in and close to the defect immediately before therapy (left), and immediately after therapy application at the abdominal cavity, under BPC 157 solution immersion (right).Particular vessel recruitment in and close to the defect.

Figure 8 Support was obtained from the presentation of MDA- and NO tissue levels in adhesion tissues.A: At 7 d post-injury, we determined nitric oxide (NO) in adhesion tissue samples using the Griess reaction.Medication (/kg, 10 mL/2 min bath/rat) at abdominal cavity was BPC 157 (10 µg) (B), Larginine (A) (100 mg), L-NAME (5 mg) alone or in combinations L-NAME + L-arginine (NA), L-arginine + BPC 157 (AB), L-NAME + BPC 157 (NB), L-arginine + LNAME + BPC 157 (ANB) or saline bath equal volume (controls) (C).aP < 0.05 at least vs control; B: At 14 d post-injury, we determined NO in adhesion tissue samples using the Griess reaction.Medication (/kg, 10 mL/2 min bath/rat) at abdominal cavity was BPC 157 (10 µg) (B), L-arginine (A) (100 mg), L-NAME (5 mg)alone or in combinations L-NAME + L-arginine (NA), L-arginine + BPC 157 (AB), L-NAME + BPC 157 (NB), L-arginine + L-NAME + BPC 157 (ANB) or saline bath equal volume (controls) (C).aP < 0.05 at least vs control; C: At 7 d post-injury, we determined oxidative stress in adhesion tissue samples was assessed by quantifying thiobarbituric acid reactivity as malondialdehyde equivalents.Medication (/kg, 1 mL bath/rat) at abdominal cavity was BPC 157 (10 µg) (B), L-arginine (A)(100 mg), L-NAME (5 mg) alone or in combinations L-NAME + L-arginine (NA), L-arginine + BPC 157 (AB), L-NAME + BPC 157 (NB), L-arginine + L-NAME + BPC 157 (ANB) or saline bath equal volume (controls) (C).aP < 0.05 at least vs control; and D: At 14 d post-injury, we determined oxidative stress in adhesion tissue samples was assessed by quantifying thiobarbituric acid reactivity as malondialdehyde equivalents.Medication (/kg, 1 bath/rat) at abdominal cavity was BPC 157 (10µg) (B), L-arginine (A) (100 mg), L-NAME (5 mg) alone or in combinations L-NAME + L-arginine (NA), L-arginine + BPC 157 (AB), L-NAME + BPC 157 (NB), Larginine + L-NAME + BPC 157 (ANB) or saline bath equal volume (controls)(C).aP < 0.05 at least vs control.
ARTICLE HIGHLIGHTS
Research background
The excise parietal peritoneum with the underlying superficial layer of muscle tissue,since the very early events to the huge bowel adhesion, may represent a model relevant to adhesion-related obstruction and a considerable number of emergency surgical presentation.The downhill course can be counteracted with the recovery by the stable gastric pentadecapeptide BPC 157 and NO-system involvement.This may be the rapid recovery of the failed blood vessels after the initial injury induction.The previous studies of vascular occlusion reveal its particular effect on the occluded vessels, and bypassing of the occlusion, in the therapy of the deep vein thrombosis and colitis ischemia/reperfusion, duodenal venous congestion and cecum perforation, bile duct ligation-induced liver cirrhosis and portal hypertension rat injuries, Pringle maneuver ischemia and reperfusion (portal triad temporary occlusion) as well as Budd-Chiari syndrome (induced by the suprahepatic occlusion of the inferior caval vein).Also, the BPC 157 cytoprotective endothelium impact can have special importance.Namely, the peritoneal lining of the cavity serves as a conduit for the blood vessels that should be markedly disturbed by parietal peritoneum injury.Thus,we considered that BPC 157 therapy is suited for the realization of the peritoneal defect healing with minimal or no adhesion formation.
Research motivation
Adhesion-related obstruction accounts for a considerable number of emergency surgical presentation, and thereby, whatever mechanisms, the continued research for preventing is more than desirable.Therefore, BPC 157 therapy, establishing vesselsadhesions-BPC 157-NO-system relation, can be suited for the realization of the peritoneal defect healing with minimal or no adhesion formation.
Research objectives
The focus was on the possible therapeutic effect of the application of the stable gastric pentadecapeptide BPC 157, and NO-agents, L-NAME, and L-arginine after the creation of a particular defect by excision of parietal peritoneum with an underlying superficial layer of muscle tissue in rats.The revealing focus was on the formation of the adhesion, which could extensively occupy and obstruct the abdominal wall and intestine and pelvic organs, with respect to the initial defect creation, and the early blood vessels presentation in and close to the peritoneal defect.The support was the presentation of the MDA- and NO-tissue level in adhesion tissues.
Research methods
In the rats with the excised parietal peritoneum with the underlying superficial layer of muscle tissue, we suggest that this formation of adhesion would be an effect on the NO-system and that the BPC 157 beneficial effect would be an effect related to the NOsystem.
The medication was BPC 157, L-NAME, L-arginine administered alone or in combinations.The medication was applied either once, at 1 min after defect creation as an abdominal bath (1 mL /rat) to perceive the initial post-injury course, blood vessel presentation (assessed by USB microscope camera, the blood vessels presentation augmentation/reduction to perceive subsequent course leading to the adhesion formation).Application was intraperitoneally once daily, first application at 30 min after surgery, last application 24 h before assessment.Alternatively, from day 7, BPC 157 was given intraperitoneally once daily as a postponed therapy to pre-existing adhesion.At these points in the injured tissue (gross and microscopy assessment),levels of MDA and NO were also assessed.
Research results
After parietal peritoneum excision with an underlying superficial layer of muscle tissue in rats, there is failed vasculature, and finally, increased adhesion formation.NO-agents, L-NAME and/or L-arginine have diverse effects, the initial weakening of blood vessel disappearance and finally, severe worsening of adhesions (L-NAME)vsthe initial weakening of blood vessel disappearance and finally, attenuation of adhesions formation (L-arginine), which counteract each other response given together.The application of the stable gastric pentadecapeptide BPC 157 with its most recent described vascular effects (“vascular recruitment”) recovers abundant vascular vessel presentation in and close to the defect, which occurs rapidly.Finally, BPC 157 attenuated bowel adhesion formation and NO- and MDA-tissue values.
Research conclusions
BPC 157 therapy can be suited for the realization of the peritoneal defect healing with minimal or no adhesion formation.In practical BPC 157 therapy realization,establishing vessels-adhesions-BPC 157-NO-system relation may be important.Finally,BPC 157, with LD1 not achieved, implemented in inflammatory bowel disease trials,with a particular effect on vessel presentation and counteracting effect on NO-agents harmful effect, free radical formation, and NO-values normalization, should be the practical hallmark of the further therapy to avoid adhesion formation.
Research perspectives
This study of abdominal wall injury has basic relationships, such as more vascular failure (vessels, since empty “disappear”) at a very early post-injury period, as the more failed outcome (i.e., the huge adhesion formation).MDA values and NO-levels increased at the initial abdominal wall defect, and thus increase during subsequent adhesions.On the contrary, the possible recovering effect is that the filled vessels“reappear”; adhesion formation attenuated.Decreasing of the increased NO- and MDA- values match with the beneficial outcome (less adhesion formation).Only the BPC 157 therapy in the damaged abdominal wall healing fulfils this combined beneficial effect.The very rapid activation of the blood vessels in the initial defect likely circumvents subsequent negative chain of events and results in the resolution of the adhesion and obstruction, decreasing of the increased NO- and MDA values match with the beneficial outcome (less adhesion formation).
 World Journal of Gastrointestinal Pharmacology and Therapeutics2020年5期
World Journal of Gastrointestinal Pharmacology and Therapeutics2020年5期
- World Journal of Gastrointestinal Pharmacology and Therapeutics的其它文章
- Oral encapsulated transforming growth factor β1 reduces endogenous levels: Effect on inflammatory bowel disease
- Do liver metastases from gastric cancer contraindicate aggressive surgical resection? A 14-year single-center experience
