Validation of Housekeeping Genes for Gene Expression Analysis in Iwagaki Oyster (Crassostrea nippona) Under Salinity Stress by Quantitative Real-Time PCR
GONG Jianwen, LI Qi, 2), *, YU Hong, LIU Shikai, and KONG Lingfeng
Validation of Housekeeping Genes for Gene Expression Analysis in Iwagaki Oyster () Under Salinity Stress by Quantitative Real-Time PCR
GONG Jianwen1), LI Qi1), 2), *, YU Hong1), LIU Shikai1), and KONG Lingfeng1)
1),,,266003,2),,266237,
Hypo-salinity can reduce the immunological reaction in, even lead to massive mortality. It is important to understand the molecular mechanism of oyster defense system, while quantitative real-time PCR can be employed inthe study. However, the accuracy of quantitative real-time PCR relies on the use of suitable reference genes. In this study, the expression stability of 14 candidate reference genes including traditional housekeeping genesEF1A, TUB, TUA, GAPDH, RO21,as well as new candidate reference genes RPL5, RPL8, RPS27, RPL14, RPL4, CO3, RPS8, RPS4, CYTB in different tissues ofunder salinity stress has been validated by quantitative real-time PCR. Ribosomal protein genes selected through expression analysis of transcriptome data fromgenerally were more stable than traditional reference genes. According to the geNorm analysis, RPL4 and RPS4 could be used as internal controls for studying gene expression inwith real-time PCR under salinity stress.
; reference gene; hypo-salinity stress; ribosomal protein genes
1 Introduction
Quantitative real-time PCR (qRT-PCR) has been wide- ly used to measure gene expression because of its high sensitivity, flexibility, and reproducibility (Heid, 1996). The variations caused by differences in samples, RNA ex- traction, efficiency of enzyme and transcriptional activity can influence the experimental accuracy (Mackay, 2002). For accurate and reliable analysis of target gene expression, normalization of qRT-PCR data with suitable internal reference gene(s) is required (Radonić, 2004). An ideal reference gene should express at stable level in all tissues, regardless of the experimental conditions or treatments (Vandesompele, 2002; Radonić, 2004). Commonly used reference genes usually are those involv- ed in basic cellular processes, such as the components of cytoskeleton, glycolytic pathway, protein folding and de- gradation (Eisenberg and Levanon, 2003). However, evidences show that transcription levels of housekeeping genes vary considerably in different tissues and under variable conditions (Greer, 2010). Therefore, selecting mul-tiple stably expressed reference genes, other than the com- monly used housekeeping genes, is important for the ac- curate normalization of gene expression levels.
is an important aquaculture species for it is edible during summer when other oysters are not available (Itoh, 2004).naturally ben- thic in shallow water along the coast of the seas of East Asia (Boudry, 2003). It is more sensitive to the salinity change of ambient seawater compared with euryha- line oysters, such as, which live in intertidal zones (Zhang, 2016). In the natural habitat, salinity declines with tidal cycles, rainfall and with drainage from the adjacent terrestrial sites (Drouin, 1985; Philippart, 2011). Hypo-salinity induces the immunological activity of oysters, such as the overexpression of heat shock proteins (HSPs) and phenoloxidase (Gagnaire, 2006; Kuchel, 2010; Li, 2016), and even lead to massive mortality (Meng, 2011).
In this study, we compared the performance of 14 candidate reference genes (consisting of 5 commonly used housekeeping genes of animals, and 9 new candidate reference genes detected fromtranscriptome) in order to identify the most stable internal controls for nor- malization of real-time PCR data inunder sa- linity stress.
2 Materials and Methods
2.1 Biological Materials
2.1.1 Unstressed samples (group A)
Adultwere cultured in the fish farm of Rongcheng, Shandong Province, China. Tissues from the mantle (M), visceral mass (V), adductor muscle (A) and gill (S) were collected from 12 healthy oysters. They were immediately placed into liquid nitrogen to free- ze and then stored at −80℃ for the subsequent analysis.
2.1.2 Salinity stressed samples (group B)
The experimentalwere maintained in 70L tanks containing aerated sand-filtered seawater (salinity: 30) for one week prior to experimentation. Then they were randomly divided into 3 groups under hypo-salinity stress in seawater with 30 (S3), 20 (S2), and 10 (S1) for one week (3 pools with 9 individuals each). The low salinity water was prepared by diluting sea water with tap water. Gill tissues of the experimental group were collected from 27 oysters under variant salinity stresses. They were im- mediately placed into liquid nitrogen to freeze and then stored at −80℃ for subsequent analysis.
2.2 RNA Isolation, Library Construction and Sequencing of C. nippona
Total RNA was extracted from the samples using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The purity and integrity of total RNA was de- termined using a Nanodrop 2000 spectrophotometer (Ther- mo, USA) and an Agilent 2100 BioAnalyzer (Agilent Technologies, USA). The RNA was pooled proportionally from three oysters in each experimental group (group A: M, V, A, S; group B: S1, S2, S3).
The mRNA was enriched by Oligo(dT) beads and then fragmented. The cDNA was synthesized with random he- xamersmRNA fragments as templates. The cDNA fragments were purified and resolved with EB buffer for end repair, single-nucleotide adenine (A) addition and adap- tor connections. After PCR amplification, the 150bp li- brary was then sequenced via Illumina HiseqTM4000.
2.3 De novo Assembly, Reads Mapping and Gene Clustering
Clean reads were obtained by removing ‘dirty’ reads containing adapter sequences, sequences with more than 10% unknown bases, and low-quality reads containing more than 40% of low quality (-value<10) bases. Clean reads from four tissues were pooled together to assemble a comprehensive reference transcriptome by Trinity v2.8.4 (Grabherr., 2011). Then clean reads from each sample were mapped against the reference transcriptome using alignment tool Bowtie2 (Langmead and Salzberg, 2012) by default parameters. RSEM v1.3.1 (Li and Dewey, 2011) was used to quantify the mapped reads. The gene abundances were calculated and normalized to the number of reads per kb per million reads (RPKM) (Mortazavi., 2008). Gene clustering was performed with RPKM in group A and group B using kmeans command of the R statistical software.
2.4 Primer Design and Real-Time qRT-PCR Assays
The primers were designed using Primer Premier 5 soft- ware (http://www.premierbiosoft.com/). A series of 5-fold of 5 dilutions of cDNA were made to determine the gene specific PCR amplification efficiency for each primer pair using the following equation: Efficiency(%)=10(−1/slope)×100%.
The purified RNA samples were reversely transcribed using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Dalian, China), following the manufacturer’s pro-tocol. Real-time qPCR was performed with Roche480 ins- trument and software, while QuantiNovaTMSYBR Green PCR Kit (Qiagen) was employed for RT-PCR. The PCR mixture contained 0.5µL diluted cDNA, 5µL 2× SYBR Green PCR Master Mix, 0.7µL forward (reverse) primers, and 3.1µL distilled water in a final volume of 10µL. Cycling conditions were 95℃ for 2min, followed by 40 cycles of 95℃ for 5s and 60℃ for 10s and then a melt curve stage after the cycling stage. The melting curve and agarose gel electrophoresis for all the genes demonstrated single peaks and bands, confirming gene-specific amplification. Real-time PCR was performed in triplicate for each sample. A no-template control was analyzed in parallel for each gene.
2.5 Statistical Analysis
Following PCR data collection, geNorm (Vandesompele, 2002) was used to rank the expression stability of reference genes. Briefly, the geNorm program is based on pairwise comparisons and stepwise exclusion of candidate genes according to their expression stability measure (M) values. In general, the lower thevalue, the higher the gene expression stability. The program recommends<1.5 to identify sets of reference genes with stable expression. The pairwise variation (GeNorm:/(+1) is used to determine the number of genes required for reliable normalization. A threshold value of 0.15 was report- ed by Vandesompele. (2002).
3 Results
3.1 Selection of New Candidate Reference Genes
We identified 5 distinct clusters representing a variety of gene expression patterns in different tissues (group A) and under different salinity stresses (group B) respective- ly. Genes in cluster 3 (1491/37451 unigenes) of group A and in cluster 1 (1954/33157 unigenes) of group B show- ed the more stable and higher expression trends (Fig.1). A total of 1472 unigenes are presented in both groups (Fig.2). Among those common unigenes, nine unigenes with high and stable expression trends were selected to be new candidate reference genes (Table 1). In addition, five commonly used reference genes, RO21, EF1A, TUA, TUB and GAPDH, were selected as candidate reference genes.

Fig.1 Clusters of genes in different tissues of C. nippona and under different salinity stresses. Different tissues include mantle (M), visceral mass (V), adductor muscle (A) and gill (S). Different salinity stresses include 30 (S3), 20 (S2), 10 (S1).

Fig.2 Venn diagram of genes in cluster 3 of different tissues and cluster 1 under different salinity stresses.

Table 1 Candidate reference genes and their primer sequences used for real-time PCR
()
()
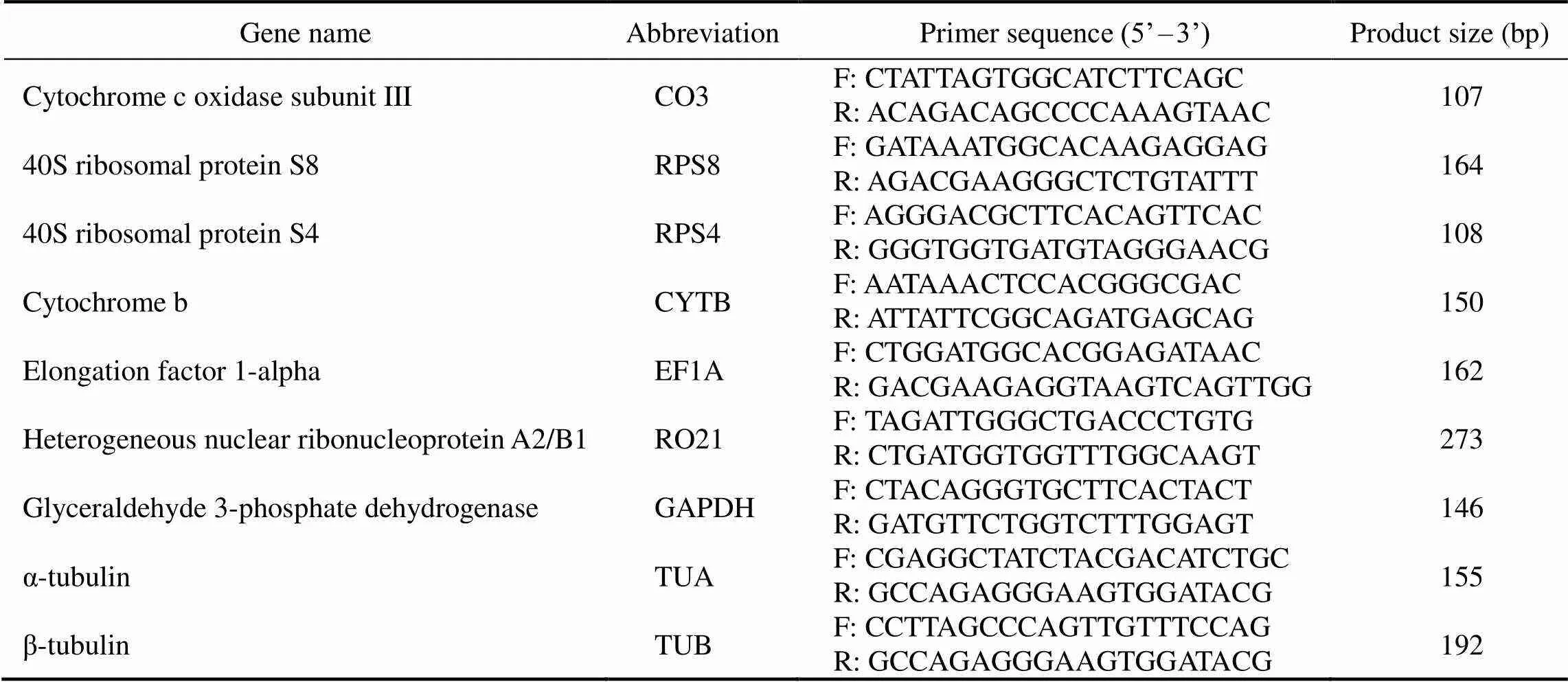
Gene nameAbbreviationPrimer sequence (5’–3’)Product size (bp) Cytochrome c oxidase subunit IIICO3F: CTATTAGTGGCATCTTCAGCR: ACAGACAGCCCCAAAGTAAC107 40S ribosomal protein S8RPS8F: GATAAATGGCACAAGAGGAGR: AGACGAAGGGCTCTGTATTT164 40S ribosomal protein S4RPS4F: AGGGACGCTTCACAGTTCACR: GGGTGGTGATGTAGGGAACG108 Cytochrome bCYTBF: AATAAACTCCACGGGCGACR: ATTATTCGGCAGATGAGCAG150 Elongation factor 1-alphaEF1AF: CTGGATGGCACGGAGATAACR: GACGAAGAGGTAAGTCAGTTGG162 Heterogeneous nuclear ribonucleoprotein A2/B1RO21F: TAGATTGGGCTGACCCTGTGR: CTGATGGTGGTTTGGCAAGT273 Glyceraldehyde 3-phosphate dehydrogenaseGAPDHF: CTACAGGGTGCTTCACTACTR: GATGTTCTGGTCTTTGGAGT146 α-tubulinTUAF: CGAGGCTATCTACGACATCTGCR: GCCAGAGGGAAGTGGATACG155 β-tubulinTUBF: CCTTAGCCCAGTTGTTTCCAGR: GCCAGAGGGAAGTGGATACG192
3.2 Real-Time PCR Amplification of Candidate Housekeeping Genes
All the primer pairs amplified single PCR product with expected size, and the specificity of amplicon was confirmed by the single peak of the melt curve and the sequencing analysis. PCR efficiencies of primers ranged from 94%–106%. The meant values of reference genes ranged from 22.02–29.36.
3.3 Expression Stability of Candidate Housekeeping Genes
RPS4 (=0.52), EF1A (=0.52) and RPL4 (=0.59) were the most stable genes in different tissues, while RPS8 (=2.40), CO3 (=2.02) and CYTB (=1.7) were the least stable. RPL4 and RPS4 (=0.21) were the most stable genes in gill tissue under salinity stress, while the most common used internal controls TUB (=1.36), RO21 (=1.05), TUA (=0.97), EF1A (=0.79) and GAPDH (=0.67) appeared less stable (Fig.3).

Fig.3 Expression stability of reference genes calculated by GeNorm in different tissues (A) and under different salinity stresses (B).
3.4 Optimum Number of Housekeeping Genes
The2/3 value was 0.12 in different tissues, while it was 0.15 in gill tissue under salinity stress (Fig.4). This suggests that RPL4 and RPS4 can be chosen as reference genes to study the gene expression levels inunder salinity stress.
4 Discussion
This is the first study to analyze the stability of potential reference genes selected from transcriptome dataset ofunder different salinity stresses. The candidate reference genes detected from transcriptome datasets are almost ribosomal protein genes and mitochondrial respi- ratory chain protein genes (CO3 and CYTB). The less stable expression of respiratory chain genes can be explained by the energy demand of the organism under different salinity conditions (Ellison and Burton, 2008). Ribo- somal protein genes generally performed better than com- mon housekeeping genes.

Fig.4 Determination of the optimal number of reference genes required for accurate normalization in different tissues (A) and under different salinity stresses (B), based on pairwise variation (Vn/n+1) between reference genes using GeNorm analysis.
The ribosome, as a catalyst for protein synthesis, is uni- versal and essential for all organisms. A mammalian ribo- some has 79 ribosomal proteins, one more than a yeast ri- bosome encoded by 137 genes, of which L is for large subunit and S is for small subunit (Warner, 1999). Ribo- some protein genes are considered good reference genes because of their participation in all types of cells for the synthesis of new ribosomes (Hisao., 2001). Several recently published reports have validated that ribosomal protein genes showed high stability in diverse abiotic and biotic conditions, indicating that they may become ano- ther source of reference genes (Shakeel, 2018). They have been widely used as internal genes in both human and other animals, as well as in plants and algae (Barsalobres-Cavallari, 2009; Rosic, 2011; Liu, 2012). RPL4 and RPS4 are the most stably expressed genes in different tissues under salinity stress in the current study. RPS4 was demonstrated to be the best housekeeping genes in the algaunder thermal stress (Rosic, 2011).
EF1A, TUB, TUA, GAPDH and RO21 are commonly used as internal controls for qRT-PCR in oysters (Boutet, 2004; Gonzalez, 2007; Meistertzheim, 2007). EF1A is a member of the G-protein family, which plays a key role in protein translation (Browne and Proud, 2002). Although EF1A has stable expression levels in the gill tissue under variant salinity stresses, it has less stable expression compared among each tissue, implying that it is not suitable to be a reference gene under salinity stress. The heterodimeric protein α, β-tubulin assembles the mi- crotubule in a head-to-tail arrangement (McKean, 2001). GAPDH functions in nuclear RNA export, DNA replication, DNA repair, exocytotic membrane fusion, cyto- skeletal organization and phosphotransferase activity (Tri- stan, 2011); RO21 is heterogeneous nuclear ribonucleo-protein (hnRNP) and plays a significant role in the regulation of mRNA-related processes (Siomi and Drey- fuss, 1997). None of these five commonly used housekeeping genes showed high stability under salinity stress, suggesting that they were unsuitable as internal controls in this situation.
The unstable expression levels of commonly used house- keeping genes mean that there is no ‘one-size-fits-all’gene that can be used for the normalization of gene expression data under all conditions (Barber, 2005). Vandesompele(2002) have proposed the use of the mean expression level of several genes for normalization. Pairwise variation in the A and B groups were both below the cut-off value of 0.15, showing that the use of RPL4 and RPS4 as reference genes is sufficient in gene expression studies inunder salinity stress, irrespective of different tissues.
In conclusion, our data suggest that the novel genes detected from transcriptome data performed better than commonly used reference genes of. The results of the present study will facilitate sensitive and accurate quantification of gene expression in, which could also be extrapolated to related oyster species.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 31772843), the Natural Science Foundation of Guangxi Province (No. AA17204080-4), the Fundamental Research Funds for the Central Universities (No. 201762014), and the Ocean Uni- versity of China-Auburn University Joint Research Cen- ter for Aquaculture and Environmental Science.
Barber, R. D., Harmer, D. W., Coleman, R. A., and Clark, B. J., 2005. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues., 21: 389-395.
Barsalobres-Cavallari, C. F., Severino, F. E., Maluf, M. P., and Maia, I. G., 2009. Identification of suitable internal control genes for expression studies inunder different experimental conditions., 10: 1.
Boudry, P., Heurtebise, S., and Lapègue, S., 2003. Mitochondrial and nuclear DNA sequence variation of presumedandspecimens: A new oyster species in Hong Kong?, 228: 15-25.
Boutet, I., Tanguy, A., and Moraga, D., 2004. Response of the Pacific oysterto hydrocarbon contamination under experimental conditions., 329: 147-157.
Browne, G. J., and Proud, C. G., 2002. Regulation of peptide-chain elongation in mammalian cells., 269: 5360-5368.
Drouin, G., Himmelman, J. H., and Béland, P., 1985. Impact of tidal salinity fluctuations on echinoderm and mollusc populations., 63: 1377-1387.
Eisenberg, E., and Levanon, E. Y., 2003. Human housekeeping genes are compact., 19: 362-365.
Ellison, C. K., and Burton, R. S., 2008. Genotype-dependent va- riation of mitochondrial transcriptional profiles in interpopulation hybrids., 105: 15831-15836.
Gagnaire, B., Frouin, H., Moreau, K., Thomas-Guyon, H., and Renault, T., 2006. Effects of temperature and salinity on hae- mocyte activities of the Pacific oyster,(Thun- berg)., 20: 536-547.
Gonzalez, M., Gueguen, Y., Desserre, G., De Lorgeril, J., Romestand, B., and Bachère, E., 2007. Molecular characterization of two isoforms of defensin from hemocytes of the oyster., 31: 332-339.
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., Adiconis, X., Fan, L., Raychowdhury, R., Zeng, Q., Chen, Z., Mauceli, E., Hacohen, N., Gnirke, A., Rhind, N., di Palma, F., Birren, B. W., Nusbaum, C., Lindblad-Toh, K., Friedman, N., and Regev, A., 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome., 29: 644-652.
Greer, S., Honeywell, R., Geletu, M., Arulanandam, R., and Rap- tis, L., 2010. Housekeeping genes; expression levels may change with density of cultured cells., 355: 76-79.
Heid, C. A., Stevens, J., Livak, K. J., and Williams, P. M., 1996. Real time quantitative PCR., 6: 986-994.
Hsiao, L. L., Dangond, F., Yoshida, T., Hong, R., Jensen, R. V., Misra, J., Dillon, W., Lee, K. F., Clark, K. E., Haverty, P., Weng, Z., Mutter, G. L., Frosch, M. P., MacDonald, M. E., Milford, E. L., Crum, C. P., Bueno, R., Pratt, R. E., Mahadevappa, M., Warrington, J. A., Stephanopoulos, G., and Gullans, S. R., 2001. A compendium of gene expression in normal human tissues., 7: 97-104.
Itoh, N., Tun, K. L., Komiyama, H., Ueki, N., and Ogawa, K., 2004. An ovarian infection in the Iwagaki oyster,, with the protozoan parasite., 27: 311-314.
Kuchel, R. P., Raftos, D. A., and Nair, S., 2010. Immunosuppressive effects of environmental stressors on immunological function in., 29: 930-936.
Langmead, B., and Salzberg, S. L., 2012. Fast gapped-read align- ment with Bowtie 2., 9: 357-359.
Li, B., and Dewey, C. N., 2011. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome., 12: 323.
Li, J., Zhang, Y., Liu, Y., Zhang, Y., Xiao, S., and Yu, Z., 2016. Co-expression of heat shock protein (HSP) 40 and HSP70 inresponse to thermal, low salinity and bacterial challenges., 48: 239-243.
Liu, C., Wu, G., Huang, X., Liu, S., and Cong, B., 2012. Validation of housekeeping genes for gene expression studies in an ice algaduring freezing acclimation., 16: 419-425.
Mackay, I. M., Arden, K. E., and Nitsche, A., 2002. Real-time PCR in virology., 30: 1292-1305.
McKean, P. G., Vaughan, S., and Gull, K., 2001. The extended tubulin superfamily., 114: 2723-2733.
Meistertzheim, A. L., Tanguy, A., Moraga, D., and Thébault, M. T., 2007. Identification of differentially expressed genes of the Pacific oysterexposed to prolonged ther- mal stress., 274: 6392-6402.
Meng, X., Dong, Y., Dong, S., Yu, S., and Zhou, X., 2011. Mortality of the sea cucumber,Selenka, exposed to acute salinity decrease and related physiological responses: Osmoregulation and heat shock protein expression., 316: 88-92.
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L., and Wold, B., 2008. Mapping and quantifying mammalian transcriptomes by RNA-seq., 5: 621-628.
Philippart, C. J., Anadón, R., Danovaro, R., Dippner, J. W., Drink- water, K. F., Hawkins, S. J., Oguz, T., O’Sullivan, G., and Reid, P. C., 2011. Impacts of climate change on European marine ecosystems: Observations, expectations and indicators., 400: 52-69.
Radonić, A., Thulke, S., Mackay, I. M., Landt, O., Siegert, W., and Nitsche, A., 2004. Guideline to reference gene selection for quantitative real-time PCR., 313: 856-862.
Rosic, N. N., Pernice, M., Rodriguez-Lanetty, M., and Hoegh- Guldberg, O., 2011. Validation of housekeeping genes for gene expression studies inexposed to thermal and light stress., 13: 355-365.
Shakeel, M., Rodriguez, A., Tahir, U. B., and Jin, F., 2018. Gene ex- pression studies of reference genes for quantitative realtime PCR: An overview in insects., 40: 227-236.
Siomi, H., and Dreyfuss, G., 1997. RNA-binding proteins as re- gulators of gene expression., 7: 345-353.
Tristan, C., Shahani, N., Sedlak, T. W., and Sawa, A., 2011. The diverse functions of GAPDH: Views from different subcellular compartments., 23: 317-323.
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., and Speleman, F., 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes., 3: research0034.1.
Warner, J. R., 1999. The economics of ribosome biosynthesis in yeast., 24: 437-440.
Zhang, G., Li, L., Meng, J., Qi, H., Qu, T., Xu, F., and Zhang, L., 2016. Molecular basis for adaptation of oysters to stressful marine intertidal environments., 4: 357-381.
. Tel: 0086-532-82031622
E-mail: qili66@ouc.edu.cn
December 26, 2019;
February 13, 2020;
June 30, 2020
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年6期
Journal of Ocean University of China2020年6期
- Journal of Ocean University of China的其它文章
- Numerical Simulation and Risk Analysis of Coastal Inundation in Land Reclamation Areas: A Case Study of the Pearl River Estuary
- Variation of Yellow River Runoff and Its Influence on Salinity in Laizhou Bay
- Cold Water in the Lee of the Batanes Islands in the Luzon Strait
- Preliminary Design of a Submerged Support Structure for Floating Wind Turbines
- Inversion of Oceanic Parameters Represented by CTD Utilizing Seismic Multi-Attributes Based on Convolutional Neural Network
- Trace-Norm Regularized Multi-Task Learning for Sea State Bias Estimation
