Influence Factors on Photochemical Production of Methyl Iodide in Seawater
CHEN Yan, LIU Shanshan, YANG Guipeng, 2), 3), and HE Zhen, 3), *
Influence Factors on Photochemical Production of Methyl Iodide in Seawater
CHEN Yan1), LIU Shanshan1), YANG Guipeng1), 2), 3), and HE Zhen1), 3), *
1)Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education; College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China 2) Laboratory for Marine Ecologyand Environmental Science,Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China 3)Institute of Marine Chemistry, Ocean University of China, Qingdao 266100, China
Methyl iodide (CH3I) is an important trace greenhouse gas in the atmosphere and an ozone-depleting substance. The influence of different environmental factors, such as the duration of illumination; the strength of illumination; the concentrations of humic acid, ferric ion (Fe3+), and iodide ion (I−); and pH, on the photochemical production of CH3I in artificial seawater (ASW) were tested by simulated solar irradiation. In addition, Yangtze River Estuary waters from inshore to offshore were used to explore the relationship between the photochemical production of CH3I and different sources of dissolved organic matter (DOM) in natural seawater (NSW). The results revealed that higher concentrations of humic acid and I−, as well as higher strengths of illumination and longer illumination durations, promoted the photochemical formation of CH3I in ASW. The addition of Fe3+accelerated the photochemical production of CH3I, but high concentrations of Fe3+inhibited the formation of CH3I. Experiments on NSW obtained from the Yangtze River estuary spiked when concentrations of DOM were high, confirming that DOM plays an important role in facilitating the photochemical production of CH3Iwithin the Yangtze River Estuary. The photochemical production of CH3I in the seawater was significantly higher under light conditions relative to dark conditions, indicating that illumination accelerated the production of CH3I.
methyl iodide; photochemical production; Yangtze River Estuary; dissolved organic matter
1 Introduction
Methyl iodide (CH3I) is an important trace greenhouse gas and environmental pollutant in the atmosphere (Reifenhäuser and Heumann, 1992; Yuan., 2016; Carpenter., 2017). It can significantly damage the ozone layer and react with other trace gases that indirectly affect the surface temperature of the earth and global climate (Du., 2014). The ocean plays an important role in the biogeochemical cycles of CH3I. Sources of CH3I in the ocean are primarily derived from its natural release (Lovelock, 1975; Manley and Dastoor, 1987; Schall., 1994; Manley and Cuesta, 1997). The mechanisms of natural CH3I production primarily include marine biotic and abiotic mechanisms (Nightingale., 1995). Research on the production of CH3I by marine organisms has been extensive (Gschwend., 1985; Manley and Dastoor, 1987; Moore., 1996; Fuse., 2003; Hughes., 2011; Hughes., 2013; Lim., 2018), but the abiotic mechanism by which CH3I is pro-duced remains unclear.
The observation that significant amounts of CH3I could be produced in seawater through photochemistry was first reported by Moore and Zafiriou (1994). Moore and Zafiriou (1994) used natural sunlight and oxygenic seawater of the North Sea to study the photochemical production of CH3I. They showed that the production of CH3I under sunlight far exceeded the blank measured in the dark sample. Goodwin. (1997) tested seawater under dark and light conditions and found that the production of CH3I under light conditions was significantly higher than that under dark conditions. These results show that light can promote the production of CH3I in seawater, but the optimal light duration and intensity require further study. Colored dissolved organic matter (CDOM) can act as the precursor to the generation of halocarbons (Hao., 2017; Song., 2017). Studies have shown that dissolved organic matter (DOM) can produce methyl chloride (CH3Cl) through a photochemical reaction in chlorinated solution (Moore, 2008). CDOM extracted from subsurface seawater can improve the photochemical yield of CH3Cl (Moore, 2008). Méndez-Díaz. (2014) studied the halogenation of DOM in seawater under non-specific sunlight conditions using simulated sunlight and natural sunlight. They suggested that volatile halocarbons (VHCs) can be produced through photochemical reactions.
Generally, the effect of light, pH, and various other parameters, such as ferric ions (Fe3+) and iodide ion (I−), on the photochemical production of CH3I in seawater has been extensively studied. Fe3+are oxidizable, affect the photochemical reaction of CDOM, and change the formation of the intermediates in the photochemical process of CDOM (Bi, 2017), thus affecting the formation of CH3I. For example, Fe3+can generate hydroxyl (OH·) through photochemical reactions, react with CDOM in the environment, and accept electrons during the ligand-to-metal charge transfer process and subsequent Fenton reaction (Wuosmaa and Hager, 1990), resulting in the generation of ferrous ion (Fe2+) and reactive oxygen species (ROS) (Abrahamsson., 2003). The photoinduced OH· can oxidize most CDOM rapidly. Therefore, the Fe(II)- CDOM complex light reaction plays a crucial role in the transformation of CDOM.
Fan. (2012) studied the factors affecting the photolysis of CDOM in water and discussed the influence of different conditions on the residual concentration of CDOM; the results showed that increasing pH could promote the CDOM photochemical reaction. Moore (2008) discussed the effect of pH on the production of CH3Cl and found that when pH increased, the content of CH3Cl increased. Deng. (1997) showed that when pH was in the range of 3–5, the hydrolysis product of Fe3+primarily existed in the form of Fe(OH)−; thus, pH also had an impact on the existence of iron (Fe) and thus affected the photochemical reaction of CH3I. Under alkaline conditions, Fe3+can form a precipitation, which reduces the iron source for the photoreaction and does not promote the photochemical reaction with humic acid. In addition, Fe3+can also combine with humic acid to produce co-precipitation, resulting in a decrease in the content of Fe3+and humic acid and a decrease in the production of CH3I (Bi, 2017).
I−is one of the precursor substances to CH3I formation in seawater. Moore and Zafiriou (1994) compared CH3I production in iodide and iodide-free offshore (Labrador Sea) and coastal water samples and showed a significant increase in CH3I production when I−was added to seawater. There is thus a need to explore the influence of I−on the photochemical formation of CH3I.Because the concentration of light-absorbing organic compounds in coastal waters is usually higher, the production of CH3I in coastal waters is approximately six times higher than in offshore waters (Labrador Sea) (Moore and Zafiriou, 1994).Therefore, the mechanisms and factors affecting the photochemical production of VHCs in seawater need to be explored.
The objectives of this study were to characterize the photochemistry of CH3I in artificial seawater (ASW) and clarify the effects of illumination time, the strength of illumination, the concentration of humic acid, Fe3+, I−, and pH on CH3I generation as well as evaluate the production of CH3I in natural seawater (NSW) from the Yangtze River under irradiation. The results of this study provide important parameters for photochemical research of CH3I in the ocean.
2 Methods
2.1 Preparation of Solutions
The NSW of the Yangtze River Estuary from inshore to offshore was collected from Zheyuke No.2 scientific survey ship from March 3 to 13 2019. The sampling stations are shown in Fig.1. Samples from surface seawater (0–5 m) were obtained using 12-L Niskin bottles attached to a conductivity-temperature-depth (CTD) Rosette (Sea-Bird 911, General Oceanics Co.) carousel system. After being brought to the laboratory, the samples were used for radiation experiments and analysis in the shore laboratory after removing the phytoplankton and bacteria sequentially by 0.45-μm and 0.2-μm polyether membranes.Samples were then transferred to 100-mL gas-tight quartz tube reactors without headspace and were then used for irradiation experiments.
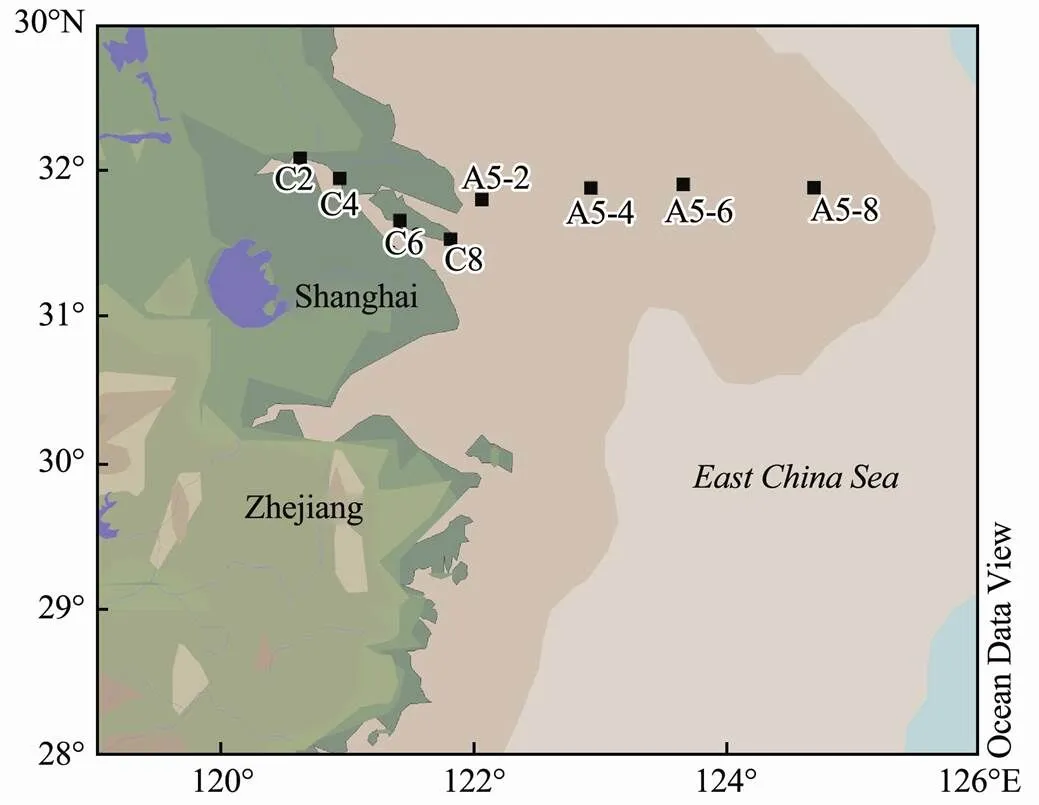
Fig.1 Sampling locations in the Yangtze River Estuary.
According to Martin’s method, the artificial seawater was composed of 24.7g sodium chloride (NaCl), 13.0g magnesium chloride hexahydrate (MgCl2·6H2O), and 9.0 g sodium sulfate decahydrate (Na2SO4·10H2O) and was dissolved in 954g ultra-pure water to obtain 1L of ASW (Cao., 2011). Humic acid (2mgL−1) and KI (0.06 ppm) were added to the artificial seawater as the precursor to produce CH3I. The samples were placed in the solar simulator under a constant temperature water bath at 20℃ and illuminated with 550Wm−2illumination intensity for 5h.Because the surface ocean waters contained I−at 100–200nmolL−1(Moore and Zafiriou, 1994), we prepared artificial seawater and set the concentration gradient of KI from 0.06ppm to 600ppm.
Reagents such as NaCl, MgCl2·6H2O, Na2SO4·10H2O, and ferric chloride hexahydrate (FeCl3·6H2O) were purchased from Sinopharm Group Chemical Reagent Co. Ltd. Potassium iodide (KI) and humic acid were purchased from Aladdin Company. All reagents were analytical grade. The standard sample of CH3I was purchased from the National Research Center for Standard Materials in Beijing.
2.2 Irradiation Experiments
The seawater solutions were purged with ultra-high- purity nitrogen to ensure that CH3I was removed to blank levels before irradiation experiments. The solutions were placed in a 100-mL airtight quartz tube reactor with a sealed bottom and an outlet in the middle of the bottom where the reaction solution was injected into the reactor with a syringe. The gas tightness of the quartz tube reactor was checked before the irradiation experiment. The solutions were placed in the airtight quartz tube with no headspace and irradiated in a Sun-test CPS solar simulator (ATLAS, Germany) under a water bath at a constant temperature of 20℃ for illumination. The solar simulator contained a xenon arc lamp with an adjustable intensity from 400 to 765Wm−2. Aside from the illumination time experiment and the illumination intensity experiment, the illumination times of other experiments were set to 25h, and the illumination intensity was set to 550Wm−2. The dark control group was wrapped in aluminum foil and was placed in quartz bottles in the solar simulator. After irradiation, the sample was sucked out of the quartz tube through a long thin tube and introduced into a 100-mL glass syringe, followed by injection into the purge and trap system. In each experiment, the blank measure did not increase in the amount of CH3I in the sample stored in the dark over the same period.
2.3 Analytical Procedure
The samples were measured using a purge and trap system and were then coupled to a gas chromatograph (GC, Agilent 6890N) with an electron capture detector (ECD). Samples (100mL) were purged at 40℃ with ultra-pure nitrogen at a flow rate of 60mLmin−1for 14min. The extract CH3I gases were then dried using two glass tubes containing magnesium perchlorate and sodium hydroxide and then were collected in a stainless steel tube (length, 30cm; i.d., 0.8mm) cooled to −190℃ with liquid nitrogen. The steel tube was finally heated with boiling water, and the desorbed gases were introduced into the GC column (Agilent DB-624; length, 60m; i.d., 0.32mm; film thickness, 3μm). CH3I was identified and quantified based on the retention times and external calibration standards. Its calibration standards were obtained from the Research Institute of National Standard Materials of China. At the same time the calibration standards were diluted twice with methanol and then were used to prepare working standards. Three parallel samples were taken for each group of experiments. The detection limit for CH3I was 0.1pptv, and the precision was 2%. Details on the methods used to analyze CH3I were reported by He. (2013).
Dissolved organic carbon (DOC) concentration was determined through catalytic high-temperature oxidation by a total organic carbon analyzer (TOC-VCPH, SHIMA DZU) (Peltzer and Brewer, 1993; Ferrari., 1996). Relative standard deviation of the measured sample was less than 2%.
3 Results and Discussion
3.1 Influencing Factors
3.1.1 Illumination time
The results of previous experiments showed that sun- light plays a significant role in the production of CH3I in seawater (Moore and Zafiriou, 1994). Several studies on the photochemical production of methyl halides suggested that the production pathways of CH3Cl and CH3I in seawater were similar (Ooki,., 2010; Yang., 2020). The same results were also obtained in the study of Yang. (2020), which found that no detectable CH3Cl was formed in the dark.The effect of illumination can be divided into the effect of illumination time and light intensity. The variation in CH3I released by samples with different concentrations of I−under different illumination times is shown in Fig.2. When the concentration of KI was low, different illumination times have little influence on the concentration of CH3I. Increasing illumination time causes fluctuations in the concentration of CH3I. Some researchers have suggested that CH3I is produced by the free combination of methyl and iodine (Moore, 1994; Xiang and Deng, 2001). Iodine and methyl are precursors to the production of CH3I. If methyl is adequate, higher iodine concentrations lead to greater production of CH3I. When KI concentration is low, precursor substances for the formation of CH3I are insufficient, and the reaction can only be carried out to a certain extent, as the reactants of the photochemical reaction have been exhausted. Other conditions also have an effect on the formation of CH3I, but the effect of these conditions was not significant; consequently, the concentration of CH3I fluctuates as the illumination time increases. In addition, the instability of iodine is also related to fluctuations in reaction yield.More CH3I was released at illumination times of 20h and 25h.
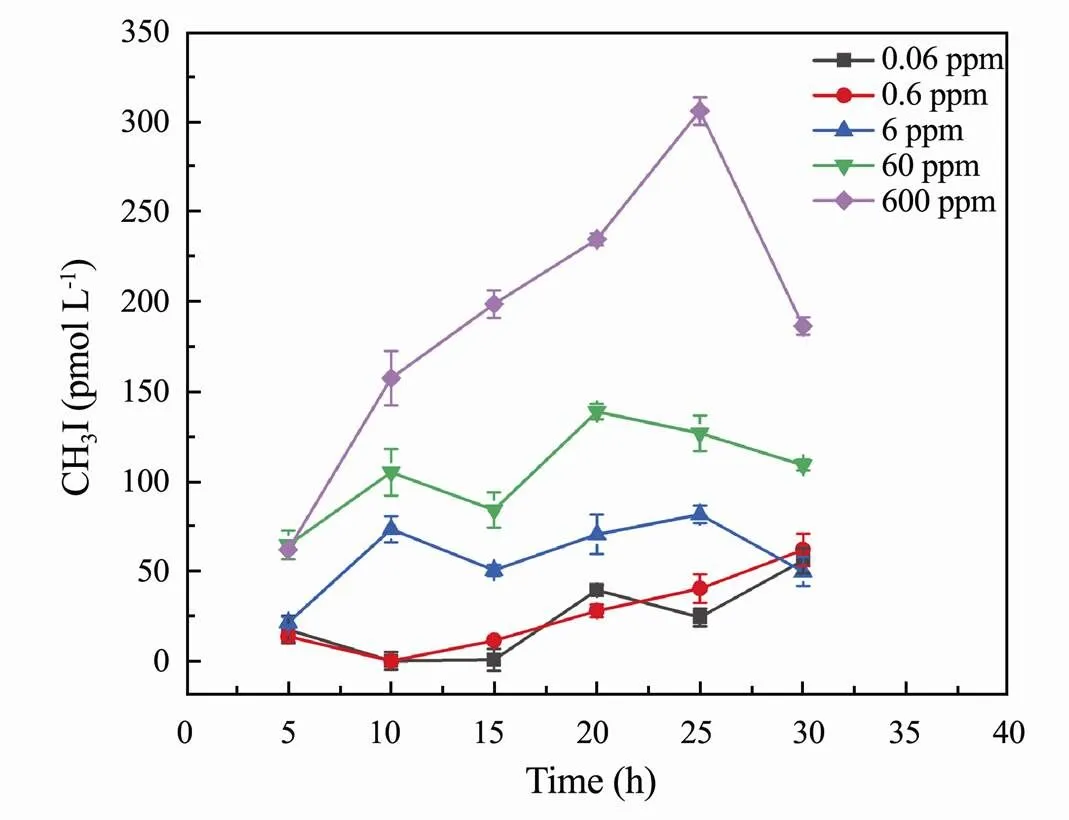
Fig.2 Effect of irradiation time on CH3I photoproduction from humic acid solution of different concentrations in ASW.
Stemmler. (2014) simulated the production of CH3I on a global scale and proposed that the photoche- mical process was the best method for explaining variation in global surface concentration of CH3I. Liu. (2020b) reported the photochemical synthesis of methyl bromide (CH3Br) from syringic acid. Their experiments showed that the concentration of CH3Br increased as the irradiation time increased and that CH3Br was not produced under dark conditions. They also studied the photochemical formation of CH3Cl in natural seawater and humic acid solution and concluded that the production of CH3Cl increased as the irradiation time increased. As mentioned above, CH3I and CH3Cl have similar photochemical production mechanisms; thus, long illumination times can promote the production of CH3I in seawater.
3.1.2 Illumination intensity
The concentration of CH3I released under illumination with different light intensities is shown in Fig.3. When the illumination time was constant, the amount of CH3I generation increased as the illumination intensity increased. When the illumination intensity was low, the increase in the generation of CH3I was faster than when the illumination intensity was high; however, overall CH3I production increased as the illumination intensity increased. The unusual inflection point from 550Wm−2to 600Wm−2most likely stemmed from the fact that CH3I can also be produced under dark conditions (Richter, 2004). Richter (2004) conducted incubation experiments in the tropical Atlantic to test the hypothesis that CH3I is produced in surface water by a photochemical pathway. He found that the production of CH3I observed in all incubations kept in the sunlight was five times higher than the production of CH3I from incubations kept in the dark. He also noted that CH3I can be produced under dark conditions.
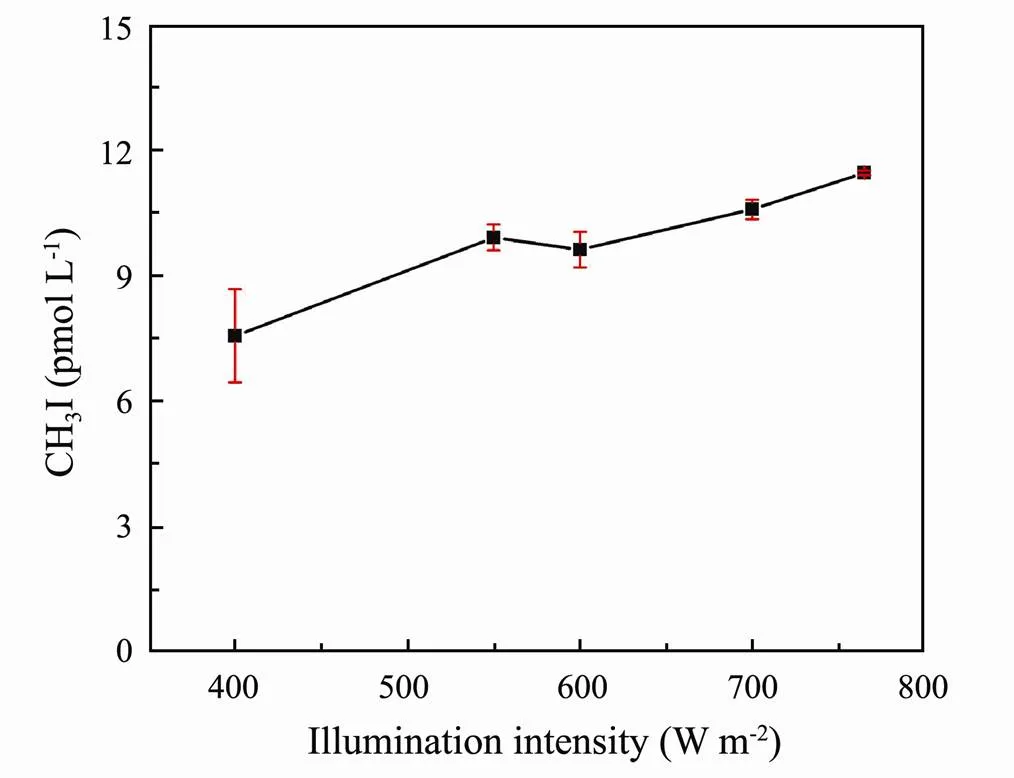
Fig.3 Effect of irradiation intensity on CH3I photoproduction in ASW.
Archer. (2007) pointed out that variation in CH3I concentration followed seasonal trends in illumination and that the concentration of CH3I was high when the illumination intensity was high during the summer. Wang. (2009) also reported the seasonality of CH3I in the open ocean, and the highest concentration of CH3I was observed in the summer. They suggested that the increase in illumination intensity could promote the formation of CH3I. Shi. (2014a) studied the photochemical production of CH3I in the nearshore environment of Kiel Fjord. Ziska. (2013) noted the high concentration of CH3I in subtropical circulation (sea area) was caused by photochemical production. Our experimental results show that the increase in illumination intensity can promote the light reaction in ASW and increase the production of CH3I, which is consistent with the research results described above.
3.1.3 Iodide ion concentration
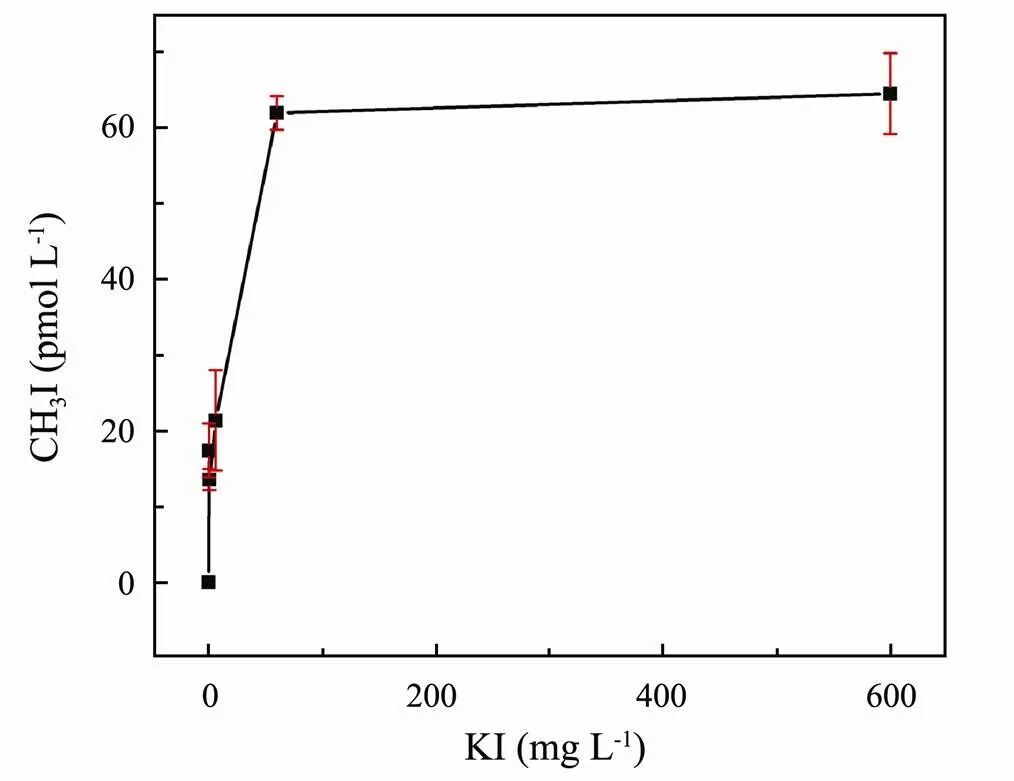
Fig.4 Effect of I− concentration on CH3I photoproduction in ASW.
3.1.4 Humic acid concentration
As the concentration of humic acid increased (2mgL−1, 4mgL−1, 8mgL−1, respectively), the amount of CH3I generated increased (Fig.5).Lin and Manley (2012) found a significant correlation between DOM and bromoform (CHBr3) production from seawater.CDOM was photochemically degraded to form VHCs (Manley and Barbero, 2001). CH3Cl is generated by the reaction of chlorine ions in seawater with CDOM under sunlight (Moore, 2008). I−is oxidized by ozone in surface seawater to form non-free radical active halogen substances (HOI and I2), which react photochemically with CDOM in seawater to form organic iodide (Xiang and Deng, 2001). CDOM can generate methyl radicals and acetyl groups through photochemical reactions. Halogens can also be oxidized by OH· to form free radicals and non-free radical active halogens in surface seawater (Martino., 2009). Active radical halogen substances are formed by the breaking of unsaturated C-C bonds and the recombination of carbon center radicals (Mopper and Zhou, 1990), while the non-free radical active halogen substance halogenates several types of organic functional groups through the substitution of electrons (Grebel., 2010). Humic acid is an important component of CDOM and is derived from algal decomposition and terrigenous constituents, with many aromatic and phenolic groups (Opsahl and Benner, 1997). Humic acid has been shown to have the ability to enhance the photochemical production of methyl halides (Keppler., 2000; Moore, 2008). CDOM can react to form transient ROS in light, which can react with humic acid and lead to their degradation (Paul., 2012). Humic acid can also trigger electron transfer after the absorption of light radiation (Gjessing and Källqvist, 1991). The energy transfer in the excited state of these active intermediates can cause the free radical reaction of other compounds, which affects the photochemical reaction of organic compounds(Bi, 2017). Therefore, higher concentrations of humic acid facilitate the generation of greater amounts of CH3I.

Fig.5 Effect of humic acid concentration on CH3I photoproduction in ASW.
3.1.5 pH
The concentrations of CH3I released by the three samples under different pHs (3.53, 6.71, and 9.04, respectively) are shown in Fig.6. Maximum CH3I production (55.9pmolL−1) occurred under a pH of 3.53 (acidic). While the amount of CH3I decreased remarkably at pH 9.04 (alkaline) and 6.71 (near neutral). The results indicated that neutral and alkaline environments inhibited photochemical reactions in water. Photochemical reactions in natural water were more likely to accelerate CH3I generation under acidic conditions.
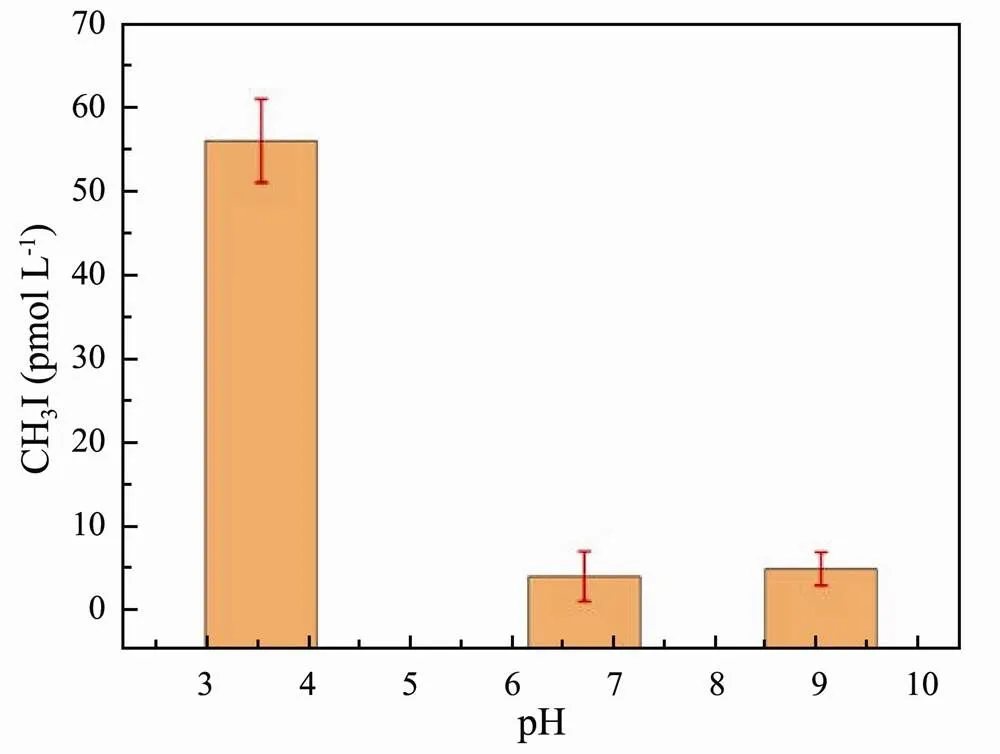
Fig.6 Effect of pH on CH3I photoproduction in ASW.
Dallin. (2009) proposed that the excited state of the benzene ring exposed to an aqueous solution enriched in Cl−was protonated. Cl−can break the carbon-oxygen bond (-O-CH3) and have a series of reactions with methyl groups (-CH3) to produce CH3Cl (Dallin., 2009). The humic acid has multiple methoxy groups. Under neutral and alkaline conditions, OH−and Cl−competed with the methyl groups attached to methoxy groups and led Cl−to the binding sites of methyl groups. These reactions reduced the production of CH3Cl (Bi, 2017). Yang. (2020) found that the production of CH3Cl decreased as pH increased over the range of 5.5–10.5. Their results showed that the yield of CH3Cl was the lowest when pH was 10.5 (Yang., 2020). We speculated that the mechanism for I−is similar to that for Cl−. Specifically, I−can break the -O-CH3bond and mediate a series of reactions with -CH3to produce CH3I. When pH increased, OH−competed with I−for -CH3, blocking the site where I−binds -CH3and producing less CH3I.
Hopkins. (2013) characterized the potential effect of ocean acidification on seawater halocarbons in the Arctic. Although the effects of ocean acidification on halocarbon concentrations were generally subtle, some significant effects on diiodomethane (CH2I2) and CHBr3were observed.Furthermore, the concentrations, the rate of net production, and the sea-to-air flux showed a significant positive response toCO2. Similar studies have shown that current increases in atmospheric CO2affect the production of important marine trace gases, revealing that concentrations of CH3I were significantly reduced under high CO2(Liss., 1997; Hopkins., 2010), and suggest that acidification (with a low pH) facilitates the release of CH3I.
3.1.6 Fe3+concentration
Fe in the ocean plays an important role in the transformation of CDOM in seawater (Balmer and Sulzberger, 1999; Soares., 2015). Thus, study of the formation of CH3I in seawater under the influence of Fe3+is important. Based on previous experiments (Bi, 2017; Liu., 2020a), we studied Fe3+in the range of 0 to 600μmolL−1. Variation in CH3I concentrations in samples with different concentrations of Fe3+(20μmolL−1, 100μmolL−1, 200μmolL−1, and 600μmolL−1, respectively) is shown in Fig.7.The addition of FeCl3·6H2O at low concentrations promoted the formation of CH3I. The production of CH3I was greatly inhibited by the addition of high concentrations of FeCl3.
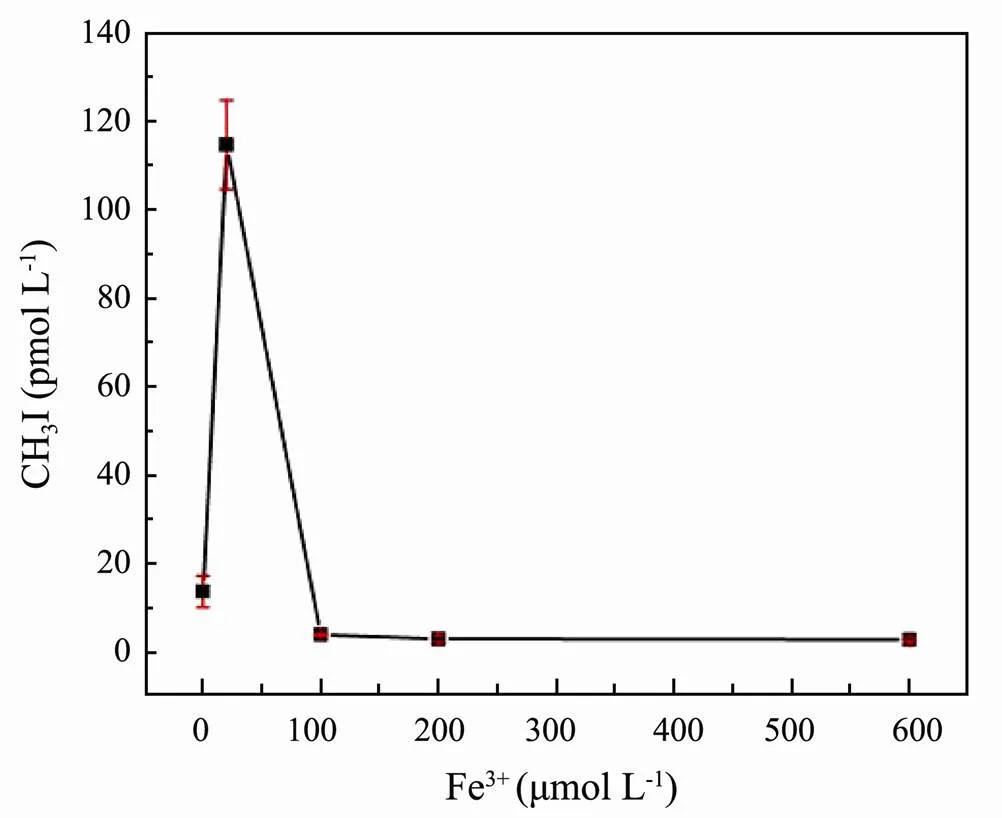
Fig.7 Effect of Fe3+ concentration on CH3I photoproduction in ASW.
As the main component of DOM, humic acid possesses a large number of hydroxyl, methyl, amino, carboxyl, and carbonyl groups, which can absorb sunlight of a specific wavelength and produce a series of active radicals, such as singlet oxygen (Balmer and Sulzberger, 1999). The oxidation property of Fe3+changes the formation of some intermediates in the photochemical process of humic acid (Soares., 2015) and then affects the formation process of CH3I. Fe3+can be reduced to Fe2+with the carboxyl on humic acid. Carbon center radicals were obtained from the decarboxylation of humic acid and combined with dissolved oxygen to generate hydrogen peroxide. The hydrogen peroxide was then reduced by Fe2+. Additionally, I−can be further oxidized by hydrogen peroxide to form iodine-free radicals, which thereby increase the amount of CH3I generated by the photochemical reaction. However, excess Fe3+can co-precipitate with high concentrations of humic acid. The amounts of humic acid and Fe3+decline because of their co-precipitation, leading to decreases in the CH3I produced by photochemical reactions. The same results have been observed by Yang. (2020), wherein the amount of CH3Cl reduced noticeably in the presence of Fe3+.
3.2 The Formation of CH3I in Natural Seawater
To study the photochemical formation of CH3I in NSW, irradiation experiments were performed using seawater collected near the Yangtze Estuary. The results of these irradiation experiments are shown in Fig.8. The production of CH3I under light conditions was higher than that in the dark, indicating that light can promote the production of CH3I in seawater. The amount of CH3I produced under dark conditions was slightly higher than the original value of CH3I in seawater. Additionally, the amount of CH3I production at station C8 was close under light and dark conditions. These results indicated that CH3I could also be produced under dark conditions. Because we used a filter membrane with a diameter of 0.2μm, the seawater used in the experiment may still contain some microorganisms. Previous studies have shown that CH3I in the ocean has microbial release production pathways, such as bacteria (Fuse., 2003) and cyanobacteria (Hughes., 2011). Based on the above analysis, we speculated that some microbial activities may release CH3I in the dark, and this possibility requires further study.
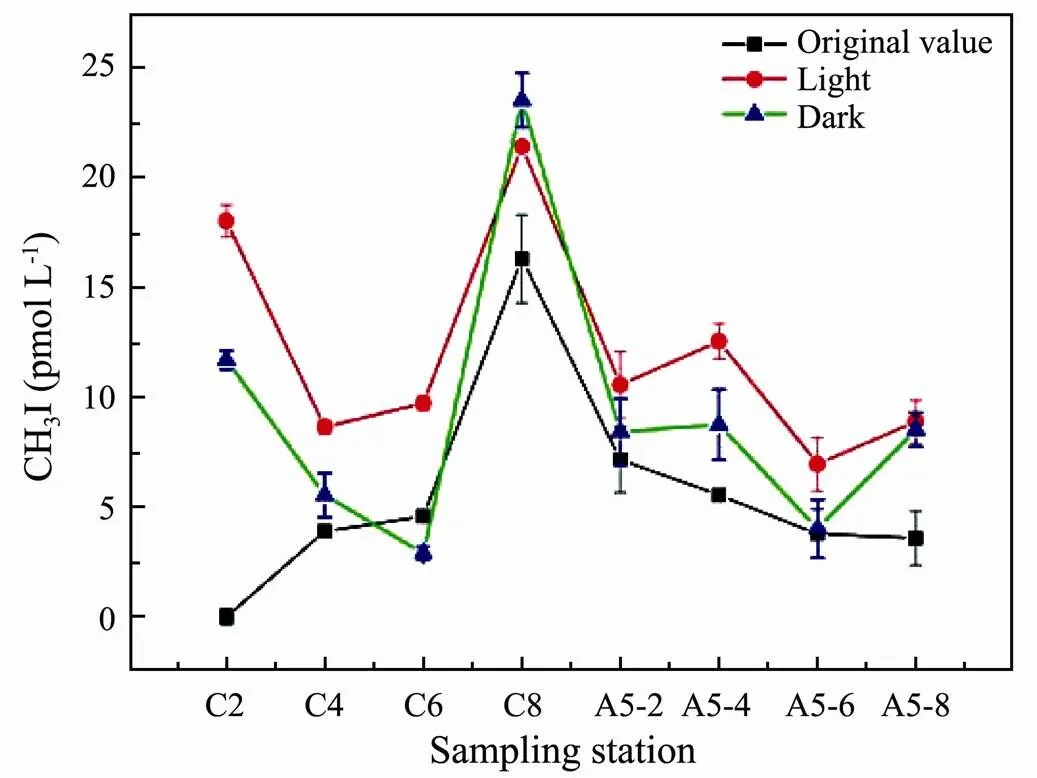
Fig.8 CH3I production in NSW from the Yangtze River Estuary.
The concentration of CH3I in the Yangtze Estuary region (C8) was higher than that in the open sea (A5-2, A5-4, A5-6, A5-8), which likely stems from the biotic sources from macroalgae and microalgae production released in the seawater and terrigenous inputs from freshwater runoff. High concentrations of nutrients, abundant sunlight, and appropriate water temperature accelerate the growth of phytoplankton (Chiang., 2004; Yuan., 2019) and may lead to the release of VHCs. Meanwhile, the content of DOC in these areas was abundant and provided sufficient precursor reactant for the photochemical reaction of seawater to produce CH3I. Richter and Wallace (2004) observed a lower accumulation of CH3I in filtered samples from the tropical Atlantic than in unfiltered samples. They suggested that this result reflects the fact that organic precursors were filtered out rather than differences in biological activity induced by filtering (Richter and Wallace, 2004). Therefore, concentrations of DOC of natural seawater samples were measured to further explore the relationship between DOC and CH3I. The analysis revealed a significant relationship between DOC and CH3I. The concentration of CH3I was significantly and positively correlated with the concentration of DOC (2=0.868) (Fig.9). This result confirmed that the photochemical generation of CH3I was associated with DOC concentration and that DOC provided precursors to the photochemical reaction, which is consistent with studies on the absorption and utilization of CDOM in the natural seawater of Kiel Fjord (Shi., 2014b).
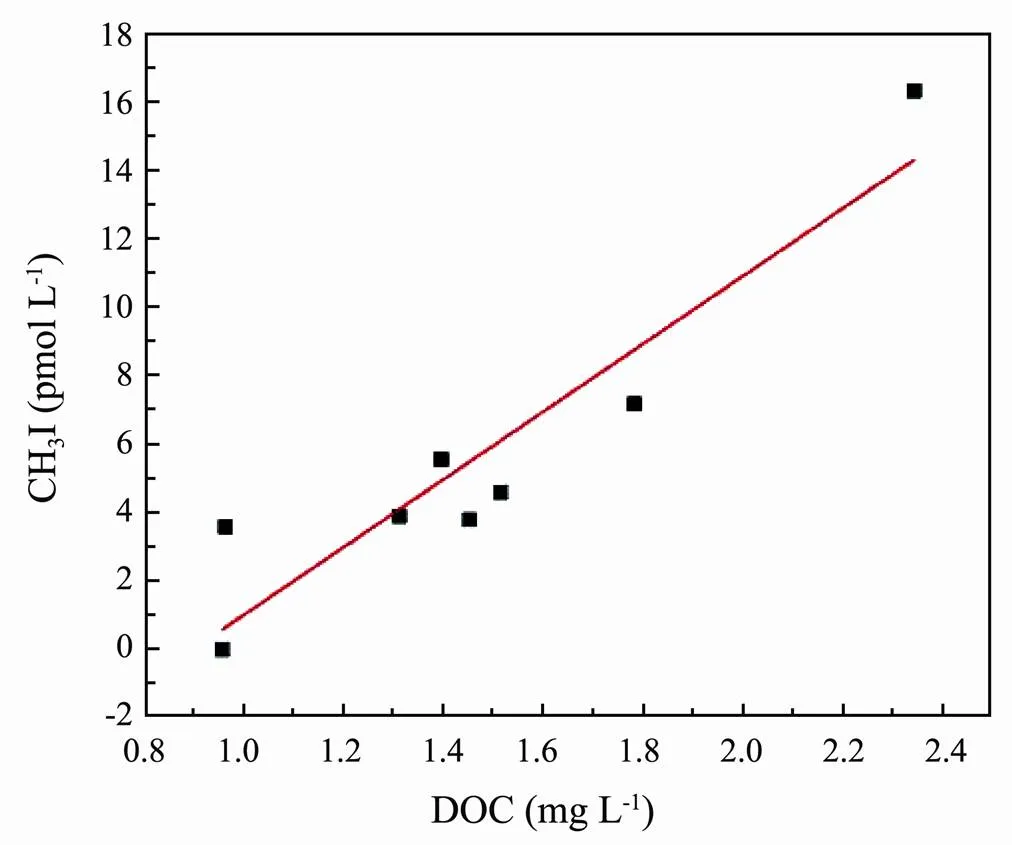
Fig.9 Relationships found between concentration of CH3I and DOC in NSW.
4 Conclusions
Our results showed that as precursor substances to form CH3I, the increase of humic acid and I-can promote the photochemical formation of CH3I. The increase in the intensity and duration of illumination led to increases in the photochemical generation of CH3I. Light and precursor substances were two significant contributors to the generation of CH3I. The addition of Fe3+can promote the photochemical production of CH3I, while excessive concentrations of Fe3+inhibited the formation of CH3I. The increase in pH inhibited the photochemical production of CH3I because of the reaction between hydroxide radicals and methyl groups. The photochemical production of CH3I in NSW was associated with DOC concentration, indicating that DOC provided precursors for the photochemical reaction.
Acknowledgements
This work was supported by the National Key Research and Development Program (No. 2016YFA0601 304), and the National Natural Science Foundation of China (Nos. 41830534, 41506088).
Abrahamsson, K., Choo, K., Pedersén, M., Johansson, G., and Snoeijs, P., 2003. Effects of temperature on the production of hydrogen peroxide and volatile halocarbons by brackish-water algae., 64 (3): 725-734.
Archer, S. D., Goldson, L. E., Liddicoat, M. I., Cummings, D. G., and Nightingale, P. D., 2007. Marked seasonality in the concentrations and sea-to-air flux of volatile iodocarbon compounds in the western English Channel., 112 (C8): C8009.
Balmer, M. E., and Sulzberger, B., 1999. Atrazine degradation in irradiated iron/oxalate systems: Effects of pH and oxalate.,33 (14): 2418-2424.
Bi, X. W., 2017. Photochemical formation of volatile halogenated hydrocarbons in seawater. Master thesis.Dalian Maritime University (in Chinese with English abstract).
Cao, X. Y., Ge, C. F., and Yang, G. P., 2011. Surface acid-base and copper (ii) adsorption properties of Jiaozhou Bay sediment., 41 (7): 137- 143 (in Chinese with English abstract).
Carpenter, L. J., Yokouchi, Y., and Atlas, E. L., 2017. Introduction to special issue on natural halocarbons in the atmosphere., 74 (2): 141-143.
Chiang, K. P., Chou, Y. H., Chang, J., and Gong, G. C., 2004. Winter distribution of diatom assemblages in the East China Sea., 60 (6): 1053-1062.
Dallin, E., Wan, P., Krogh, E., Gill, C., and Moore, R. M., 2009. New pH-dependent photosubstitution pathways of syringic acid in aqueous solution: Relevance in environmental photochemistry., 207 (2): 297-305.
Deng, N. S., Wu, F., Luo, F., and Liu, Z., 1997. Photodegradation of dyes in aqueous solutions containing Fe(III)-oxalato complexes., 35 (11): 2697-2706.
Du, H. N., Xie, W. X., Cui, Y. Q., Chen, J. L., and Ye, S. Y., 2014. Research advances in methyl bromide in the ocean., 25 (12): 3694-3700 (in Chinese with English abstract).
Fan, X. M., Li, X. G., Tang, X. L., Zhou, X., Liu, B., and Zou, H. L., 2012. Study on photochemical degration of dissolved organic matter in aqueous solution., 34 (5): 191-196 (in Chinese with English abstract).
Ferrari, G., Dowell, M. D., Grossi, S., and Targa, C., 1996. Relationship between optical properties of chromophoric dissolved organic matter and total concentration of dissolved organic carbon in southern Baltic Sea region., 55 (3): 299-316.
Fuse, H., Inoue, H., Murakami, K., Takimura, O., and Yamaoka, Y., 2003. Production of free and organic iodine byspp., 229 (2): 189-194.
Gjessing, E. T., and Källqvist, T., 1991. Algicidal and chemical effect of UV-radiation of water containing humic substances., 25 (4): 491-494.
Goodwin, K. D., North, W. J., and Lidstrom, M. E., 1997. Production of bromoform and dibromomethane by giant kelp: Factors affecting release and comparison to anthropogenic bromine sources., 42 (8): 1725-1734.
Grebel, J. E., Pignatello, J. I., and Mitch, W. A., 2010. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters., 244 (17): 6822.
Gschwend, P. M., MacFarlane, J. K., and Newman, K. A., 1985. Volatile halogenated organic compounds released to seawater from temperate marine macroalgae., 227 (4690): 1033-1035.
Hao, Z., Yin, Y., Cao, D., and Liu, J., 2017. Probing and comparing the photobromination and photoiodination of dissolved organic matter by using ultra-high-resolution mass spectrometry., 51 (10): 5464-5472.
He, Z., Yang, G. P., Lu, X. L., and Zhang, H. H., 2013. Distributions and sea-to-air fluxes of chloroform, trichloroethylene, tetrachloroethylene, chlorodibromomethane and bromoform in the Yellow Sea and the East China Sea during spring., 177: 28-37.
Hopkins, F. E., Kimmance, S. A., Stephens, J. A., Bellerby, R. G. J., Brussaard, C. P. D., Czerny, J., Schulz, K. G., and Archer, S. D., 2013. Response of halocarbons to ocean acidification in the Arctic., 10 (4): 2331-2345.
Hopkins, F. E., Turner, S. M., Nightingale, P. D., Steinke, M., Bakker, D., Liss, P. S., and Bender, M. L., 2010. Ocean acidification and marine trace gas emissions., 107 (2): 760-765.
Hughes, C., Franklin, D. J., and Malin, G., 2011. Iodomethane production by two important marine cyanobacteria: Prochlorococcus marinus (CCMP 2389) andsp. (CCMP 2370)., 125 (1): 19-25.
Hughes, C., Johnson, M., Utting, R., Turner, S., Malin, G., Clarke, A., and Liss, P. S., 2013. Microbial control of bromocarbon concentrations in coastal waters of the western Antarctic Peninsula., 151: 35-46.
Keppler, F., Eiden, R., Niedan, V., Pracht, J., and Schöler, H. F., 2000. Halocarbons produced by natural oxidation processes during degradation of organic matter., 403: 298-301.
Lim, Y. K., Phang, S. M., Sturges, W. T., Malin, G., and Rahman, N. B. A., 2018. Emission of short-lived halocarbons by three common tropical marine microalgae during batch culture., 30 (1): 341-353.
Lin, C. Y., and Manley, S. L., 2012. Bromoform production from seawater treated with bromoperoxidase., 57 (6): 1857-1866.
Liss, P. S., Hatton, A. D., Malin, G., Nightingale, P. D., and Turner, S. M., 1997. Marine sulphur emissions., 352 (1350): 159-169.
Liu, H., Pu, Y. Y., Tong, T., Zhu, X. M., Sun, B., and Zhang, X. X., 2020a. Photochemical generation of methyl chloride from humic acid: Impacts of precursor concentration, solution ph, solution salinity and ferric ion., 17 (2): 503.
Liu, H., Tong, T., Pu, Y. Y., Sun, B., Zhang, X. X., and Yan, Z. Y., 2020b. Insight into the formation paths of methyl bromide from syringic acid in aqueous bromide solutions under simulated sunlight irradiation., 17 (6): 2081.
Lovelock, J. E., 1975. Natural halocarbons in air and in the sea., 256 (5514): 193-194.
Lu, X. L., Yang, G. P., Song, G. S., and Zhang, L., 2008. Advances in the studies on the marine chemistry of methyl iodine., 27 (2): 103-110 (in Chinese with English abstract).
Manley, S. L., and Barbero, P. E., 2001. Physiological constraints on bromoform (CHBr3) production by(Chlorophyta).,46 (6): 1392- 1399.
Manley, S. L., and Cuesta, J. L., 1997. Methyl iodide production from marine phytoplankton cultures., 42 (1): 142-147.
Manley, S. L., and Dastoor, M. N., 1987. Methyl halide (CH3X) production from the giant kelp, macrocystis, and estimates of global CH3X production by kelp., 32 (3): 709-715.
Martino, M., Mills, G. P., Woeltjen, J., and Liss, P. S., 2009. A new source of volatile organoiodine compounds in surface seawater., 36 (1): 329-342.
Méndez-Díaz, J. D., Shimabuku, K. K., Ma, J., Enumah, Z. O., Pignatello, J. J., Mitch, W. A., and Dodd, M. C.,2014. Sunlight-driven photochemical halogenation of dissolved organic matter in seawater: A natural abiotic source of organobromine and organoiodine., 48 (13): 7418-7427.
Moore, R. M., 2008. A photochemical source of methyl chloride in saline waters., 42 (6): 1933-1937.
Moore, R. M., and Zafiriou, O. C., 1994. Photochemical production of methyl iodide in seawater., 99 (D8): 16415-16420.
Moore, R. M., Webb, M., Tokarczyk, R., and Wever, R., 1996. Bromoperoxidase and iodoperoxidase enzymes and production of halogenated methanes in marine diatom cultures., 101 (C9): 20899- 20908.
Mopper, K., and Zhou, X., 1990. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes., 250 (4981): 661-664.
Nightingale, P. D., Malin, G., and Liss, P. S., 1995. Production of chloroform and other low-molecular weight halocarbons by some species of macroalgae., 40 (4): 680-689.
Ooki, A., Tsuda, A., Kameyama, S., Takeda, S., Itoh, S., Suga, T., Tazoe, H., Okubo, A., and Yokouchi, Y., 2010. Methylhalides in surface seawater and marine boundary layer of the Northwest Pacific., 115: C10013.
Opsahl, S., and Benner, R., 1997. Distribution and cycling of terrigenous dissolved organic matter in the ocean., 386 (6624): 6769-6776.
Paul, A., Dziallas, C., Zwirnmann, E., Gjessing, E. T., and Grossart, H. P., 2012. UV irradiation of natural organic matter (NOM): Impact on organic carbon and bacteria., 74 (3): 443-454.
Peltzer, E. T., and Brewer, P. G., 1993. Some practical aspects of measuring DOC–Sampling artifacts and analytical problems with marine samples., 41 (1-3): 243-252.
Reifenhäuser, W., and Heumann, K. G., 1992. Determinations of methyl iodide in the Antarctic atmosphere and the south polar sea., 26 (16): 2905-2912.
Richter, U., 2004. Factors influencing methyl iodide production in the ocean and its flux to the atmosphere. PhD thesis, Kiel University.
Richter, U., and Wallace, D. W. R., 2004. Production of methyl iodide in the tropical Atlantic Ocean., 312 (23): 12, DOI: 10.1029/2004GL020779.
Schall, C., Laturnus, F., and Heumann, K. G., 1994. Biogenic volatile organoiodine and organobromine compounds released from polar macroalgae., 28 (7): 1315- 1324.
Shi, Q., Marandino, C., Petrick, G., Quack, B., and Wallace, D., 2014a. A time series of incubation experiments to examine the production and loss of CH3I in surface seawater., 119 (12): 8242-8254.
Shi, Q., Petrick, G., Quack, B., Marandino, C., and Wallace, D., 2014b. Seasonal variability of methyl iodide in the Kiel Fjord., 119 (3): 1609- 1620.
Soares, P. A., Batalha, M., Souza, S. M. A. G. U., Boaventura, R. A. R., and Vilar, V. J. P., 2015. Enhancement of a solar photo-Fenton reaction with ferric-organic ligands for the treatment of acrylic-textile dyeing wastewater., 152: 120-131.
Song, G. S., Li, Y. J., Hu, S. Z., Li, G. J., Zhao, R. H., Sun, X., and Xie, H. X., 2017. Photobleaching of chromophoric dissolved organic matter (CDOM) in the Yangtze River Estuary: Kinetics and effects of temperature, pH, and salinity., 19 (6): 861-873.
Stemmler, I., Hense, I., Quack, B., and Maier-Reimer, E., 2014. Methyl iodide production in the open ocean., 11 (16): 4459-4476.
Wang, L., Moore, R. M., and Cullen, J. J., 2009. Methyl iodide in the NW Atlantic: Spatial and seasonal variation., 114 (C7): C7007.
Wuosmaa, A. M., and Hager, L. P., 1990. Methyl chloride transferase: A carbocation route forbiosynthesis of halometabolites., 249 (4965): 160-162.
Xiang, W., and Deng, N. S., 2001. Advances in research of natural formation of volatile halohydrocarbon in seawater., 25 (9): 21-23.
Yang, Q., Guo, Y., Yue, E., Zhang, S., Blatchley III, E. R., and Li, J., 2020. Methyl chloride produced during UV254 irradiation of saline water., 384: 121263.
Yuan, D., He, Z., and Yang, G. P., 2019. Spatiotemporal distributions of halocarbons in the marine boundary air and surface seawater of the Changjiang estuary and its adjacent East China Sea., 140: 227-240.
Yuan, D., Yang, G. P., and He, Z., 2016. Spatio-temporal distributions of chlorofluorocarbons and methyl iodide in the Changjiang (Yangtze River) Estuary and its adjacent marine area., 103 (1-2): 247-259.
Ziska, F., Quack, B., Abrahamsson, K., Archer, S. D., Atlas, E., Bell, T., Butler, J. H., Carpenter, L. J., Jones, C. E., Harris, N. R. P., Hepach, H., Heumann, K. G., Hughes, C., Kuss, J., Krüger, K., Liss, P., Moore, R. M., Orlikowska, A., Raimund, S., Reeves, C. E., Reifenhäuser, W., Robinson, A. D., Schall, C., Tanhua, T., Tegtmeier, S., Turner, S., Wang, L., Wallace, D., Williams, J., Yamamoto, H., Yvon-Lewis, S., and Yo- kouchi, Y., 2013. Global sea-to-air flux climatology for bromoform, dibromomethane and methyl iodide., 13 (17): 8915-8934.
. E-mail:zhenhe@ouc.edu.cn
January 21, 2020;
August 12, 2020;
September 24, 2020
(Edited by Ji Dechun)
 Journal of Ocean University of China2020年6期
Journal of Ocean University of China2020年6期
- Journal of Ocean University of China的其它文章
- Numerical Simulation and Risk Analysis of Coastal Inundation in Land Reclamation Areas: A Case Study of the Pearl River Estuary
- Variation of Yellow River Runoff and Its Influence on Salinity in Laizhou Bay
- Cold Water in the Lee of the Batanes Islands in the Luzon Strait
- Preliminary Design of a Submerged Support Structure for Floating Wind Turbines
- Inversion of Oceanic Parameters Represented by CTD Utilizing Seismic Multi-Attributes Based on Convolutional Neural Network
- Trace-Norm Regularized Multi-Task Learning for Sea State Bias Estimation
