Tailoring soft magnetic properties of Fe-based amorphous alloys through C addition
Lingxiang Shi,Xiaolu Qin,Kefu Yao
School of Materials Science and Engineering,Tsinghua University,Beijing,100084,China
ABSTRACT It is known that developing Fe-based amorphous alloys with the saturation flux density(Bs)higher than 1.65 T is a major challenge.In present work,effects of C addition on magnetic properties of Fe-based amorphous alloys were systematically studied.It has been found that the addition of C can significantly increase the saturation flux density (Bs) and the magnetic flux density at 800 A/m (B800) of the Fe-B-C-Si-P amorphous alloys.After the addition of C,the Bs of the amorphous alloys increase from 1.61 T (Fe83B13Si3P1) to 1.65-1.71 T (Fe83B13-xCxSi3P1,x=1.5,2,3 and 4).The Fe83B10C3Si3P1 amorphous alloy possesses excellent soft magnetic properties with high Bs of 1.71 T and low Hc of 1.5 A/m.It shows that density increase of the alloys and weakened metalloid-sp/metal-d bonding caused by C addition contribute to the increment of Bs.It suggests that the newly developed high-performance amorphous alloys possess great potential in application.
Keywords:Amorphous metals Magnetic properties Alloy design
1.Introduction
The increasingly serious environmental issues and energy crisis strongly entail decreasing the wasteful dissipation of energy.For power applications,building high-efficiency and high-performance devices such as transformers and electric motors for electric vehicles has become of great interest.Particularly,soft magnetic materials in such devices play a crucial role in determining the efficiency.Soft magnetic materials with high saturation flux density(Bs),low coercivity(Hc)and low core loss (P) will lead to higher efficiency for these devices.Consequently,Fe-based amorphous alloys,characterized by low Hcand low P,has received intensive research in recent years [1,2].Nevertheless,compared to the widely used traditional silicon steel,their biggest deficiency is the lower Bs.Therefore,recent related research has mainly focused on improving the Bsof Fe-based amorphous alloys.
For Fe-based amorphous alloys,Bsis chiefly a composition-determined parameter that reflects the total magnetic moment per unit volume.Therefore,a higher Fe content(wt.%)usually means higher Bs[3,4].For instance,when a large amount of transition metal (e.g.Zr,Hf,Nb,Ta,Mo and W) is alloyed into the Fe-based amorphous alloys,due to the large relative atomic mass,the Bsis usually not very high(typically around 1-1.4 T [5-8]).
Therefore,in order to increase the Fe content (wt.%),in recent years,for the composition design of high-BsFe-based amorphous alloys,metalloids with small relative atomic mass (B,C,Si and P) are usually considered as the main alloying elements [9-13].Thus,research in recent years has focused on Fe-metalloids systems,which have relatively high Bsof around 1.65 T [10-13].However,the Bsof these Febased amorphous systems are generally lower than 1.7 T.Therefore,developing Fe-based amorphous alloys with a substantial improvement of Bsis still a major challenge in this field.
In this work,a series of high-BsFe-based amorphous alloys with the composition of Fe83B13-xCxSi3P1(x=0,1.5,2,3 and 4)were designed and successfully developed.After the addition of C,Bsis significantly improved.Specifically,the Fe83B10C3Si3P1amorphous alloy exhibits a combination of high Bs(1.71 T)and low Hc(1.5 A/m).Furthermore,we systematically investigated the effect of C addition on magnetic properties and crystallization behavior.
2.Experimental procedures
Fe83B13-xCxSi3P1(x=0,1.5,2,3 and 4) ingots were produced by induction melting the mixture of pure Fe (99.98 mass%),B (99.9 mass%),C (99.999 mass%),Si (99.999 mass%) and pre-alloyed Fe3P in an argon atmosphere.Alloy ribbons with thickness of about 19 μm were produced by single copper roller melt-spinning method in an argon atmosphere.For annealing,the alloy ribbons were sealed in a quartz tube with high-purity argon gas to prevent oxidation,and then the quartz tube was put in a tube furnace preheated to annealing temperature for different annealing times followed by water quenching to room temperature.The density was measured by the Archimedean method.The structure of as-spun ribbons was identified by X-ray diffraction (XRD,Rigaku D/max 2500) with a Cu Kα source and highresolution transmission electron microscopy(HRTEM,JEOL).The onset temperatures of the first and second crystallization (Tx1,Tx2)and Curie temperature (TC)were determined by differential scanning calorimetry(DSC,Netzsch STA 449 F3) at a heating rate of 0.67 K/s.Bsand Hcof alloy ribbons were measured using a vibrating sample magnetometer(VSM,Lake Shore) and a DC B-H loop tracer (Linkjoin MATS-2010SD)under a maximum applied field of 800 kA/m and 800 A/m,respectively.The parameter of B800,which is the magnetic flux density measured at applied magnetic field of 800A/m,was measured using a DC B-H loop tracer (Linkjoin MATS-2010SD).The experimental uncertainties in Bsand B800measurement are within 1%and less than 3%for the Hcmeasurement.Temperature dependence of magnetization for the amorphous alloys was measured by a superconducting quantum interference device (SQUID,Quantum Design) magnetometer.
3.Result and discussion
Fig.1 shows the XRD patterns of the free side of the melt-spun Fe83B13-xCxSi3P1(x=0,1.5,2,3 and 4) ribbons.No sharp diffraction peaks corresponding to crystalline phases were observed,indicating the formation of single amorphous phase for all ribbons.
This indicates that even for a system with B as the main metalloid,B and C can be mutually substituted to a large extent without sacrificing amorphous forming ability (AFA).This phenomenon may originate from the similarity between B and C:Boron and carbon are adjacent elements in the periodic table with similar atomic size,valence and electronegativity.Since mainstream commercial Fe-based amorphous alloys nowadays usually use B as the main metalloids[14],the strategy of replacing B for C is widely applicable.
To investigate the influence of C addition on crystallization behavior,DSC curves of the Fe83B13-xCxSi3P1(x=0,1.5,2,3 and 4)amorphous alloys were measured and analyzed (Fig.2).No obvious glass transition can be detected for all produced alloys,which is common for Fe-based amorphous alloys with Fe content higher than 80 at.%[10,15-17].In the DSC curves,there are two exothermic peaks for all the produced amorphous alloys.The two peaks from low to high temperature correspond to first and second crystallization,respectively.The first and second crystallization processes indicate the precipitation of crystalline α-Fe and Fe-metalloid compounds,respectively.Tx1and Tx2denote the onset temperatures of the first and second crystallization,respectively.As can be seen from the DSC curves,the addition of C gradually reduces Tx1and increases Tx2,resulting in a rise in Tx2-Tx1.It indicates that C addition would affect the thermal stability and crystallization behavior of the Fe-based amorphous alloys.It should be noted here that the addition of C is beneficial for obtaining Fe-based nanocrystalline alloys with single crystalline α-Fe phase due to the increment of Tx2-Tx1.
The magnetic properties of the Fe83B13-xCxSi3P1(x=0,1.5,2,3 and 4) amorphous alloys were also systematically investigated.The results are shown in Table 1 and Fig.3:With the increment of C,Bsrises initially,then reaches a maximum at C=3 at.%; finally,as C content further increases,Bsstarts to drop.In particular,when C=3 at.%,the Bsof the system rises to as high as 1.71 T,which is one of the highest values of Bsamong that of the Co-free Fe-based amorphous alloys[10,11,18,19].This indicates that the appropriate addition of C can effectively improve the Bsof the Fe-based amorphous alloys.
Then,understanding the mechanism behind the beneficial effect of C on Bswould be important.As the temperature rises from 0 K(absolute zero),the Bsof the system decreases continuously.When the temperature exceeds TCof the material,the material transforms from ferromagnetic to paramagnetic.Therefore,the Bsat room temperature is mainly determined by the following factors:First,the Bsat 0 K,which is the reflection of the magnitude of magnetic moment.Second,TCand the shape of the Bsversus temperature curve.Obviously,when the Bsat 0 K is similar,a higher TCor a slower rate of Bsdrop with rising temperature will result in an increase in the Bsat room temperature.Third,as mentioned above,since Bsessentially measures the total magnetic moment per unit volume,when the total magnetic moment is about the same,the higher the density of the material,the larger the Bsof thesystem [20,21].The densities of Fe83B13-xCxSi3P1(x=0,1.5,2,3 and 4) amorphous alloys are 7.32,7.34,7.36,7.38 and 7.39 g/cm3,respectively.Indeed,the density of the developed alloys increases monotonically with the content of C,which partly explains the rise of Bs.Still,increment in density alone cannot account for the all of the change of Bs:First,Bsof the alloys does not increase monotonically with the content of C.Bsreaches the maximum value when C=3 at.%.Then,as the C content continues to increase,Bsbegins to decrease.Second,the magnitude of Bsrise(about 6%from C=0 to C=3 at.%)is much larger than the increase in density (less than 1%).In addition,as shown in Table 1,there is no obvious increase of TCafter the addition of C.
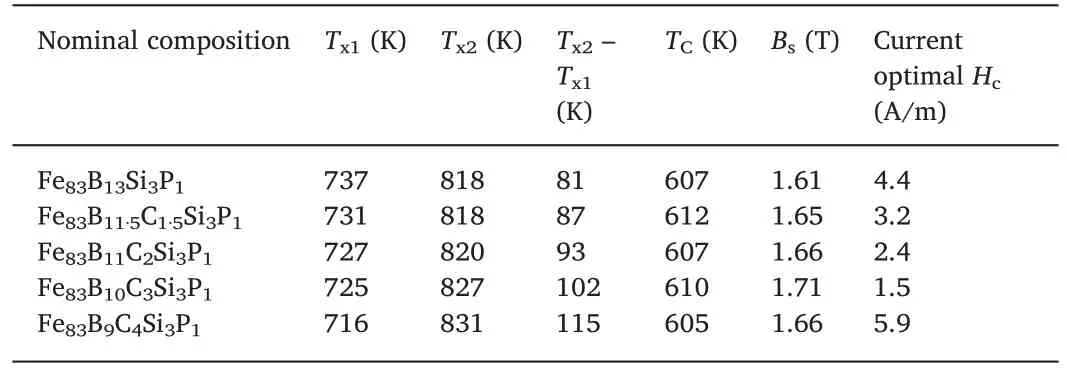
Table 1 Thermal and magnetic properties of the produced Fe-B-C-Si-P amorphous alloys.
Therefore,in order to better understand the cause of the variation of Bs,temperature dependence of the Bswas also measured.Three alloys were chosen:The C-free Fe83B13Si3P1alloy with the lowest Bs,the Fe83B10C3Si3P1with the highest Bs,and the Fe83B9C4Si3P1alloy with higher C but lower Bs.As shown in Fig.4,the shapes of Bs-T curves between 5 K to room temperature are very similar for all three amorphous alloys.The variation of room-temperature Bsis mainly caused by the difference of Bsat near 0 K.
For metallic glasses,local environment(coordination numbers,type and distance of nearest neighbors) has a huge impact on ferromagnetic behaviors.From the perspective of electronic structure,metalloid-sp/metal-d bonding causes moment reduction in 3d-based amorphous alloys,which in turn lead to the decrease in Bsat around 0 K [22].Meanwhile,in respect of topology,metalloids with smaller atomic radius have smaller theoretical coordination numbers [23,24],which reduces the metalloid-sp/metal-d bonding,hence the increase of the Bsof amorphous alloys.Therefore,since C (0.077 nm) has a smaller atomic radius than B (0.082 nm) [25],substitution of B by C tends to result in higher Bs.Nevertheless,from the perspective of exchange interaction,according to the Bethe-Slater curve [26],the strength of exchange interaction of Fe-based amorphous rises with the increase of ra/r3d,where rais the atomic radius of ferromagnetic elements and r3dis the radius of their 3d shell of electrons.Since C has the smallest atomic radius among the metalloids and the negative value of mixing enthalpy(which reflects the strength of atomic bond force)of the Fe-C pair is the largest among Fe-X (X=B,C,Si,P) [25],the Fe-Fe distance in Ccentered clusters will be lower,resulting in a weaker exchange interaction.Consequently,there is also a tendency towards lower TCand Bs.
The variation of Bsand the fluctuation of TCcan be explained by the above two conflicting effects of C addition.Clearly,when small amount of C is added (≤3 at.%),the diminishment of metalloid-sp/metal-d bonding overshadows effect of the decline in exchange interaction.As a result,Bsrises monotonically.When C content further increases,the effect of weakened exchange interaction becomes dominant,as a result,both TCand Bsstart to decrease.Thus,there is some optimal C content to form optimum combination of clusters for the maximum Bs.Even so,the Bsof Fe-based amorphous alloy with 4 at.% C is still much higher than the Fe83B13Si3P1alloy without C,indicating that the addition of C is generally beneficial for improving the Bsof Fe-based amorphous alloys.
Fig.5 shows the annealing temperature dependence of B800for the Fe83B13-xCxSi3P1(x=0,1.5,2,3 and 4) amorphous alloys.Due to the small applied field,B800is a comprehensive reflection of Bs,Hcand permeability.It can be seen that as the annealing temperature rises,initially,B800of all alloys begins to increase,then,at about 573 K,B800reaches the plateau,and finally,after 663 K,B800begins to decline(not shown in the figure).On the one hand,the initial increase of B800can be attributed to the enhanced magnetic softness caused by structural relaxation [27-31].On the other hand,when temperature further rises,the amorphous alloys might start to crystallize,causing magnetic softness to deteriorate,hence the reduction of B800.Furthermore,as shown in Fig.5,the B800of the C-added alloys is higher than that of the C-free Fe83B13Si3P1amorphous alloy at every annealing temperature,which also indirectly confirms that the addition of C can actually improve the Bsof the system.Besides,after the addition of C,the annealing temperature required for B800to reach the maximum is reduced,which indicates that the addition of C can facilitate the relaxation process for better magnetic softness at a lower temperature.
The above discussion on B800is further corroborated by the change in Hc.As shown in Fig.6,as the annealing temperature rises to around 603 K,the amorphous alloys are sufficiently relaxed,the Hcof the system reaches to the minimum value,then,as the temperature further increases towards crystallization temperature,Hcbegins to rise again.Nonetheless,unlike B800,Hcremains steady or even slightly rises at low annealing temperature (543 K).This indicates that the contributing factors that inhibit B800and cause higher as-spun Hcare not exactly the same.Specifically,spontaneous magnetization below TCmay play an important part in maintaining high Hc.Therefore,only after annealing temperature is near or above TC(around 603 K in this case) can the amorphous alloys be magnetically sufficiently relaxed.
As discussed above,the Fe83B10C3Si3P1alloy possesses the highest Bsamong the five developed amorphous alloys in this study.Therefore,further research is needed to study the magnetic properties of the Fe83B10C3Si3P1amorphous alloy.In order to prove that its high Bsis not caused by partial crystallization,the structure of as-spun Fe83B10C3Si3P1ribbon was examined by high-resolution transmission electron microscopy (HRTEM).Fig.7 shows the HRTEM image and selected-area electron diffraction (SAED) pattern of the melt-spun Fe83B10C3Si3P1ribbon.The featureless contrast and halo ring typical of amorphous phase confirm the amorphous nature of the melt-spun ribbon.
Moreover,we investigated the soft magnetic properties of the Fe83B10C3Si3P1amorphous alloy annealed at the optimal annealing temperature (603 K) for different periods of time.As shown in Fig.8,even at the optimum annealing temperature,Fe83B10C3Si3P1amorphous alloys annealed for different periods of time exhibit distinct magnetic properties.When annealed at the optimal annealing temperature,B800of Fe83B10C3Si3P1amorphous alloy gradually increases with the increase of annealing time.When annealing time exceeds 15 min,B800reaches the plateau and then remains steady.On the other hand,as the annealing time increases,the Hcfirst gradually drops to the lowest value,and then slightly increases.As shown in Fig.8,the time required to reach the lowest Hcis actually longer than the time needed for B800to reach the plateau.
In addition,for the Fe83B10C3Si3P1amorphous alloy,its Hcafter annealing for 30 min is 1.5 A/m,which is much lower than its Hcvalue(4.7 A/m) after annealing for 15 min.The above results suggest that even at the optimal annealing temperature,magnetic properties can still be further tailored and optimized.
4.Conclusion
In this work,we systematically studied the effect of C addition on the soft magnetic properties of high-BsFe-based amorphous alloys.Overall,the addition of C can improve the comprehensive performance of Fe-based amorphous alloys:
1).The addition of C can significantly enhance Bsof the Fe-based amorphous alloy.When 1.5 to 4 at.% of C is added,Bsof the Febased Fe83B13-xCxSi3P1amorphous alloys is enhanced from 1.61 T to 1.65-1.71 T.In particular,the Fe83B10C3Si3P1amorphous alloy possesses excellent soft magnetic properties with high Bsof 1.71 T,high B800of 1.64 T and low Hcof 1.5 A/m.
2).The increment of Bsby C addition can be attributed to both density increase of the alloys and weakened metalloid-sp/metal-d bonding.Between these two factors,weakened metalloid-sp/metal-d bonding leads to larger magnetic moment and plays a major role.
3).The addition of C in Fe-B-Si-P alloys would reduce the onset temperature of the first crystallization peak Tx1while increase the onset temperatures of second crystallization peak Tx2,resulting in a rise in Tx2-Tx1.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by National Key Basic Research and Development Program (Grant No.:2016YFB00300500) and National Natural Science Foundation of China (Grant No.:51571127).
 Progress in Natural Science:Materials International2020年2期
Progress in Natural Science:Materials International2020年2期
- Progress in Natural Science:Materials International的其它文章
- Mechanical behavior and microstructure evolution for extruded AZ31 sheet under side direction strain
- Kinetic analysis and strain-compensated constitutive models of Ti-42.9Al-4.6Nb-2Cr during isothermal compression
- First-principles comprehensive study of electronic and mechanical properties of novel uranium hydrides at different pressures
- Crystallite growth characteristics of Mg during hydrogen desorption of MgH2
- Influence of Cr content on the microstructure and mechanical properties of CrxFeNiCu high entropy alloys
- Microstructural characterization and hydrogenation performance of ZrxV5Fe(x=3-9) alloys
