Synthesis,characterization of a DOPO-based polymeric flame retardant and its application in polyethylene terephthalate
Xing Ao,Y Du,Dn Yu,Wei Wng,Weizhong Yng,Bin Sun,∗,Meifng Zhu
a State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,College of Materials Science and Engineering,Donghua University,201620,Shanghai,China
b Key Laboratory of Science & Technology of Eco-Textile,Ministry of Education,College of Chemistry,Chemical Engineering and Biotechnology,Donghua University,201620,Shanghai,China
c Shanghai Different Chemical Fiber Co.,Ltd,China
ABSTRACT A high molecular weight DOPO-based flame retardant (DP-DE) containing phosphorus linked pendent groups was synthesized by a two-step esterification and polycondensation reaction,and characterized by Fourier transform infrared spectroscopy (FTIR),13C nuclear magnetic resonance (13C NMR) and gel permeation chromatography (GPC).After added into polyethylene terephthalate (PET) via melt-blending method,the flame retardancy of DP-DE were monitored by thermo-gravimetric analysis (TGA),limiting oxygen index (LOI) and underwriter laboratories(UL-94)test.The results showed that DP-DE display good flame retardancy in PET.And the thermal stability,char residue yield and LOI values of the composites increased with the increase of DP-DE content.The flame retarded mechanism of DP-DE was investigated by pyrolysis-gas chromatogram-mass(PY-GCMS),in situ FTIR,scanning electron microscope (SEM) and laser Raman spectroscopy (LRS).DP-DE was evidenced to release phosphorus acid and its derivatives,which catalyze carbonization and produce compact carbonaceous layers.Through protecting the polymeric materials substrate from further degradation and inhibiting the pyrolysis degree,both the amount and thermal stability of carbonaceous layers of DP-DE were improved,and hence enhancing the flame retarded performance of PET/DP-DE composites.
Keywords:High molecular weight DOPO-Based Flame retardant PET Mechanism
1.Introduction
Polyethylene terephthalate (PET) is a very important thermoplastic material displaying excellent properties such as fatigue resistance,chemical resistance,excellent mechanical strength and good processability,and it is commonly used as fibers,membranes,engineering plastics and bottles [1,2].However,PET resin is quite flammable and can burn easily and violently,thus preventing them from fulfilling fireprotection requirements in some special fields.The urgent flammability problem of PET has become an important issue and attracted extensively attention over the last few years.
Many methods have been developed to solve those challenges,among them,introducing flame retardant additives into PET via melt processing is the most common and practical one [3].The commonly applied additives can be mainly classified into halogen-containing flame retardants and non-halogenated flame retardants.The halogencontaining flame retardants have been proven to be toxic and environmentally hazardous [4].Among the non-halogenated additives,phosphorus-containing flame retardants have aroused public attentions as promising modifiers in the field of flame-retardant science due to their advantages of high flame-retardant efficiency and environment friendliness.
Among the various classes of phosphorus-containing flame retardants,9,10-dihydro-9,10-oxa-10-phosphaphenanthrene-10-oxide(DOPO) and its derivatives became popular due to their high thermostability caused by the existence of phenanthrene ring and O=P-O bonds in the molecule,strong durability of flame retardation as well as little side effect on the mechanical properties of polymers [5].Various researches have been reported on the application of DOPO-based flame retardants in polymers,which possess good fire inhibition characteristics and excellent char forming ability [6-8].König [9]synthesized a new phosphorus flame retardant methyl-DOPO with the excellent flame retarding behavior in polyurethane foam.Lin [10-12]found that the fire behavior of epoxy resins is significantly improved after modification by DOPO-based flame retardant with the phosphorus contents as low as 2-3 wt%.Nevertheless,the currently developed DOPO-based derivatives are of small molecular weight,as a result,they have poor compatibility with high molecular weight polymers and are easy to leach out.However,there are few papers about polymeric DOPO-based flame retardants with mentioned high molecular weight up to now[13-16].For example,Wang[16]synthesized a DOPO based polymeric flame retardant by condensation reaction between SPDPC and DOPOBQ.And it was found that with 10 wt%flame retardants added in epoxy resin,the system meets the UL-94 V0 classification and the LOI value of 30.2.Consequently,high molecular weight DOPO-based flame retardants have a broad development potential and will become one of the research emphases in the future since they are not volatile,have good flame retardance and compatibility with polymers as well as do not migrate from the volume of polymeric material to its surface [17].
In this study,a high molecular weight DOPO-based flame retardant(DP-DE) was synthesized via a two-step method.The first step is the melt polycondensation between one kind of DOPO-based derivative(DDP) and ethylene glycol (EG),through which DDPE oligomer with hydroxyl groups as the terminal groups was obtained.The second step is the esterification between DDPE and a tetracarboxylic acid D-P.Then,DP-DE was melt-blended with PET resin to enhance its flame resistance.PET/DP-DE composites with different DP-DE contents were finally obtained.The structure of DP-DE was characterized by Fourier transform infrared spectroscopy (FTIR) and1H,13C nuclear magnetic resonance(13C NMR).The molecular weight and molecular weight distribution were determined by gel permeation chromatography (GPC).The thermal performances of DP-DE were investigated by thermogravimetric analysis(TGA)and differential scanning calorimetry (DSC).The flame retardancy and thermal behaviors of PET/DP-DE composites were investigated by TGA,limiting oxygen index(LOI)and underwriter laboratories-94 (UL-94) test.The flame retarded mechanism of DP-DE in PET was analyzed by pyrolysis-gas chromatogram-mass(PY-GC-MS),in-situ FTIR,scanning electron microscope (SEM) and laser Raman spectroscopy (LRS),respectively.
2.Experimental
2.1.Materials
PET (bright chip,intrinsic viscosity:0.64dl/g) produced by Jiangsu Hengli Chemical Fiber Co.,Ltd.was used in this study.(9,10-Dihydro-10-(2,3-dicarboxypropyl)-9-oxa-10-phosphaphenanthrene 10-oxide(DDP)) was supplied by Zhejiang Research Institute of Chemical Industry Co.,Ltd.(Zhejiang,China).Ethylene glycol (EG),Pentaerythritol (PER),p-toluene sulfonic acid (p-TSA),N,N-dimethylformamide (DMF) and ethanol were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Tetracarboxylic acid D-P was prepared in authors Lab.Deionized water was purchased from Shanghai Jingchun Water Technologies Co.,Ltd.(Shanghai,China).
2.2.Synthesis of DP-DE
Typically,the DP-DE was synthesized in a 500 mL three-necked flask equipped with mechanical stirrer,a N2inlet valve and a condenser.DDP (1 mol),EG (1.2 mol) and p-TSA (0.01 mol,as a catalyst)were introduced.In order to remove the moisture and residual air,the reactor was purged with nitrogen alternatively before heating the reactor,then the reactor was immersed in an oil bath preheated to 170°C,and the melting mixture was stirred vigorously for 2 h.After that,the temperature of the system was increased to 190 °C for 2 h under a vacuum of 0.1 MPa.Thereafter,a light-yellow melt DDPE was obtained and D-P was added into this system according to a certain molar ratio(DDPE:D-P=4:1).The reaction parameters were changed to 200 °C,0.1 MPa for 3 h.Finally,the obtained DP-DE melt was poured into a beaker and cooled at ambient temperature to form a solid matter.The solid matter was crushed and dissolved in DMF,and then precipitated from DMF by adding deionized water,filtered,washed with plenty of ethanol,after that,dried in vacuum at 25 °C for 24 h.The synthetic procedure is illustrated in Scheme 1.
Briefly,the organic precursor D-P was synthesized by the esterification reaction and pre-condensation reaction between DDP and PER.The synthesis procedure of tetracarboxylic acid D-P is the same as that of DDPE with DDP and PER as monomer.And its molar ratio was 2:1.The synthetic procedure is illustrated in Scheme 2.
2.3.Preparation of PET/DP-DE composites
The PET was dried in the vacuum oven at 80°C for 18 h,then heated up to 130 °C for 12 h,meanwhile the DP-DE was dried in the vacuum oven at 30 °C for 24 h to remove moisture before use.Then PET was mechanically mixed with different amounts of DP-DE(0 wt%,2.6 wt%,5.1 wt%,8.0 wt%,11.6 wt%,respectively with different phosphorus content),thereafter,the mixtures were added to a two-screw extruder at the temperature around 260 °C to prepare PET/DP-DE composites.
2.4.Measurements
The Fourier transform infrared(FTIR)spectra were recorded using a Nicolet-20sx-B infrared spectrometer(USA)from 500 cm-1to 4000 cm-1with a spectral resolution of 4 cm-1and 32 scans.The samples were crushed into power,then mixed with certain amount of KBr powder and pressed into a tablet.The in situ FTIR spectra were recorded to monitor the thremodegradation process of composite in the range of 20-480 °C with a heating rate of 10 °C/min in air atmosphere.13C nuclear magnetic resonance (13C NMR) spectrum was recorded at 400 MHz on a Bruker 400 AVANCE spectrometer,with chloroform-d (CDCl3) as solvent and TMS (tetramethylsilane) as internal standard.Gel permeation chromatography (GPC) measurement was performed on Malvern GPC/SEC system with DMF as the mobile phase at the flow rate of 1 mL/min at 40 °C,and polystyrene was used as the standard specimen.
Thermogravimetric analysis (TGA) was performed using a Netzsch TG209 F1 thermoanalyzer.The sample with weight around 5-10 mg was put in the ceramic crucible and heated from ambient temperature to 800 °C at a heating rate of 20 °C/min in nitrogen atmosphere.Differential scanning calorimetry (DSC) measurement was operated using TA Q20 in nitrogen atmosphere.Approximately 5 mg sample was put in the alumina crucible and then underwent a heating and cooling cycle from 30-300 °C at the rate of 20 °C/min.The limiting oxygen index (LOI) were determined using a ZR-1 oxygen index meter(Qingdao,China) according to GB/T 2406.2-2009 standard with samples of which the dimensional size was 100 mm×10 mm×4mm.The underwriter laboratories-94 (UL-94) test was conducted on a LX-8820D-type vertical burning test instrument according to the GBT2408-2008 testing procedure using the samples of which the dimension was 130 × 13 × 3.2 mm3.
The scanning electron microscopy (SEM) measurement was performed by a HITACHI S-4800 microscope.All sample surfaces were coated with gold before observation.The laser Raman spectroscopy(LRS) measurement was carried out by Renishaw in Via-Reflex Raman micro spectrometer with excitation by a 514 nm helium-neon laser line focused a micrometer spot on the sample surface,and scanning in the 800-2000 cm-1region.Pyrolysis-gas chromatogram-mass (PY-GC-MS)analysis of PET and PET/DP-DE composites were measured by HP5890SA PY-GC-MS spectrometer (pyrolysis temperature:700 °C).
3.Results and discussion
3.1.Structural characterization of DP-DE
The structure of DP-DE was characterized by FTIR,13C NMR and GPC respectively.the corresponding results are shown in Fig.1 and Table 1.As shown in Fig.1(a),DP-DE shows typical O-H stretching vibration absorption at 3458 cm-1,C-H absorptions at 3065 cm-1and 2958 cm-1,C=O absorption at 1733 cm-1,P=O and C-O absorption at 1236 cm-1,P-O-C (aromatic) stretching absorption at 1201 cm-1,1119 cm-1and 913 cm-1[18,19].The molecular weight and molecular weight distribution of DDPE and DP-DE were determined by GPC as shown in Fig.1(b) the corresponding data are summarized in Table 1.The weight average molecular weight and polydispersity index of DPDE are 7961 g/mol and 1.76,respectively.The13C NMR and1H NMR spectrum was used to obtain more information of DP-DE structure in Fig.1(c) and (d).13C NMR spectrum are:d(C1)=28.79ppm,d(C2)=31.41ppm,d(C3)=35.19ppm,d(C4)=36.48ppm,d(C5-9)=62.35-68.72ppm,d(C10)=120.41-162.59ppm,d(C11)=170.75ppm,d(C12)=172.57ppm.1H NMR spectrum:d(H1)=0.83ppm,d(H2)=2.29ppm,d(H3)=2.58ppm,d(H4)=3.59ppm,d(H5,6,7)=4.17ppm,d(H8,9)=4.64ppm,d(H10)=7.24-8.03ppm.
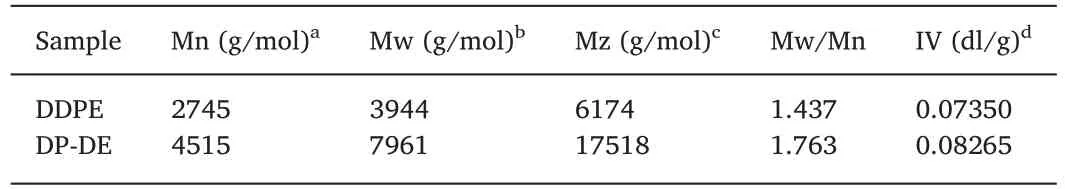
Table 1 GPC data of DDPE and DP-DE.
3.2.Thermal performances of DP-DE
The thermal stability of DP-DE was studied by TGA,and the corresponding TGA and DTG curves in nitrogen and air are shown in Fig.2(a).The initial decomposition temperature(Tonset),the maximum decomposition temperature (Tmax),and the yield of charred residue at 600 °C are summarized in Table 2.The Tonsetof DP-DE in air was 360.6 °C,which is slightly lower than that in nitrogen (364.9 °C) and much higher than the processing temperature of PET (240-290 °C),therefore DP-DE can satisfy the processing requirements of PET.Both in nitrogen and air,DP-DE underwent a decomposition step at 350-440°C with a maximum decomposition peak at around 413 °C,corresponding to the scission of phosphate ester bonds and the forming of carbonaceous layers [20],while in nitrogen,the other step appeared at 440-500 °C due to the difference of decomposition ways,which might be caused by the different decomposition actions of thermolabile fragments produced in the first step [3].The melting point of DP-DE was obtained from DSC curve in Fig.2(b),the Tmvalue of DP-DE was about 80.53 °C which is able to meet the requirements of general storage and processing.The small endothermal peak may be attributed to the star form of molecular structure of DP-DE which endow the organic flame retardant less capability to crystallize [21].
3.3.Thermal degradation behavior of PET/DP-DE composites
In order to investigate the thermal stability of PET and PET/DP-DE composites as well as explore the potential flame-retardant mode of action,TGA was performed.The temperature-dependent weight loss and the derivative of the weight loss curves for PET and PET/DP-DE composites in nitrogen and air at a heating rate of 20°C/min are illustrated in Fig.3.The characteristic temperatures of thermal degradation including Tonset,T50%and Tmaxas well as the yield of char residues at 700 °C are summarized in Table 3.
Compared with pure PET,the overall onset degradation temperatures of PET/DP-DE composites are little lower caused by the much lower decomposition temperature of DP-DE[22].T50%and Tmaxare the common parameters to characterize the thermal stability of polymers.Table 3 shows that the T50%and Tmaxvalues of composites resins increased with the increase of DP-DE contents in the system,suggesting the composite with higher DP-DE content to be more stable at high temperature.In addition,the char residues of polymers at high temperature are related to flame retardation as the yield of char residues formed in the thermal decomposition is proportional to the LOI value of polymer [23].The char yield of pure PET at 700 °C was 2.81%,while after melt blended with DP-DE,the char yield of the composites increased from 9.59% to 12.99%,indicating improved flame retardancy.
The DTG curves exhibit that the complete thermal degradation occurs in two steps for pure PET while only one step for PET/DP-DE composites.The common step from 350°C to 550°C is attributed to the decomposition and carbonization of PET chains,while the second step for PET over 550 °C might be caused by the decomposition of thermolabile fragments produced in the first step.The reason for the disappearance of the second step for PET/DP-DE composites might be as follows.The phosphorus groups in DP-DE first decomposed at low temperature to produce phosphorus-containing compounds,then crosslinking carbonization occurred between these compounds and PET chains,the formed phosphorus-rich char residue acted as a protective layer on the polymer surface against heat and oxygen diffusion,thus preventing the further decomposition of PET by raising the second decomposition temperature to a higher level which was much higher than 550 °C [18].
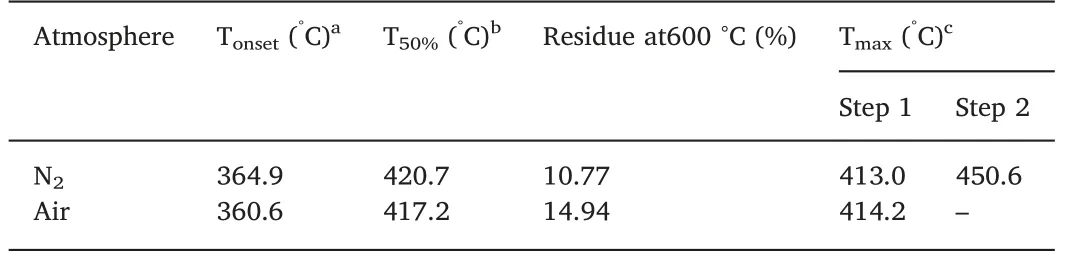
Table 2 TG and DTG data of DP-DE in Air and N2 at a heating rate of 20 °C/min.
The TGA results show that DP-DE can reduce the onset decomposition temperature of PET and promote the formation of carbonaceous layers on the surface.Meanwhile,DP-DE can form a protective layer on the carbonaceous layers,which can improve the thermal stability and yield of char residues,thus improving the flame retardancy of PET effectively.More direct evidences are given by SEM photographs in the following discussion.
3.4.Flame retardant properties of PET/DP-DE composites
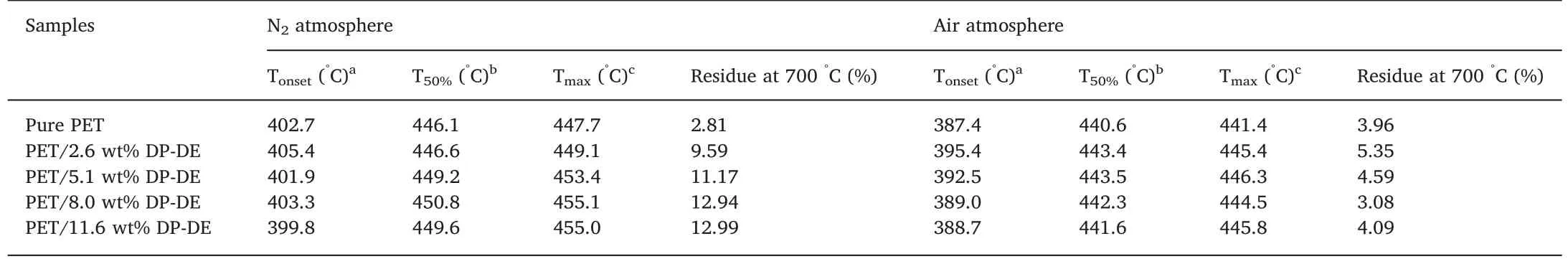
Table 3 TG and DTG data of PET and PET/DP-DE composites in N2 at a heating rate of 20°C/min.
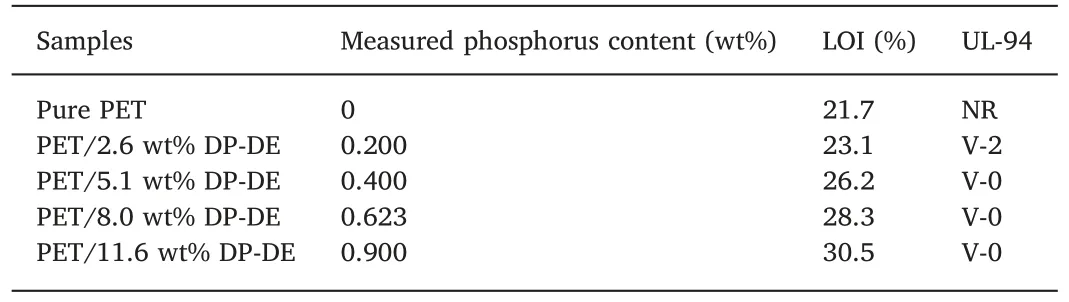
Table 4 LOI values and UL-94 test data of PET/DP-DE composites.
The flame-retardant properties of PET/DP-DE composites with different DP-DE contents were preliminarily estimated by TGA,then evaluated by LOI value and UL-94 tests.The corresponding data are shown in Table 4.It can be seen that the LOI value of pure PET was 21.7%,indicating that PET is quite flammable.The flame retardation of PET/DP-DE composite was enhanced with the increase of DP-DE contents as its LOI value increases from 23.1% to 30.5%.The UL-94 test results confirmed the flame retardancy of DP-DE.Compared with the NR rating of pure PET,the UL-94 rating of composite reached to V-0 rating when the DP-DE content reached to 5.143 wt%.TGA,LOI values and UL-94 test results together indicate that DP-DE can work as an effective flame retardant in PET to enhance its flame retardation and enlarge its application fields.
3.5.Flame retardant mechanism of DP-DE in PET
In order to investigate the mechanism of DP-DE in PET,PY-GC-MS was adopted to investigate the change of gaseous phase during the pyrolysis process.The related spectra and data are shown in Fig.4 and Table 5.As can be seen,the addition of DP-DE had little effect on the pyrolysis products of PET,indicating that the decomposition mechanism of PET is not changed.However,some relative concentrations of the products changed dramatically.The concentrations of large molecules from primary pyrolysis such as CH2=CHC6H4COOCH=CH2and C6H5COOCH2CH2OOCC6H5increase,while the concentrations of small molecules from further pyrolysis,which also play a critical role in the burning process such as CO2and CH3CHO decrease [24].These illustrate that DP-DE can promote the initial decomposition of PET,which is consistent with the reduced onset decomposition temperatures in TGA,inhibit the further degradation of PET and decrease the production of combustible gases,and therefore suspending combusting and spreading.
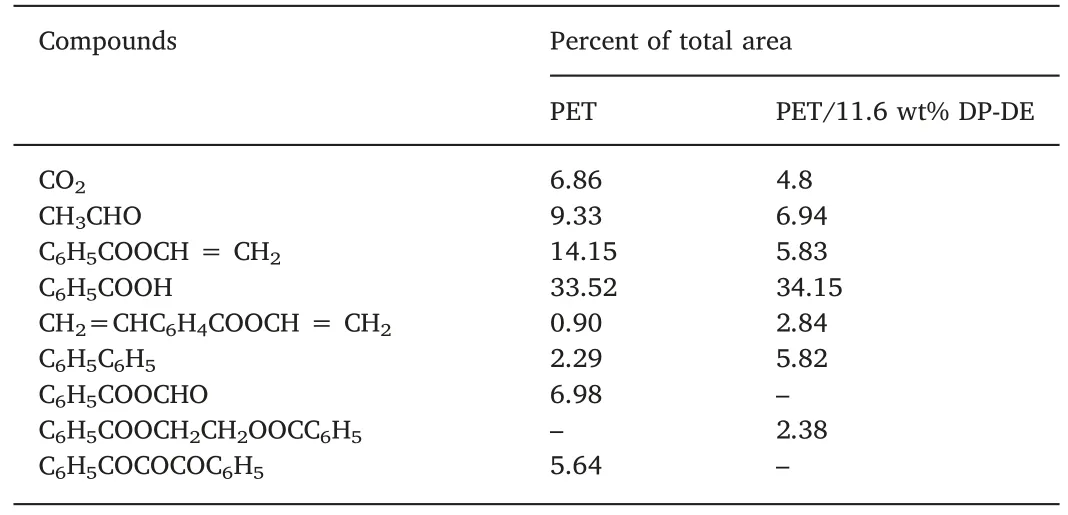
Table 5 Identification of the main components in gas chromatography.
The in situ FTIR spectra monitored the degradation process of PET/11.6 wt% DP-DE at different temperatures as shown in Fig.5.
The O-H stretching vibration shifts from 3431 cm-1to 3513 cm-1due to the reduction of hydrogen bond through the dehydration reaction during the degradation process.The thermal stability of polymer is usually studied by the intensity changes of -CH2,-CH3and C=O vibration peaks at 2963 cm-1,2907 cm-1and 1722 cm-1,respectively.As shown in Fig.5,the intensities of above peaks decreased rapidly with increasing the temperatures,and almost disappeared at 460 °C,indicating that PET chains have degraded seriously.This is consistent with TG spectrum of PET/DP-DE in N2atmosphere,where 460 °C corresponds to 69.4% weight loss (the actual weight loss in air will be bigger than that in N2).The absorption bands associated with the aromatic rings at 1600-1400 cm-1decreased substantially and almost disappeared at around 420°C,while new bands appeared subsequently at 1563 cm-1and 1375 cm-1,corresponding to C=C stretching vibration,indicating that the main chains of PET were broken gradually to form graphite char residues with increasing the thermo-oxidative degradation temperature.
It can be observed that he absorption band of P=O at 1250 cm-1was still clear over 460 °C,suggesting the existence of poly (phosphoric acid) at high temperature.The wide peaks at about 1000 cm-1can be assigned to the various phosphorus oxides and phosphate-carbon complexes,and the intensities of new peaks at 817 cm-1and 739 cm-1over 440 °C increased with the increasing temperature.The peaks at 1018 cm-1and 739 cm-1belong to PO2/PO3in phosphate carbon complexes,while the peaks at 1088 cm-1and 817 cm-1were assigned to the symmetric and asymmetric vibration of P-O-P,indicating the formation of compounds containing P-O-P in the char residuals,such as P2O5and P4O10.The P-O-P group is considered as a crosslinker linking to different aromatic species,resulting in the formation of phosphoruscarbon complexes [25].In addition,the wide band around 2300 cm-1can be assigned to CO2from degradation of PET or O-H stretching vibration in phosphorus compounds with its intensity increases apparently over 400 °C [26].
The analysis result of in situ FTIR shows that DP-DE could produce phosphate or poly(phosphoric acid)which cover on the matrix surface to promote the formation of carbonaceous layers during the degradation process.The layers can work as a barrier to insulate heat and oxygen,thus interrupting the combustion process.
In order to better understand the flame-retardant mechanism of DPDE,the surface morphologies of the residue chars were observed by SEM microphotographs as an important criterion to define the efficacy of DP-DE to the char formation[27].The detailed images are shown in Fig.6.
For original PET,the residue is a fractured char with lots of cracks and big holes dispersing on its surface due to insufficient char-formation on the burning materials surface.It is susceptible to crack and cannot effectively prevent the heat and flammable gas from the underlying PET during combustion,thus the flame-retardant property is poor [28].However,for PET/DP-DE composites,the SEM images displayed the continuous,intact and intumescent outer surfaces.They are the insulating barriers to prohibit combustible gases and feedback of heat from reaching the underlying substrate,consequently reduce the heat release rate and slow the combustion process considerably.These surfaces became more compact with less cavities and pores as the DPDE content increased from 2.6 wt%to 11.6 wt%.This may be caused by that,the decomposition products of PET could react with phosphoric acid or polyphosphoric acid(decomposition products of DP-DE)to form crosslink network,the network could cover on the char surface and enhance its mechanical performance,protect it from destroying by flammable gases and heat,so as to improve the integrity,thermal stability and flame retardation of carbonaceous layers.
To further study the chemical structure of char residues,Raman spectroscopy may be more helpful,as it is a suitable method to characterize carbonaceous materials formed during combustion.The spectra of residual chars for PET and PET/DP-DE composites are shown in Fig.7.All testing samples exhibited two characteristic bands,namely Gband(1580 cm-1,corresponds to an E2gmode of hexagonal graphite and is related to the vibration of sp2-bonds carbon atoms in graphite layers,showing the graphitic structure) and D-band (1360 cm-1,associated with vibration of carbon atoms with dangling bonds in the plane terminations of disordered graphite or glass carbons,representing the unorganized carbon structure)[29-31].As the relative ratio of IDand IG(IDand IGare the integrated intensities of D and G bands,respectively)is inversely proportional to an in-plane microcrystalline size,the bigger value of ID/IGindicates the smaller the size of carbonaceous microstructures,suggesting better flame retardation.Fig.7 shows that the ID/IGratio of composite is higher than that of pure PET,indicating that composite possesses smaller carbonaceous microstructure and better flame retardation,which also proves the former fire test results.These observations provide a positive evidence for the formation of polyaromatic species or graphitic structures,also prove the conclusion of in situ FTIR.
According to the results of PY-GC-MS,in situ FTIR,SEM and Raman,the possible flame-retardant mechanism of DP-DE is that,DP-DE firstly was oxidized to release various phosphoric acid and its derivatives in the heating process,the acids as the dehydrant reacted with PET,then promoted its initial decomposition and the formation of stable charred structures containing P-O-P and P-O-C complexes in the condensed phase.The formed carbonaceous layers can prevent the evolution of combustible gases,slow down heat and mass transfer,and prevent further degradation of the underlying polymer,consequently reducing the flammability of PET [32].
4.Conclusions
The high molecular weight DOPO-based flame-retardant DP-DE has been successfully synthesized by the reaction between DDP-containing oligomer and tetracarboxylic acid.Its chemical structure and performances have been analyzed by FTIR,13C NMR and TGA,etc.DP-DE containing phosphorus linked pendent groups show good flame retardancy in PET matrix due to its special structure and high phosphorus content.TGA results show that the thermal stabilities and char residues of PET/DP-DE composites are enhanced.The LOI values of the composites increase to 30.5% when 11.6 wt% DP-DE are added.The UL-94 of the composites reaches to V-0 rating.The flame retarded mechanism of DP-DE has been investigated by PY-GC-MS,in situ FTIR and SEM,etc.The results show that DP-DE exerts its function in PET mainly through promoting the initial decomposition of PET by releasing phosphoric acid and its derivatives to form stable carbonaceous layers on the polymeric substrate,decreasing the concentration of combustible gases and inhibiting the transfer of heat and mass under the protection of carbonaceous layers and acids.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was supported by Shanghai academic and technological leaders’ program (16XD1424500),the National Natural Science Foundation of China (No.51473035) and the Program for Innovative Research Team in University (IRT1221).
 Progress in Natural Science:Materials International2020年2期
Progress in Natural Science:Materials International2020年2期
- Progress in Natural Science:Materials International的其它文章
- Introduction of manganese based lithium-ion Sieve-A review
- Hollow bismuth ferrite combined graphene as advanced anode material for sodium-ion batteries
- Magnetically separable and visible light-active Ag/NiCo2O4 nanorods prepared by a simple route for superior photodegradation of atrazine in water
- Solid electrolyte composite Li4P2O7-Li3PO4 for lithium ion battery
- Wearable supercapacitor self-charged by P(VDF-TrFE) piezoelectric separator
- Enhanced superconducting performance in solution-derived YBCO-BaZrO3 composite film
