Solid electrolyte composite Li4P2O7-Li3PO4 for lithium ion battery
Evvy Krtini,Vlentin Yprii,Heri Joi,Mykel Mnwn,Cipt Pnghegr,Whyuiningsih
a Center for Science and Technology of Advanced Materials,National Nuclear Energy Agency,South Tangerang,15314,Indonesia
b Faculty of Engineering,Leeds University,UK
c Energy Engineering,Jakarta State Polytechnic,Depok,Indonesia
d Polytechnique Institute of Nuclear Technology,Yogyakarta,Indonesia
ABSTRACT The researches on solid electrolyte have been significantly increasing due to the safety problem in lithium ion battery.The lithium phosphates are chosen due to environmentally friendly.In the present study Li4P2O7 was synthesized by solid state reaction using NH4H2PO4 and Li2CO3 with the ratio 1:2 at various temperatures of 600 °C,800 °C and 900 °C.The products were characterized by x-ray diffraction,scanning electron microscopy and impedance spectroscopy.The x-ray diffraction showed that all samples consisted of two phases.It was found that the products consisted of 52.44% Li4P2O7 and 47.56% LiPO3; 93.56% Li4P2O7 and 6.44% Li3PO4; and 46.27% Li4P2O7 and 53.67% Li3PO4 under the synthesizing temperature of 600 °C,800 °C and 900 °C,respectively.The highest ionic conductivity of 3.85×10−5 S/m was achieved for composite Li4P2O7-Li3PO4 with the highest content of 93.56%Li4P2O7.This conductivity is higher compared with single phase of LiPO3,Li3PO4 and Li4P2O7.The increase in ionic conductivity may be due to the mixed anion effects related to the phosphate networks,and it also corresponds to the existence of anorthic phase Li4P2O7 with the space group P −1(2).The crystal lattice analysis showed that the reactant Li4P2O7 consisted of diphosphate groups P2O7.The lithium tetrahedral LiO4 were linked to P2O7 groups formed a continuous framework containing large voids,available for Li+ ion transport,and thus it exhibited high conductivity.A composite Li4P2O7-Li3PO4 is a promising solid electrolyte for solid state battery.
Keywords:Solid electrolyte LiPO3 Li3PO4 Li4P2O7 Ionic conductivity Lithium ion battery
1.Introduction
Recently,the study on lithium ion battery is paid more attention due to its various advantages,such as higher power,better energy density,lighter,longer life cycles.Lithium ion battery has various applications in electronic devices and promising energy storages for electric vehicles and renewable energy resources [1,2].Nevertheless,the safety still remains the main issue in developing lithium ion battery due to several fact,i.e.combustion that occurs when overcharging caused by separator materials and liquid electrolyte [3].Considering the safety of the lithium ion battery,the solid electrolytes with lithium ion conductivity are intensively investigated at present because of their use in power sources[4-6].Solid electrolyte based Phosphate Oxide materials are easy for preparation,and has strong glass forming character,low melting points and simple composition.Lithium phosphate (Li3PO4) is one of the promising solid electrolyte materials for lithium-ion battery because of its good application in thin film solid state battery [4].Although lithium phosphate (Li3PO4) consisted of the best glassy solid electrolytes,this material possesses very low conductivity due to a large bulk resistance [7].Therefore Li3PO4thin layer materials are widely used as the solid electrolyte in the thin film battery to reduce the resistance value [8].Many studies have aimed to increase the conductivity of phosphate-oxide-based conductors.Some of these approaches included mixing two or more different kinds of anion such as Al2O3and TiO2to the Li2O-P2O5system to produce new systems of Li2O-Al2O3-Ti2O-P2O5(LATP) [9]and Li2SO4-Li2O-P2O5[10]and Li2O-V2O5-P2O5[7].Recently,the composite montmorillonite (MMT)material containing alumina and silica was added to modify the Li2O-P2O5system due to its low cost,non-toxicity,and natural abundance [11].The electrochemical characterization indicated that the conductivity value of the samples was greater than that of Li2O-P2O5or Li3PO4.
The crystal structure of Li3PO4has been previously studied by x-ray diffraction[12].A crystalline form of Li3PO4had been prepared by two different methods,wet chemical reaction (WCR) and solid-state reaction (SSR).The WCR results showed that Li3PO4belongs to orthorhombic unit cell of β-Li3PO4with space group Pmn21 [13].Meanwhile,Li3PO4powder prepared by solid-state reaction belongs to orthorhombic unit cell of γ-Li3PO4with space group Pmnb and another phase of Li4P2O7[14].The impurity of Li4P2O7was due to phase transformation in solid state reaction during quenching of molten mixture from high temperature[15].Previous data on crystal structure of Li4P2O7at room temperature have been obtained by X-ray analysis of single crystals,but the results are controversial.One model was monoclinic,space group P21/n,and another model was interpreted as triclinic,space group P-1.The latest result by Voronin et al.confirmed the transformation from triclinic low temperature to high temperature phase of triclinic [5].In this case,a possibility of triclinic low phase is more relevant,though the sample was heated up to 800 °C.The quenching was intended to avoid crystallization at lower temperature[16].Therefore,the high form of monoclinic Li4P2O7could not be obtained at room temperature,though was quenched from above the melting point of the Li4P2O7.The crystals are always the low temperature form (anorthic).These facts indicated that the diffraction pattern of the Li4P2O7on this solid state reaction sample belong to the low temperature form of anorthic Li4P2O7(P-1 (2)) [17].The crystal lattice of Li4P2O7consists of diphosphate groups P2O7arranged by two PO4tetrahedral having a common corner [5].The lithium tetrahedral LiO4are linked to each other and to P2O7groups by a common vertex or edge,and form a continuous framework containing large voids,available for Li+ion transport.Thus,the lithium diphosphate Li4P2O7has rather high conductivity (~10−1S/m at 925 К).In contrast,the high phase (symmetry P n m a (62)) of γ-Li3PO4was obtained during solid state reaction,as described in previous work [3].
Although the Li3PO4and Li4P2O7ionic conductors have been intensively reported,there is only few papers that present complete discussion on both crystals.Further investigation need to be done to study the mixed crystals Li3PO4-Li4P2O7phenomena.It still remains the question whether the Li4P2O7is responsible to the increase in conductivity in Li3PO4.The aim of the present study is to synthesize Li4P2O7by solid state reaction using NH4H2PO4and Li2CO3with the ratio 1:2 at various temperatures of 600 °C,800 °C and 900 °C,and to characterize the reactant by x-ray diffraction,a scanning electron microscopy,and impedance spectroscopy,in order to investigate the influence of the phase composition and the homogeneity on the ionic conductivity.
2.Experimental
The solid electrolyte Li4P2O7was prepared by solid state reactions.Appropriate amount of NH4H2PO4crystal were ground into fine powder in an agate mortar before mixed with Li2CO3.The ratio between NH4H2PO4and Li2CO3is 1:2.The mixtures were placed into three ceramic-crucibles,then were stirred for approximately 5 min to ensure they mixed well.They were sintered in a furnace up to three different temperatures of 600 °C,800 °C and 900 °C,in order to allow reactants disintegrates and avoid solids ejecting from the crucible by decomposing gases.After heating the mixtures at 600 °C,at 800 °C and 900°C.800 °for 3 h,they were quenched into liquid nitrogen (N2).The solid state reactions mechanism is described below by equation (1).
As shown in the reaction,the target of the reaction was obtaining Li4P2O7.When the three products reached room temperature,the appearance after quenching(both)were shown to be white solid bulk,and rather hygroscopic especially for the 600 °C.Therefore further treatment in the dry atmosphere was necessary.They were finely ground again and reheated up to 120 °C for 3 h to remove any moisture left,resulting white fine powder.The products were denoted by the composites 600 °C,800 °C and 900 °C,for the samples with heating temperatures at 600 °C,800 °C and 900 °C,respectively.The detail experiment has been described elsewhere [6,18].
The three samples then were characterized by several methods as described below.The crystalline phase analysis of the powder samples were characterized by X-Ray Diffractometer (XRD)with the scan range from 10 to 80 [2θ],step scan of 0.02 [2θ]and time per step of 27 [s].The microstructure of the powder sample was measured by a scanning electron microscope(SEM).The electrical properties were carried out in the frequency range from 42 Hz to 1.5 MHz using impedance spectroscopy (IS).The sample preparation and characterization were performed at the Integrated Battery Laboratory,National Nuclear Energy Agency,Indonesia.
3.Results and discussion
3.1.Crystal structure
The X-ray diffraction data from various quenching temperatures(600 °C,800 °C and 900 °C) are analysed by Rietveld refinement method.Fig.1 shows a good agreement between the experimental data and the fitted curve,further confirmed by the r-factors below 5%for all the refinements.The complete result is tabulated in Table 1.In general,the refinements results for all samples 600 °C,800 °C and 900 °C that sintered at various temperatures consisted of two phases.The phase contents of the samples are as follows.The sample 600 °C consisted of 52.44% Li4P2O7and 47.56% LiPO3,the sample 800 °C consisted of 93.56% Li4P2O7and 6.44% Li3PO4,and the sample 900°C consisted of 46.27% Li4P2O7and 53.67% Li3PO4.The heating temperatures and quenched rate leads to different phases and fraction.Based on the phase diagram,at 600 °C,the materials have not fully melted yet,and it tent to solidify quickly forming glasses LiPO3.Similar process occurred when preparing the glasses LiPO3from the parent materials Li2O-P2O5[16,19].The product can be called composites since it consisted a glass and crystalline materials,similar to other compositions which contained two crystalline phases.
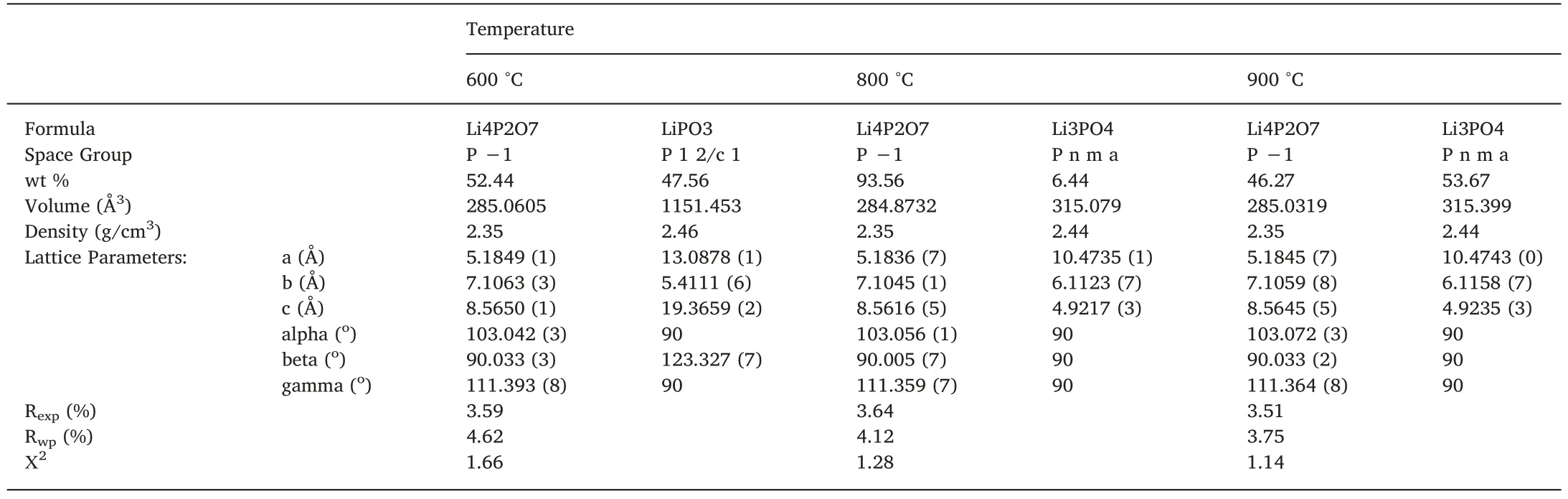
Table 1 Rietveld refinement result of quenched samples at 600 °C,800 °C and 900 °C.
At higher temperatures 800 °C,the molten mixture of Li2O-P2O5stoichiometrically formed the target material Li4P2O7with the maximum 93.56%.Interestingly the rest of the reactant was belong to 6.44% Li3PO4.The formation of crystal Li3PO4might due to chemical reaction at high temperature,some lithium and oxygen are bond to the un-bridging Li4P2O7,following reactions Li2O+Li4P2O7~2(Li3PO4).Similar process was obtained by heating the materials up to 900 °C,then quenched into liquid nitrogen.However,by further increasing temperature the fraction of Li4P2O7reduced,which may due to over solubility limit of Li2O-P2O5.Those results showed that no single phase of Li4P2O7could be obtained through the solid state reaction mechanism,and the quenching rate would give various products of the composites.However,the optimum product of 93.56% Li4P2O7was achieved at quenching temperature 800 °C.
The crystal structure of Li4P2O7was interpreted as triclinic,space group P-1,as confirmed by Voronin et al.[5].In this study,a possibility of triclinic low phase is more relevant,though the sample was heated up to 800 °C.The quenching was intended to avoid crystallization at lower temperature [16].The crystals are always the low temperature form (anorthic).These facts indicate that the diffraction pattern of the Li4P2O7on this solid state reaction sample belongs to the low temperature form of anorthic Li4P2O7(P-1(2))[17].The lattice parameters of anorthic phase for composite 800 °C are a=5.1836 (7) Å,b=7.1045(1)Å and c=7.1045(1)Å,and for composites 600°C and 900°C are similar,as listed in Table 1.The crystal structure of anorthic Li4P2O7is depicted in Fig.2a.
Fig.2c and d shows the difference between the high and low symmetry of Li3PO4.The crystal structure of γ-Li3PO4belongs to the orthorhombic phase P n m a (62) called high symmetry structure,meanwhile for β-Li3PO4belongs to the orthorhombic phase P m n 21(31) called low symmetry structure.The lattice parameters of the orthorhombic unit cells of the two polymorphs are:β-Li3PO4:a=6.1150,b=5.2394,c=4.8554 Å and γ-Li3PO4:a=6.1147,b=10.475,c=4.9228 Å.Hence the β-γ transformation was accompanied by an increase in volume of~1.4%which was largely caused by expansion of the c-axis,as shown in Fig.2d.In this case,the high symmetry phase of γ-Li3PO4was obtained during solid state reaction,as described in previous work[3].The formation of γ-Li3PO4was due to heating treatment applied to the sample above their β to γ transition temperature of 800°C that was high enough to transform the β to γ-Li3PO4phases.The crystal structure of Li3PO4obtained by solid state reaction belongs to orthorhombic unit cell of γ-Li3PO4with space group of Pmna,which is confirmed by the lattice parameters a=10.4735(1)Å,b=6.1123(7)Å,and c=4.9217 (3)Å,reflecting the high symmetry structure γ-Li3PO4(Table 1,Fig.3d).This heat treatment affects the length and strength of the bonds,resulting in the volume expansion of the crystal structure of the β-Li3PO4and transform to γ-Li3PO4,as shown in Fig.2c and d,respectively [12,17].Thus the ionic conductivity of γ-Li3PO4prepared by solid state reaction was~3 × 10−7S/m,which is higher than the ionic conductivity of β-Li3PO4obtained via wet chemical reaction~4 × 10−8S/m [12].The increasing ionic conductivity of γ-Li3PO4may be due to the mixed crystal structures that increased Li-ion mobility,and also the volume expansion due to the transformation from β to γ-Li3PO4phases.The results of this study have shown the existing of mixed crystalline phases in new composite of Li4P2O7-γLi3PO4that always occur when preparing either Li3PO4or Li4P2O7via solid state reaction.
Fig.2(a,b,c,and d) shows the crystal structure of all three phases that present in the samples,namely LiPO3,Li4P2O7,β and γ-Li3PO4,respectively.All lithium and phosphorus atoms bound to 4 oxygen atoms make tetrahedral coordination.The average bond distances of Li-O are about 1.953 Å for Li4P2O7,1.963 Å for LiPO3and 1.975 Å for Li3PO4.This meaning that the lithium atom in LiPO3is tightly bound followed by Li4P2O7and Li3PO4,respectively.The complete list of bond distances are shown in Table 2.The density of LiPO3(2.46 g/cm3) was also higher compared to Li3PO4(2.44 g/cm3)and Li4P2O7(2.35 g/cm3),indicating that the lithium atom is less free to move inside the LiPO3crystal followed by Li3PO4and Li4P2O7.
3.2.Microstructure analyses
The morphology analysis of Li3PO4for the samples 600 °C,800 °C and 900 °C are shown in Fig.3(a-d).All the entire images of SEM showed the morphology of composites obtained by backscattered electron.In this image,the backscattered electron determined the density difference of atom in LiPO3,Li3PO4,or Li4P2O7.Fig.3a shows the morphology of Li3PO4prepared by wet chemical reaction for comparison.The atoms with larger density would produce brighter colour than the atoms with smaller density [20].The grains were well dispersed homogenously [20].As shown in Fig.3b,the specimen covering most entire volume with brighter colour might come from the agglomeration of glasses LiPO3after quenched at 600 °C.This may be due to lower temperature than the melting of Li3PO4and Li4P2O7.The sample quenched at lower temperature tent to produce glasses [9,16].The SEM image shows distinct pictures of two phases containing LiPO3and Li4P2O7but it is rather difficult to calculate the average particle size from the SEM data.The difference in heat treatment and quenching rate produced two phases with different compositions,various morphology and sizes.
3.3.Electrical properties
The shape of Impedance spectra of the samples synthesized at 600°C,800°C and 900°C are shown in Fig.4.The spectrum of Li3PO4is also shown for comparison.The spectra were obtained from the range frequency of f=42 Hz-5 MHz.There of them were clear semicircle loops observed in all spectra,and a semicircle could be analogous to an electrical circuit consisting of a resistor and a capacitor in parallel connection.Impedance diagram of sample 800 °C exhibited two semicircles which correspond two capacitive loops with two capacitive time constant.The first loop give resistance value at R1~1.61 × 106Ω for first loop and~2.35 × 106Ω for second loop.The higher frequency region in this observation can be interpreted as the response of the bulk to the electric field.The lower frequency region can be interpreted as response from grain boundary in the sample.Moreover,the resistance,R1,of the sample synthesized at 600 °C was 1.63 × 107Ω while at 800°C was 1.83×108Ω.In addition,the sample synthesized at 900°C has only one semicircle and exhibited the resistance of 1.43 × 107Ω.Distorted and depressed semicircles may arise from the overlap of semicircles with various time constants,distributed microscopic properties,etc.[11].Fig.4 shows the Impedance Spectra plot for Li3PO4and all samples,and the resistance properties of fitted semicircle is summarized in Table 3.
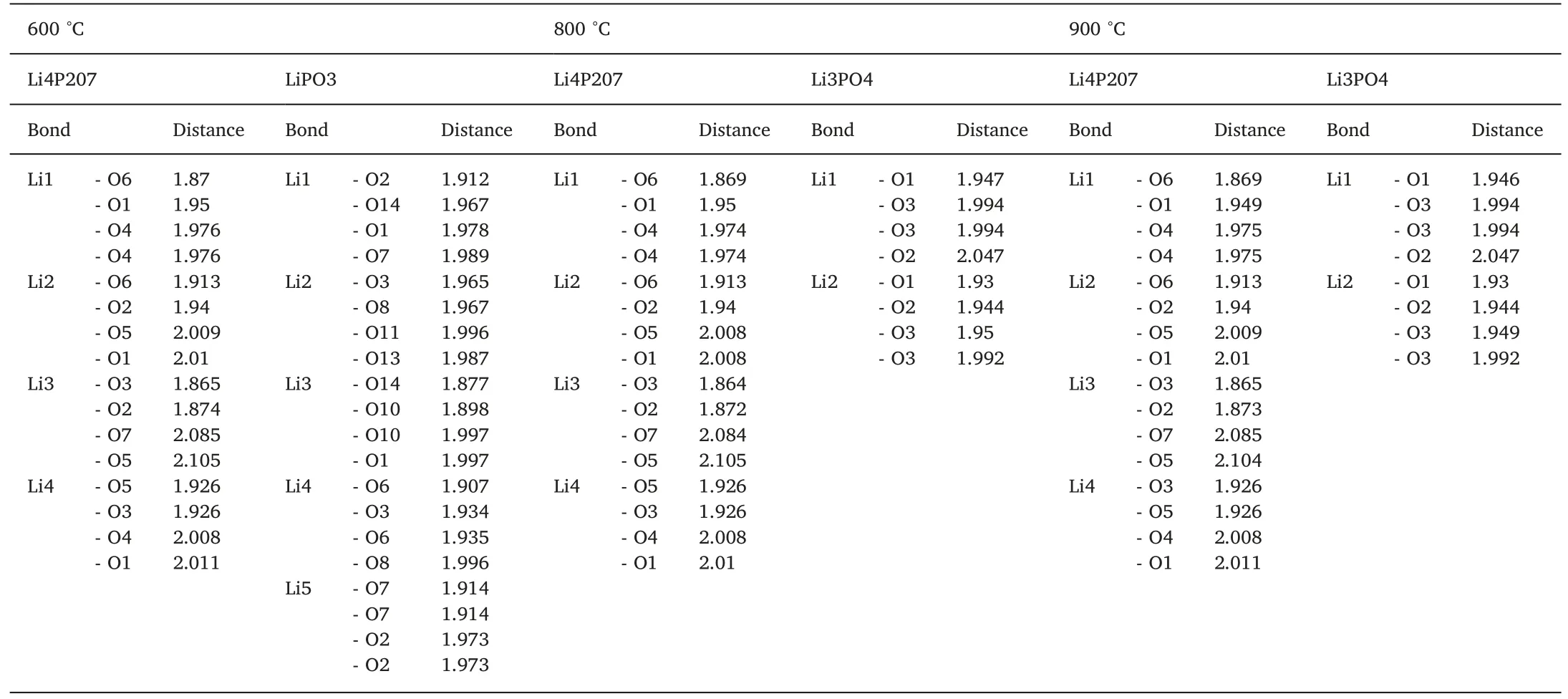
Table 2 Bond distance calculation of quenched samples at 600 °C,800 °C and 900 °C.

Table 3 Semicircle fitting parameters of Cole-Cole Plots and Conductivities samples at 600 °C,800 °C and 900 °C,including Li3PO4.
The conductivity curve data obtained from LCR measurement are fitted according to Jonscher power law on equation (2) as shown in Fig.5.The fitted curves are represented as dashed line in Fig.5.The conductivity obtained from measurement was total conductivity(σtotal)determining both DC labeled as σDCand AC conductivity represented as Aωnwhere n is power law constant (0 < n < 1).The composite 900 °C data curve fitted with the power law while the others,600 °C and 800 °C,only fit at the lower frequency.
Furthermore,the conductivity data measured by LCR meter are listed in Table 3.As shown in Fig.5,the highest conductivity was owned by composite at 800 °C by the value of 3.85 × 10−5S/m.Moreover,the composite 600°C exhibited the lowest conductivity value 2.78 × 10−7S/m and the composite 900 °C conductivity was 4.52 × 10−6S/m.The highest conductivity (3.85 × 10−5S/m) was reached at 800°C,which is higher than the total conductivity of Li3PO4prepared by wet chemical reaction and solid state reaction,namely 6.30×10−6S/m and 7.70×10−6S/m,respectively[17].It is slightly higher compared with bulk conductivity investigated by Ayu eat al.2.7×10−5S/m[12].The underlying lithium ion diffusion mechanism are more mobiles,in the system which consist mixed phases,such as Li3PO4and Li4P2O7.The lithium diphosphate Li4P2O7has rather high conductivity(~10−1S/m at 925К)[5].The addition of excess Li2O into the glass former system P2O5will raise the ionic conduction,which may be due to lithium ions Li+diffusion and enlargement of the phosphate network [3,7].It was found that the heat treatment during formation and the quenching rated are also responsible on forming the final product of the composites.The synthesized temperature variation leads the different phase compositions.The synthesis of only single phase Li3PO4or Li4P2O7could not be achieved by solid-state reaction.However,the new composite Li4P2O7-Li3PO4produced better conductivity that can be applied as solid electrolyte in a rechargeable battery.
4.Conclusion
The synthesized of single phase of Li4P2O7can't be achieved by solid state reactions.The products of the solid state reaction contain two phases of Li4P2O7and LiPO3or Li3PO4,depending on the quenching temperatures 600°C,800°C and 900°C.The highest Li4P2O7volume fraction of 93.27% can be achieved for the synthesizing temperature of 800 °C,with the rest product of 6.63% Li3PO4.This composite Li4P2O7-Li3PO4exhibits the highest ionic conductivity of 3.85×10−5S/m,which is two orders higher compared with the single phase of Li3PO4and Li4P2O7.The best composite is achieved for lithium-phosphate system.The new composite Li4P2O7-Li3PO4is a promising candidate for solid electrolyte in all solid state lithium ion battery.The application of the composite solid electrolyte will improve not only the safety due to the high temperature resistance,but also replacing both liquid electrolyte and the separator,which commonly used in commercial battery.
Declaration of competing interest
There is no conflict of interest.
Acknowledgement
This work is financially supported by the Ministry Research Technology and Higher Education through the Research Grant of National Innovation System Consortium (INSINAS) with the contract no.01/INS-1/PPK/E2/2018 and IRPK-2018-148.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pnsc.2020.01.020.
 Progress in Natural Science:Materials International2020年2期
Progress in Natural Science:Materials International2020年2期
- Progress in Natural Science:Materials International的其它文章
- Mechanical behavior and microstructure evolution for extruded AZ31 sheet under side direction strain
- Kinetic analysis and strain-compensated constitutive models of Ti-42.9Al-4.6Nb-2Cr during isothermal compression
- First-principles comprehensive study of electronic and mechanical properties of novel uranium hydrides at different pressures
- Crystallite growth characteristics of Mg during hydrogen desorption of MgH2
- Influence of Cr content on the microstructure and mechanical properties of CrxFeNiCu high entropy alloys
- Microstructural characterization and hydrogenation performance of ZrxV5Fe(x=3-9) alloys
