Magnetically separable and visible light-active Ag/NiCo2O4 nanorods prepared by a simple route for superior photodegradation of atrazine in water
Ahme Shawky,Maha Alhaa,R.M.Mohame,Nasser S.Awwa,Hala A.Ibrahium,e
a Nanomaterials and Nanotechnology Department,Advanced Materials Division,Central Metallurgical R&D Institute (CMRDI)P.O.Box 87 Helwan,11421,Cairo,Egypt
b Department of Mechanical Engineering,School of Engineering,The University of Tokyo,7-3-1 Hongo,Bunkyo-ku,Tokyo,113-8565,Japan
c Chemistry Department,Faculty of Science,King Abdulaziz University,P.O.Box 80200,Jeddah,21589,Saudi Arabia
d Research Centre for Advanced Materials Science (RCAMS),King Khalid University,P.O.Box 9004,Abha,61413,Saudi Arabia
e Department of Biology,Nuclear Materials Authority,P.O.Box 530,El Maadi,Egypt
ABSTRACT Atrazine (ATZ) is a known herbicide used in agriculture and caused many concerns according to its toxicity.Herein,we have prepared NiCo2O4 nanorods that can work as a photocatalyst under visible light exposure by Ag loading through a simple route.The characterizations revealed the role of Ag for improving the structural,optical and optoelectronic properties of the produced photocatalyst.The Ag loading with 5 wt% confirms the minimization of the bandgap from 3.6 down to 2.57 eV with enhanced visible light absorption and sustaining the magnetic properties.The Ag/NiCo2O4 nanorods showed a superior photodegradation of ATZ within 20 min at 2.0 g L-1 with excellent recyclability.The enhancement of the photocatalytic activity is referred to the local surface plasmon resonance of Ag-loaded photocatalyst.This reduces the electron-hole recombination and outperforms the unloaded sample.This work is highlighting the application of magnetically separable spinel photocatalysts for the removal of herbicides and water remediation.
Keywords:Spinel photocatalyst Visible light Photodegradation Atrazine Magnetic separation
1.Introduction
Water pollution represents the current main issue facing humankind.Still,the use of herbicides is necessary for the agriculture and food industry [1,2].Atrazine (ATZ),is one of the globally used herbicides that can govern the grass and broadleaf weeds.Another function for ATZ is shielding the crops from other surroundings like humidity and nutrients.The problem of accumulation of ATZ in water become a significant matter [3,4].So,it was disqualified from many countries due to according to its harmful effects [3,5,6].Thus,searching for a sustainable way to eliminate ATZ becomes a must.Photocatalysis signifies a promising tool for the removal of organic pollutants[7-9].It is simple,green,and alternative to the most common techniques like reverse osmosis and coagulation [10,11].Oxide semiconductors are a typical example of photocatalysts such as TiO2,ZnO,and others[9,12,13].However,these conventional photocatalysts are suffering from disadvantages that are affect the realization of its application.Some of these are instability [14],enormous bandgap energy (Eg) that limiting the light absorption [7-9],separation,and recovery of the spent photocatalyst [15].Hence,it is crucial to advance a stable photocatalyst that compatible with visible light and having a feature being separable and recoverable as well.
Currently,the spinel-structured binary oxide photocatalysts have grown much attention due to its various applicability in environmental remediation,water splitting,and CO2reduction [16].This type of material has magnetic and optical properties that could be tuned during the preparation process [17].Between them,Nickel cobaltite (NCO)showed promising advances in various fields,including supercapacitors[18],solar cells [19],and catalysis according to its flexibility of assembly and ecofriendly[20,21].However,the fast recombination of the photogenerated carriers represents the central issue of the NCO application [22].Consequently,some efforts are made by coupling with other materials to overcome this problem [23,24].Among that,the decoration of NCO with metal nanoparticulate would enhance the light absorption,reduce the optical bandgap (Eg),and increase the charge separation due to the surface plasmon resonance effect (SPR) [25,26].Moreover,there was no published studies on the photocatalytic removal of herbicides by NCO.Thus,combining a facile synthesis for NCO and loading with metal nanoparticles would be of great significance for the elimination of ATZ.
Herein,we prepared NCO nanorods followed by photo-assisted deposition of Ag on it.The examination of obtained compounds bares the suppression of structural and optical properties ofNCO through Ag loading.A notable decrease of Eg,enhancing light absorption and improving carrier separation is caused by the presence of Ag over NCO.The application of this photocatalyst for the photooxidation of ATZ herbicide in water is observed.The adjusted Ag loading to NCO displayed a total photodegradation of ATZ in just 20 min with superb reproducibility.This study investigates the usage of spinel-structured photocatalysts for water purification under visible light exposure.
2.Experimental section
2.1.Materials
Nickel(II) nitrate hexahydrate,(Ni(NO3)2·6H2O,≥98.5% (KT)),Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O,≥98%),hexamethylenetetramine (HMTM,≥99.0%),Trisodium citrate dihydrate(HOC(COONa)(CH2COONa)2·2H2O (TSC),≥99.0%),and silver nitrate(AgNO3,≥99.0%) were purchased from Merck.The above materials used directly without additional decontamination.
2.2.Synthesis procedure
0.15 g of Ni(NO3)2·6 H2O,0.3 mg of Co(NO3)2·6H2O,0.5 g of HMTM,and 0.12 g of TSC were mixed in a beaker containing 100 mL of deionized water (DI H2O,14-18 MΩ).Under mechanical stirring,the solution was heated to 100○C for 6 h.The product was ultra-centrifuged after being cool down to the room temperature.Then,the obtained material was rinsed by H2O and ethanol and dried in an oven at 80○C before further annealing at 350○C for 3 h yielding black solid powder denoted as NCO.
The Ag loading procedure was done by a simple photo-assisted deposition reported elsewhere [27].The obtained black solid powder was dispersed by sonication in 100 mL H2O.Then,a pre-calculated quantity of AgNO3solution was poured drop-wisely to the above suspension before light exposure by a 300 W UV lamp for 24 h.The Agloaded samples were obtained after drying the dispersion for 10 h at 80○C.The Ag content in the black solid powder was calculated as 1.0,2.0,3.0,4.0 and 5.0 wt%,and denoted as 1Ag/NCO,2Ag/NCO,3Ag/NCO,4Ag/NCO,and 5Ag/NCO respectively.
2.3.Characterization of the produced photocatalysts
X-ray diffraction (XRD,Bruker series axis D8) was employed with Cu Kα radiation with 1.54 Å wavelength to give information about the crystalline phase of the Ag/NCO samples.In addition,X-ray photoelectron spectroscopy (XPS,Thermo Scientific) was performed for surface structure,chemical state determination,and quantitative analysis of elements within the produced powders.Transmission electron microscope(TEM,JEOL,JEM-1230)was applied by dropping 10 μL of the suspended sample on a carbon-coated grid and dried before investigation.The specific surface area of the photocatalysts (SA) was determined by the BET method from N2adsorption-desorption isotherms using a Chromatech device(Nova 2000)at 77 K.The optical properties,including light absorption and photoluminescence emission spectra(PL),were measured using a UV-VIS-NIR spectrophotometer V-570(Jasco) and RF-5301 fluorescence spectrophotometer (Shimadzu),respectively in ambience.The estimated Egfor the prepared samples was evaluated from the diffuse reflectance spectra (DRS).The photocurrent intensity of the prepared photocatalysts was investigated by an electrochemical workstation (Zahner Zennium).The magnetic properties were analyzed by a vibrating magnetometer,VSM 9600-1 (LDJ),at room temperature with a peak field intensity of 15 kOe.Lastly,the oxidized species for the photocatalytic process were detected using a Bruker EPR A300 spectrometer to characterize the electron spin resonance (ESR) spectra at room temperature.
2.4.Experimental setup
Commercial Xenon lamp (300 W) was used with a 420 nm optical cut-offfilter.Before light exposure,the adsorption-desorption equilibrium was established by engaging the aqueous solution of 50 ppm ATZ mixed with the prepared photocatalyst in murky condition for 30 min.During light irradiation,Liquid chromatography with LC-20A chromatograph (Shimadzu),employing a C18 column,was employed to perceive the variation of ATZ concentration in water.The photodegradation efficiency (P) was estimated using the formula;
in which C0and Ctrepresent the concentrations of ATZ before and after light exposure time(t),respectively.The gas product was passed over a solution of 0.2 M NaOH to perceive the existence of CO2.And then,through the addition of an aqueous solution of barium nitrate,the formation of another white precipitate was clearly seen.The other mineralized Cl and NO3ions were monitored at different time light by ion chromatography (DX-300) equipped with AS4A-SC column and a CDM-II conductivity detector.
3.Results and discussion
3.1.Structure and morphology of the products
Fig.1 demonstrates the XRD graphs of the synthesized samples of NCO (as in a) and the Ag-loaded ones as in b,c,d,e,and f for the 1.0,2.0,3.0,4.0,and 5.0 Ag wt.%,correspondingly.The diffraction peaks reflecting the crystalline planes that are all in coincidence with the facecentered cubic crystalline phase of NiCo2O4(JCPDS No.73-1702) [28].There were no additional peaks observed after the loading of Ag to the product.However,the shifting of the (311) peak to lower value was seen of the expanded portion,as in Fig.S1(supplementary materials).This indicates a little distortion to the lattice of NCO by deposition of Ag on its surface [29].There was no indication for other phase formation or impurities on the Ag-loaded samples,as in Fig.1b-f That is revealing the homogenous spreading of Ag on the produced NCO surface.The peak shift values and corresponding crystallite size(D)of NCO obtained from the Scherrer equation are presented in Table 1.The D values increased from 2.4 nm in the pure NCO to 3.0 nm for the 3Ag/NCO and decreased to 2.7 nm for the 5Ag/NCO.We expect the little change in the D values,which is referring to the induced strain on the NCO crystallites upon deposition of Ag [28].
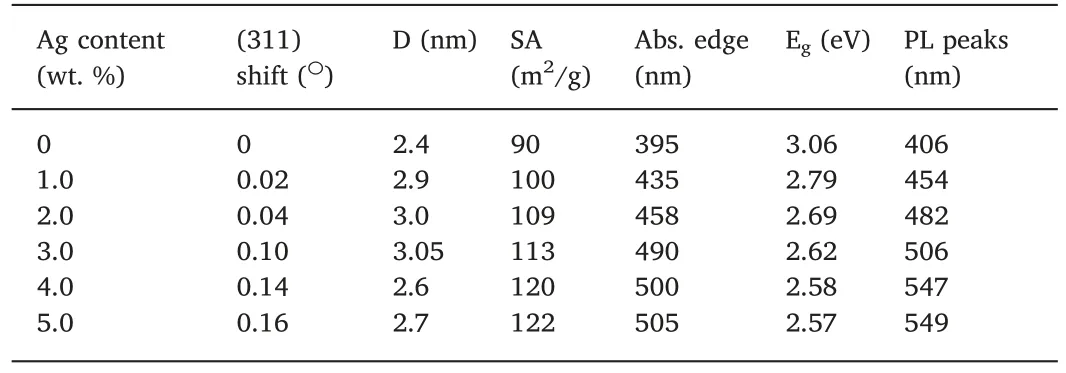
Table 1 Effect of Ag loading on structural and optical properties of synthesized NiCo2O4 nanorods represented by main (311) diffraction peak shift,crystallite size (D)specific surface area (SA),absorption edge (Abs.edge),estimated band gap energies from DSR spectra,and PL emission peaks.
XPS investigations of the 5Ag/NCO sample is displayed in Fig.2.The satellite peaks of 2p3/2and 2p1/2experiential at 855.5 and 872.3 eV in Fig.2A is referring to the Ni2+of the spinel structure[30].Similarly,the 2p3/2and 2p1/2peaks detected at 786.8 and 801.9 eV in Fig.2B indicates the oxidation state from the Co3+of the NCO [23].The O1s peak in Fig.2C at 529.3 eV is reliable for the spinel structured NCO as well[31].The 3d5/2and 3d3/2observed at 368.1 and 374.2 eV are approving the existence of deposited Ag0on the exterior of NCO[26].The amount of Ag in the sample was calculated by quantitative analysis of spectra as 4.8 wt% that is agreed with ICP analysis (4.6 wt%).Therefore,the metallic Ag can be successfully controlled on the surface of NCO.
The TEM images for the unloaded NCO and 5Ag/NCO are presented in Fig.3A and B,respectively.The NCO sample is showed nanorod morphology.The nanorod diameter was 30-50 nm and bundled as in Fig.3A.Through the deposition of 5 wt% of Ag on the surface of NCO,the bundle size reduced into less accumulated nanorods.Uniformly 5.0-8.0 nm-sized Ag nanodots were crystallized on the surface of the NCO nanorods as shown by white arrows in Fig.3B and the corresponding histogram in Fig.S2(supplementary materials),indicating the successful deposition Ag on NCO.The existence of Ag on the NCO nanorods was clarified by investigating the high-resolution imaging of the 5Ag/NCO as in Fig.2C.The differentiation of the lattice spacing Ag nanodots from NCO was observed.The lattice spacing of the spherical Ag nanodots is 2.4 Å that agrees with the (111) plane [26,32].The lattice spacing of the NCO nanorod was measured as 4.8 Å for the(111)and 2.87 Å for (200) planes [33,34].These results indicate the deposition of Ag nanodots over the NCO surface.
The Ag loading has an additional effect on SA of the synthesized NCO photocatalysts as in Table 1.The SA of the NCO sample was initially 90 m2g-1.Upon loading of Ag,the SA improved to 100,109,113,120,and 122 m2g-1by increasing the expanse of Ag at 1,2,3,4,and 5 wt%,correspondingly.This enhancement of SA may attribute to the de-bundling of the NCO nanorods and the introduction of smallsized Ag dots into the photocatalyst.This indicates the Ag dots can show an upshot on surface properties subsequently the photocatalytic performance [35].
3.2.Optical properties of synthesized samples
Fig.4a indicates the absorption spectra of all produced samples.It was found that pure NCO never showed any absorption in the visible regime.Nevertheless,by loading NCO with Ag,the visible light absorption was noticed through the shifts of the absorption edges to the higher value of 435,458,450 and 500 nm for 1.0,2.0,3.0 and 4.0 wt% of Ag loading to the pure NCO at 395 nm,respectively as in Table 1.The highest absorption of the Ag/NCO sample observed at 5.0 wt % of Ag loading at 505 nm did not differ that much for further increase of Ag loading,indicating the saturation for the light absorption.This result emulates the SPR consequence by depositing Ag particulates on the surface of NCO that enhance light absorption in the visible region[36,37].The calculated Egof the products from DRS is also abbreviated in the inset of Fig.4a and Table 1.The Egreduced by increasing Ag content on the NCO sample from 3.06 to 2.57 eV for the 5 Ag/NCO,correspondingly.
We have applied PL spectra measurements in Fig.4b as a supplementary study to investigate the optical performance of the Ag/NCO photocatalysts.The PL represents the changeover of the photoinduced electrons from the conduction band(CB)to the valence band(VB).The PL peak for the pure NCO is detected at 406 nm.As Ag introduced to NCO,we noticed that PL spectra exhibit a clear redshift by increasing the Ag content as listed in Table 1.This is due to the accumulation of the Ag nanoparticles that have its own PL [38].In addition,the formation of a sub-states introduced by Ag at lower energy levels than NCO's CB is a clear evidence for the PL peak shift due to the reduction of the Egand hence a better charge separation [39].In addition,the peak intensity of the PL is also reduced by loading Ag into NCO indicating suppression of the photoinduced charges.The lowest PL peak observed at 549 nm was of the minimum intensity.This is also signifies the Ag deposition on the surface of NCO that advance the recombination of the photogenerated carriers,and then resulting in enhancement to the photocatalytic performance [40].
3.3.Magnetic properties of Ag/NCO
The magnetic separation is an essential step for the industrial realization of the photocatalysts.It is known that NCO is initially spinel ferrimagnet [41].The effect of depositing 5 wt % Ag dots on the magnetic properties of NCO is demonstrated in Fig.5.Under the external fields up to 5.0 KOe,the samples displayed a hysteresis loop with a saturation magnetization of 65.1 emu g-1compared with 58.5 emu g-1for 5Ag/NCO and NCO,respectively.The remnant magnetization of the Ag-loaded sample was close to the pure NCO at 30.5 and 28.6 emu g-1,singly while the coercive field intensity was almost the same at 1.03 KOe for both samples.Thus,the Ag loading did not change the magnetic behavior of the NCO and the magnetic separation of the photocatalyst can be easily realized [42].The inset image of Fig.5 evidenced the separation of Ag/NCO from solution.
3.4.Performance of Ag/NCO photocatalysts
3.4.1.Inspiration of Ag amount
Fig.6a shows the photocatalytic efficiency of ATZ oxidation under light exposure time via employing the as prepared NCO photocatalysts.The photodegradation of ATZ increased as time goes.The outcomes of Fig.6a exposed that photooxidation of ATZ prior to Ag deposition on NCO is the lowest (16%).In contrast,The Ag-loaded NCO at 1.0,2.0,3.0,and 4 wt% to NCO,the photooxidation of ATZ improved significantly to 50,55,74,and 99%,respectively,within 60 min as seen in Fig.6b.Therefore,the introduction of Ag has a considerable consequence on the photocatalytic oxidation of ATZ,although Ag colloidal solution did not show any noticeable photocatalytic activity (Fig.S3,supplementary materials).The complete photodegradation of ATZ occurs under visible light irradiation after 60 min without any additives using the 5Ag/NCO photocatalyst.
Next,The ATZ photooxidation was studied via the first-order kinetic model as in eq.(2) [7];
where K is the reaction rate constant and t is the light exposure time.The K values were calculated by plotting ln(C0/C)versus t as perceived in Fig.6c.The K values clearly augmented by cumulative Ag content in NCO from 0.003 min-1in the pure NCO reaching 0.049 min-1for the 5Ag/NCO photocatalyst as seen in Fig.6d.This high performance is referred to the precision of the surface structure and shifting the light absorption into the visible regime.In addition to that,the recombination suppression of the photogenerated carriers is confirmed from PL spectra in Fig.4b[32].To assist this finding,the photocurrent transient response of the different Ag-loaded amount into NCO was studied,as in Fig.7.The photocurrent response for the 5Ag/NCO was recorded initially at 0.04 mA cm-2compared to 0.0039 mA cm-2for the unloaded NCO photocatalyst.This increase of about one order of magnitude is mentioned to the enhancement of the charge separation by the introduction of Ag dots to the pure NCO photocatalyst and improving the electrical properties of the whole photocatalyst [26].
3.4.2.Influence of photocatalyst dose on the photocatalytic performance
Turning to the dose of the photocatalyst,Fig.8a-c displays the change of the photocatalytic performance of ATZ oxidation with a time of light irradiation.The trials were established by altering the expanse of 5Ag/NCO between 0.5 and 2.5 g L-1.The perceptible efficiency was observed through increasing the dose from 0.5 to 2.0 g L-1.In 2.0 g L-1dose,the photodegradation of ATZ reached 100% in 20 min compared to 55,60,and 80%for 0.5,1.0,and 1.5 g L-1,similarly as in Fig.8b.The mineralization of ATZ was tested for this dose of the photocatalyst,as in Fig.S4(supplementary materials).We found that the mineralization of ATZ was about 64%after 20 min of light irradiation while reached 98%after 60 min.
The first-order reaction kinetics for the ATZ photodegradation is confirmed in Fig.8c.The K value existed for each photocatalyst amount of the 5Ag/NCO photocatalyst.It was observed that the value of K was further reached 0.152 min-1at the dose of 2.0 g L-1.Alternatively,the further raising of the dose to 2.5 g L-1diminished the photocatalytic performance to 70%.This may attribute to the fact of baring a lot of probe molecules to the plenty of active sites of the photocatalyst [43].However,the cumulative dose of the Ag/NCO can perform as a blockade in front of exposed light passed to the ATZ molecules resulting the reduction of the photoactive sites,and hence the photocatalytic performance [8,9,13].
The photocatalytic reproducibility of the spent photocatalyst can be seen in Fig.9.Our optimized 5Ag/NCO showed an excellent recycling ability through the application of five times,keeping both structural and magnetic properties (Fig.S5,supplementary materials).The photodegradation of ATZ could sustain 94%of the original efficiency by the fifth use.There were no residues in forms of Ag,Ni,or Co ions from the spent photocatalyst in the treated water,indicating the high stability of the optimized photocatalyst.Thus,the 5Ag/NCO demonstrates well applicability as a gainful magnetic photocatalyst for upcoming separation and purification in industrial purposes.Based on the above results,our presented Ag/NCO photocatalyst reveals the best performance photocatalyst up to now.Table 2 listing the recent research papers using different photocatalysts for ATZ photooxidation compared to this work [44-50].This proves the significance of the simply prepared Ag/NCO is superior among the current literature conclusions.
3.4.Photocatalytic process
The ESR spectra for the optimized photocatalyst were measured in water to identify the radical formation,as shown in Fig.10.As seen in Fig.10a,the photogenerated holes detected by TEMPO-h+initially showed high intensity upon light illumination while decreased within 10 min.Similarly,the formed·OH species were identified by DMPO-·OH that increased over time,as shown in Fig.10b.This indicates that the photogenerated holes produce·OH radicals responsible for the oxidation process.The DMPO-·O2-also showed signal enhancement of upon shining the light that signifies the formation of·O2-by the photoexcited electrons as in Fig.10c.This means that both holesand electrons are contributing to the photocatalytic process in the presence of oxygen.
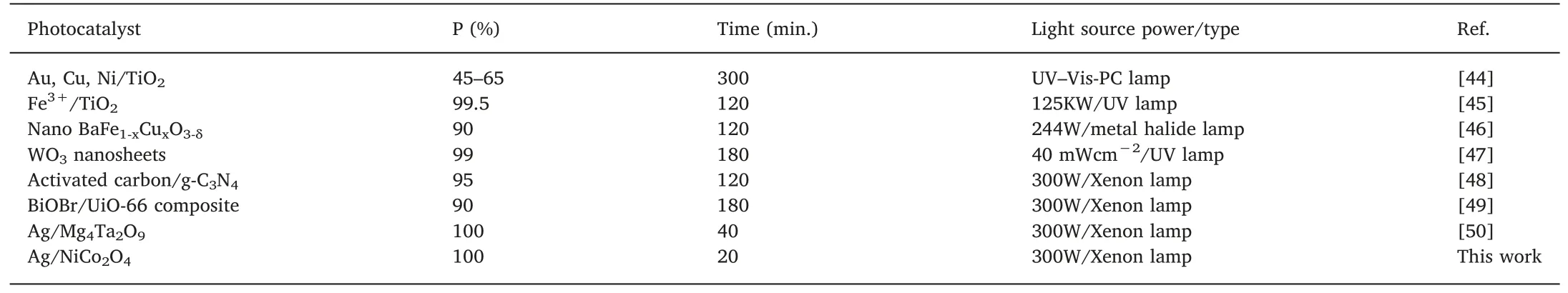
Table 2 Recent published results on photooxidation of ATZ under visible-light illumination using various photocatalysts compared to this work.
The proposed photocatalytic process of ATZ degradation via Ag/NCO photocatalyst is presented in Fig.11.By light absorption,the photogenerated carriers (h+and e-) are separated into VB and CB
The photoexcited electrons assist to transfer to the mid-gap state introduced by the presence of Ag dots and sufficiently the reaction with the oxygen producing oxygen radicals (4).While the holes generates hydroxyl radical by interaction with adsorbed water molecules as in(5)
The oxidation agents in (4) and (5) can subsequently degrade ATZ into CO2,the main product,that inveterate by Barium nitrate solution and the produced reaction gas,forms white precipitation.The XRD analysis of this precipitates confirms the production of BaCO3(Fig.S6 in supplementary materials).The other ion products NO3-and Cl-as evidenced in Fig.8d by ion chromatography confirm the complete mineralization of ATZ as in equation (6)
Thus,the produced photocatalyst of this study can be used in practical applications for cleaner environmental applications like herbicide elimination.
4.Conclusions
A simple procedure has been developed to achieve a visible light-responsive and magnetically separable Ag-loaded NiCO2O4.The Ag-loaded samples show the improvements of structural,optical,and optoelectronic properties with keeping the ferrimagnetic nature of the product.Moreover,the obtained photocatalyst exhibit a superior photocatalytic activity for the photodegradation of Atrazine as a universally applied herbicide in industry.The complete photodegradation is realized in a short time(20 min)under visible light.The optimized 5Ag/NCO photocatalyst shows an excellent reproducibility after five cycles of usage,keeping almost the same performance.This work is opening the door for realizing the application of visible light-active magnetic spinel-structured photocatalysts in water purification.
Declaration of competing interest
There is no conflict of interest.
Acknowledgment
Authors extend their appreciation to the Research Center for Advanced Materials (RCAMS) at King Khalid University for supporting this work through research project program under grant number RCAMS/KKU/006-19.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pnsc.2020.01.021.
 Progress in Natural Science:Materials International2020年2期
Progress in Natural Science:Materials International2020年2期
- Progress in Natural Science:Materials International的其它文章
- Introduction of manganese based lithium-ion Sieve-A review
- Hollow bismuth ferrite combined graphene as advanced anode material for sodium-ion batteries
- Solid electrolyte composite Li4P2O7-Li3PO4 for lithium ion battery
- Wearable supercapacitor self-charged by P(VDF-TrFE) piezoelectric separator
- Enhanced superconducting performance in solution-derived YBCO-BaZrO3 composite film
- FeCo-based hybrid MOF derived active species for effective oxygen evolution
