Introduction of manganese based lithium-ion Sieve-A review
Ding Weng,Hoyue Dun,Yong Hou,Jing Huo,Lei Chen,Fng Zhng,Jido Wng
a State Key Laboratory of Tribology,Department of Mechanical Engineering,Tsinghua University,Beijing,100084,China
b The Education Ministry Key Lab of Resource Chemistry,Shanghai Key Laboratory of Rare Earth Functional Materials,Shanghai Normal University,Shanghai,200234,China
c China Center for Information Industry Development,Beijing,102200,China
ABSTRACT With the large-scale use of lithium-ion batteries,the global demand for lithium resources has increased dramatically.It is essential to extract lithium resources from liquid lithium sources such as brine and seawater,as well as recycled waste lithium-ion batteries.Among various liquid lithium extraction technologies,lithium ionsieve (LIS) adsorption is considered to be the most promising method for its low energy consumption and environment-friendly.This method has advantages of excellent lithium uptake capacity,high selective,and good regeneration performance.In this review,we summarized the development of lithium manganese oxides(LMO)-type LIS,including the chemical structure,lithium intercalation/de-intercalation mechanism,preparation methods and forming technology of this material.The problems in the industrial application of ion-sieves are put forward,and the future research directions are prospected.
Keywords:Ion-sieve Lithium adsorption Lithium manganese oxides
1.Introduction
Lithium is a strategic resource with high electrochemical activity and energy density.This lightest metal is widely applied in rechargeable lithium-ion batteries [1,2].Due to the rapid growth of the electric vehicle industry and the advent of hybrid electrical-vehicles,and energy storage,the market demands for lithium are increasing across the world [3,4].
Today,lithium is extracted from granitic pegmatite ores and lithium water resources including salt-lake brine and seawater.As reported by Grosjean,C et al.[5],brines in some areas of the world contain a large amount of Li+,comprising 62% of the world's complete lithium resource.Therefore,a high potential exists for recovering lithium from brines as long as an efficient lithium extracted technology can be developed.
Thus far,several methods have been utilized for extracting lithium from brines,such as evaporative crystallization method [6],co-precipitation method [7],solvent extraction method [8],and adsorption method[9].The most commonly used is the evaporative crystallization method.However,this method takes several months to concentrate on the brines and requires many carbonates,so the production cost and environmental contamination is severe.In the case of the co-precipitation method,agents such as AlCl3are added to the brines followed by adjusting the pH with NaOH to obtain lithium aluminum hydroxide.This method,however,has strict requirements on temperature and pH,and it is only suitable for low-grade brine.Solvent extraction method has the advantages of high purity and simple process for lithium extraction,but its pollution is severe,and it will cause corrosion to equipment.Adsorption method has been recognized as one of the most promising approaches for lithium extracting from brine owing to the most cost-effective and environment-friendly advantages.
Lithium ion-sieve (LIS)is a lithium ion adsorbent with low toxicity,low cost,high chemical stability,and high Li+uptake capacity,which is considered as the most promising adsorbent[9-12].In general,LIS is classified into lithium manganese oxides (LMO) and lithium titanium oxides(LTO)types.The LMO-type LIS is the most popular adsorbent for lithium owing to its superior lithium selectivity,high lithium adsorption capacities,and excellent regeneration performance.In comparison,the LTO-type LIS has much stable molecular structure because of its large titanium-oxygen bond energy.However,compared to the LMOtype LIS,the lithium adsorption rate of the LTO-type LIS was relatively slow [13].More seriously,this adsorbent is of limited use in lithium recovery from aqueous solution when electrical potential is applied[14].
In this review,we summarize the chemical structure and adsorption mechanism of different LMO-type LIS.Emphasis is placed on preparation methods of LMO precursors and forming technologies for powder adsorbent (e.g.,granulation,membrane formation,and foaming),followed with some prospects regarding LMO-type LIS (see Fig.1).
2.Ion-sieve effect and LIS
Since ion-sieve oxide first prepared by Volkhin et al.,in 1971,it has attracted more and more attention in the past decades due to its unique properties and performance as adsorbent [15-17].Ion-sieve adsorbent is a kind of inorganic material,in which the template ions are introduced into an inorganic compound by redox or ion exchange reaction,and a heating process obtains the compound oxide.Then the template ions are eluted from their crystal positions by eluent,and the vacancy crystal sites are still retained.Thus the inorganic material obtained with regular crystal sites could only accommodate ions,and the ionic radii of which are smaller than or equal to that of the target ions structure.This material tends to accept template ions and form the best crystal configuration.Therefore,the template ions have a reliable screening and memory effect,which can adsorb target ions from the coexistence of the multiple ions.This effect is called “ion-sieve effect”.For LIS,only lithium ion can enter the vacancy,due to that it has the smallest ion radius among all metal ions.The preparation process of ion-sieves and ion-sieve effect are shown in Fig.3 (see Fig.2).
3.LMO overview
LMO-type LIS has become the most widely researched and promising selective lithium adsorbent currently due to its high adsorption capacity,excellent regeneration performance,and high selectivity.In this chapter,the details on the spinel structures of LMO precursors,types of LMO and lithium intercalation/de-intercalation mechanisms will be discussed.
3.1.Types of LMO
Since LMO-type LIS has been synthesized [18],some of that have been well developed [10,19].Currently,only a few LMO-type LIS having precursors with high lithium adsorption capacities have been synthesized,such as λ-MnO2,MnO2·0.3H2O,and MnO2·0.5H2O,which are derived from LiMn2O4,Li1.33Mn1.67O4(Li4Mn5O12),and Li1.6Mn1.6O4(Li2Mn2O5)(see Table 1).
Chitrakar [9]presented a phase diagram including additional proton manganese oxides shown in Fig.4.In this figure,the precursors of LMO-type LIS are divided into two types:redox reaction region on the vertical plane and ion exchange region on the horizontal plane.Their lithium uptake capacities from the aqueous solutions are summarized in Table 2 (see Table 3).
The theoretical adsorption capacity of different adsorbents depends on the molar ratio of Li and Mn of different precursors.Generally,with the increase of Li/Mn molar ratio in the precursor,the theoretical adsorption capacity improves.MnO2·0.5H2O has the highest theoretical adsorption capacity with its precursor's Li/Mn molar ratio of 1:1.But,in practical application,the adsorption capacity is often lower than the theoretical value.
3.2.Spinel structures of LMO precursors
Invariably,structure determining nature,thus the selective adsorption of lithium by LMO-type LIS is attributed to its precursors’spinel structures [26-30].Among many precursors,the LiMn2O4is the most representative one,as shown in Fig.530.
LiMn2O4is a spinel structure with Fd3m space group.In this structure,Li+occupies the 8a sites of tetrahedrons,Mn3+and Mn4+alternately occupy the 16d sites of octahedrons,and oxygen anion occupies the 32e sites of the face-centered cubes.Therefore,LiMn2O4can be represented as (Li)8a[Mn(III)Mn(IV)]16dO4.Li+could move along 8a→16c→8a→16c channel without causing the collapse of the overall frame,which is the structural basis of lithium intercalation/de-intercalation cycle in spinel-type LMO [31].
In spinel LiMn2O4,the ratio of two metal cations Li and Mn is 1:2;however,this stoichiometric proportion can be relaxed somewhat under certain conditions [32].For instance,the corresponding precursor of another LMO-type LIS MnO2·0.3H2O is Li1.33Mn1.67O4(Li4Mn5O12),which has a different ratio of Li and Mn compared with LiMn2O4.Li1.33Mn1.67O4(Li4Mn5O12) presents higher theoretically lithium capacity than that of λ-MnO2because more lithium ions can be extracted from it or inserted into it.However,the neutron diffraction studies by Ammundsen et al.29showed that the process of lithium reinsertion applies only to tetrahedral locations,not octahedral locations;it may be expressed by the following equation:
Other typical lithium-rich LMO precursor is Li1.6Mn1.6O4(Li2Mn2O5),whose corresponding LIS is MnO2·0.5H2O.In this compound,extra lithium ions may be located in the interstitial sites of the spinel structure with a definite location [28].This is evidenced by the fact that the proportion of cations and anions(4:5)is different from that of the typical spinel compounds (3:4).Unfortunately,there have been no published Li1.6Mn1.6O4structural models so far.Perhaps,future research should focus on this issue to combine the development of LIS with excellent lithium absorption properties.
3.3.Lithium intercalation/de-intercalation mechanisms
In the past several decades,there are many different explanations of Li+intercalation/de-intercalation mechanism in LMO.Among them,the redox mechanism,ion exchange mechanism,and composite mechanism are the three main theories.
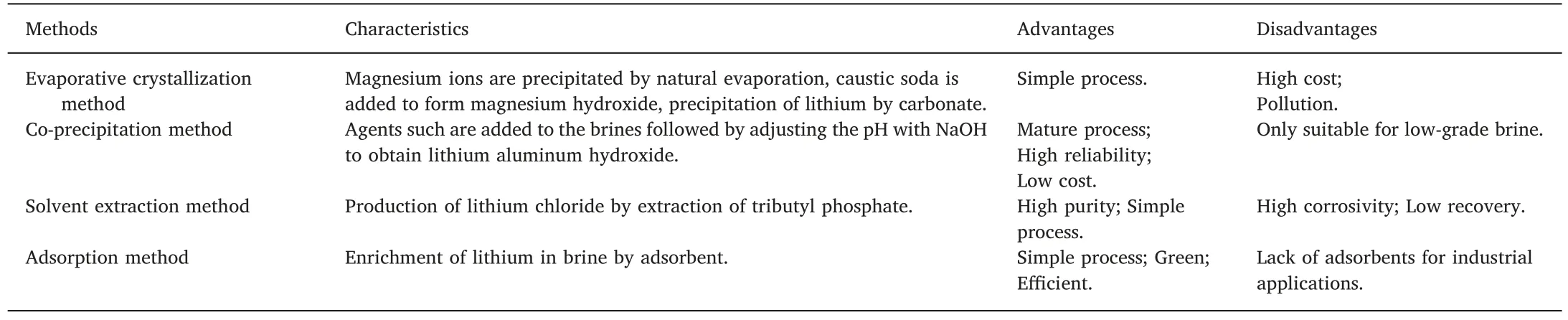
Table 1 Comparison of lithium extracting methods.
3.3.1.Redox mechanism
In the early research by Hunter [18],the lithium intercalation/deintercalation in LiMn2O4was considered to be a pair of redox reactions:
In equation (2),the (),[ ],□represent 8a tetrahedral sites,16d octahedral sites,and vacancy respectively.This reaction is the disproportionation of Mn III under acidic conditions.In this process,only Mn III on the surface is transformed into Mn II and Mn IV.The manganese in the manganese oxides obtained are all Mn IV,and the tetrahedral position of 8a is a vacancy,which is named as λ-MnO2.This process is similar to a mechanism proposed by Kozawa [33]for the cathodic reduction of λ-MnO2in the alkaline electrode.
Ooi et al.[34]also studied the intercalation mechanism of Li+in λ-MnO2.They pointed out that the mobility of lithium ions and electrons in λ-MnO2structure is independent.Because of the alternating distribution of Mn III and Mn IV,electron migration can be easily realized in the spinel structure.Therefore,the Li+intercalation process can be divided into the following two steps:
In the above equations,x is the number of moles of inserted Li+per mole of Mn atoms in the solid,(s)represents the surface of the particle.Equation (3a) shows that the insertion of lithium ions into tetrahedral vacant sites of the λ-MnO2framework,accompanied by the reduction of Mn IV to Mn III; Equation (3b) shows that migration of excess positive charge to the surface of λ-MnO2with the oxidation of hydroxide ions in the aqueous phase.According to this model,the oxidation site of OH-does not need to be adjacent to the insertion site of Li+,so the insertion of Li+in λ-MnO2will not destroy the spinel structure.
According to the redox mechanism,manganese in LMO-type LIS will be dissolved during recycling,which will affect the structural stability of spinel.Therefore,the phenomenon that the adsorption capacity of lithium ion-sieves decreases gradually during recycling can be explained.However,the disadvantage of this mechanism is that it can hardly explain the phenomenon that the adsorption capacity of ionsieve increases with the increase of solution pH.
3.3.2.Ion exchange mechanism
At present,researchers mostly study the adsorption properties of lithium ion-sieves based on ion exchange mechanism.Shen and Clearfield [35]proposed that the intercalation/de-intercalation of Li+in LMO-type LIS follows the ion exchange mechanism:
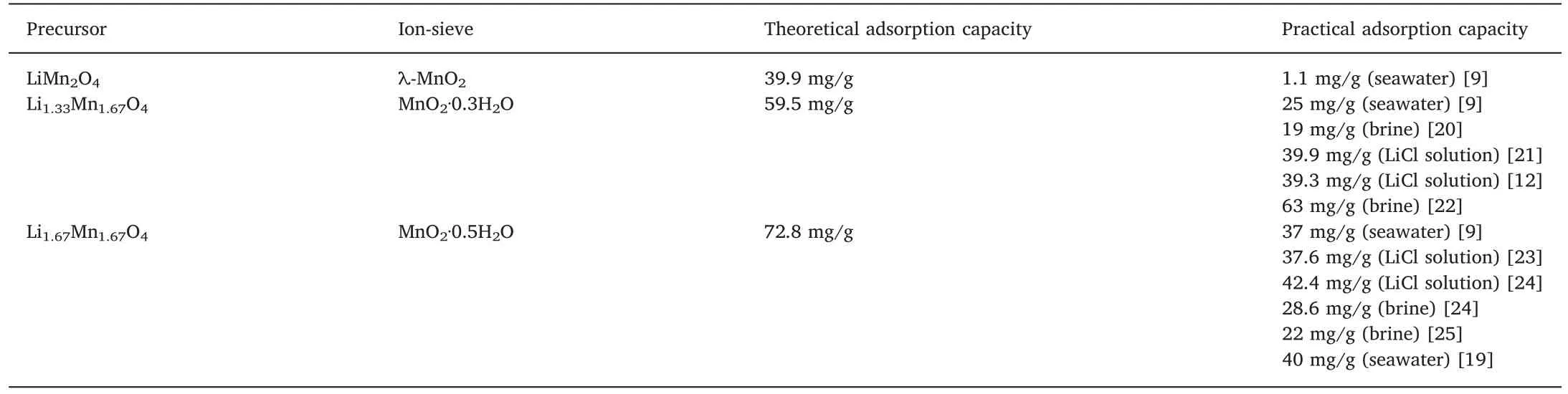
Table 2 Adsorption capacity of different types of LMO.
Besides,the presence of hydroxyl groups on the surface of LMO-type LIS was confirmed by infrared spectroscopy,which is related to the tetrahedral position of vacancy 8a in the spinel structure.According to this mechanism,the 8a tetrahedral sites of LMO-type LIS is not the vacancy explained by redox mechanism but protonated.
Koayaka et al.[36]proved that the adsorption capacity of lithiumion is proportional to the content of hydrogen ion by analyzing the relationship between the composition and adsorption capacity of several spinel λ-MnO2.However,they believed that the reason for the selective adsorption of lithium ions in λ-MnO2is not based on the socalled ion-sieve effect,but only on the ion exchange reaction between lithium ions and protons.
According to this mechanism,lithium ions in solids can be entirely replaced by protons,and the sites of Mn III and Mn IV in crystals remain unchanged in Li+/H+ion exchange.Moreover,because the spinel structure is retained,it has high selectivity and excellent renewability for Li+.Also,the phenomenon that the adsorption capacity of ion-sieve increases with the increase of solution pH also proves the existence of ion exchange reaction.However,the disadvantage of this mechanism is that it can hardly explain the dissolution of manganese and the gradual decrease during recycling.
3.3.3.Composite mechanism
Although the above two mechanisms can explain many phenomena of lithium recovery from aqueous solution,they both have limitations.Therefore,some researchers put forward a composite mechanism based on these two mechanisms.
Ooi et al.[37]investigated the intercalation reaction of lithium ions in different types of spinel ion sieves and divided the lithium insertion sites into three groups:a redox-type site,a Li+specific ion exchange site,and a Li+nonspecific ion exchange site.The proportion of each site depends on the preparation conditions.The oxidation state of manganese in the precursor of heat treatment is an essential factor affecting the formation of insertion sites.
Feng et al.[38]concluded the sources of redox and ion exchange sites.Generally,spinel precursors with trivalent manganese provide redox sites,whereas if the precursors contain only tetravalent manganese,they provide ion exchange sites.Furthermore,they found that the proportions of the two types of sites depend on preparation conditions.Ion exchange sites are formed mainly at temperatures lower than 500 °C,and redox sites are formed at higher temperatures.The intercalation/de-intercalation reaction of Li+takes precedence over ion exchange sites.The intercalation/de-intercalation reaction of Li+explained by composite mechanism is shown in Fig.6.
The composite mechanism can explain the process of lithium intercalation/de-intercalation by LMO in theory,but it is too complex to verify in practice.
4.Synthesis of LMO-type LIS
In general,the synthesis of LMO-type LIS is required two stages:preparation of precursors and acid treatment.Most of the precursorscould be obtained in only one step.However,for Li1.6Mn1.6O4,it takes two steps,including the synthesis of orthorhombic and its heat treatment.We discussed the preparation methods of precursors,thermal treatment of o-LiMnO2,and acid treatment.Furthermore,the doping modifications are discussed in this chapter.
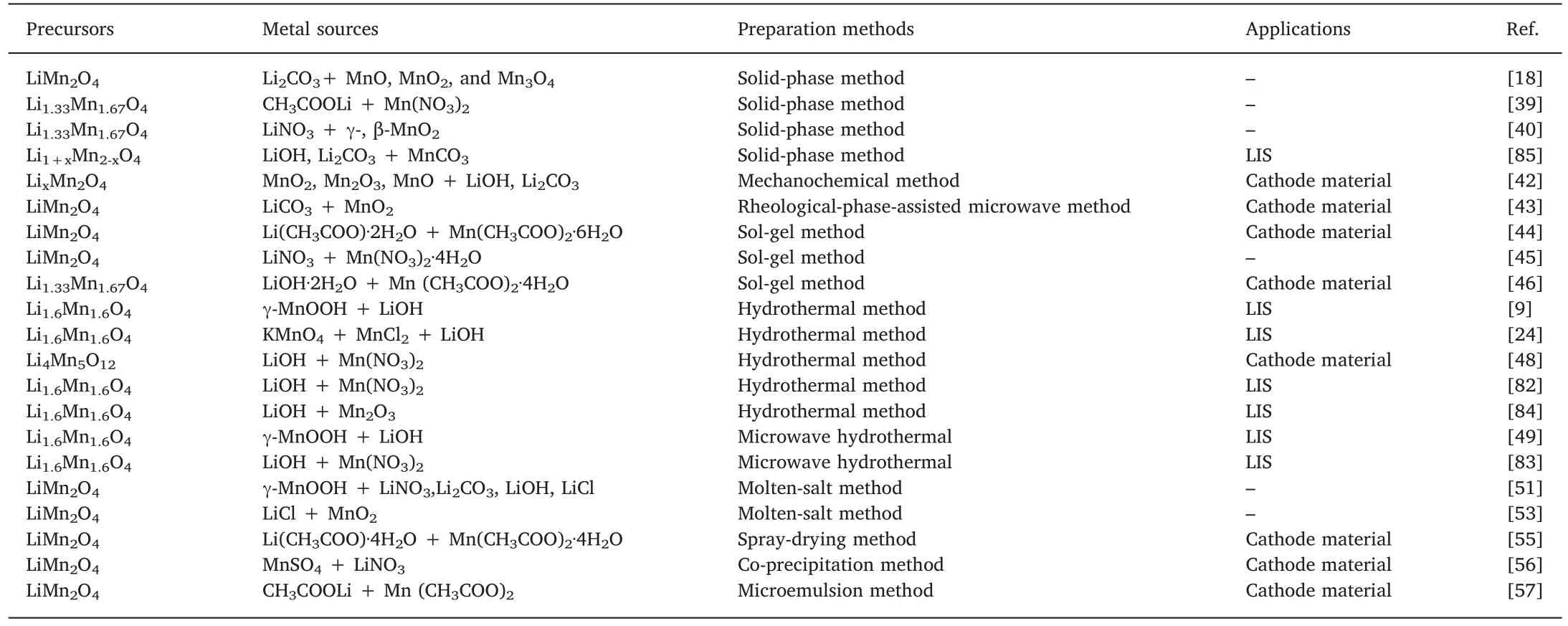
Table 3 Some common LMO precursors obtained by different preparation methods.
4.1.Preparation methods of precursors
Generally,the adsorption capacity of LMO-type LIS depends mainly on the preparation methods of its precursors.On the other hand,the different ratio of Li and Mn in raw materials will affect the crystallinity of the product,thus affecting the regeneration ability of the adsorbent.For decades,several synthesis methods of precursors have been developed,which can be divided into the solid-phase synthesis method and soft-chemical synthesis method.
4.1.1.Solid-phase synthesis methods
The solid-phase combustion method is one of the most popular approaches to prepare LMO-type LIS precursors.In this method,lithium salt and manganese salt are mixed according to a certain stoichiometric ratio,and calcination at an appropriate temperature for a period of time to obtain the corresponding products.
Hunter [18]prepared LiMn2O4by mixing Li2CO3and several manganese oxides (MnO,MnO2,and Mn3O4) at 850 °C in air.Takada et al.[39]prepared well-crystallized Li1.33Mn1.67O4(Li4Mn5O12)powder by heating a eutectic mixture of lithium acetate CH3COOLi and manganese nitrate Mn(NO3)2.Yang et al.[40]prepared Li1.33Mn1.67O4(Li4Mn5O12) spinels by using LiNO3and different manganese sources(γ-,β-MnO2,hollandite,and H+-form birnessite manganese oxides) at 400 °C.The pH titration results showed that more uniform acidic sites are contained in the spinel obtained from a precursor with a relatively smaller pore size (tunnel size).Chitrakar et al.[41]obtained lowcrystallized orthorhombic LiMnO2by mixing γ-MnOOH (or Mn2O3),and LiOH·H2O under steam atmosphere at 120 °C,then heated it at 400 °C in the air to prepared spinel Li1.67Mn1.67O4(Li2Mn2O5).The corresponding LMO-type LIS(MnO2·0.5H2O)has an adsorption capacity of 4.75 mmol g-1(33 mg g-1).
The conventional solid-phase method is simple and easy to apply,however,due to the uneven contact and inadequate reaction of raw materials,the size and distribution of the prepared powders are large and inhomogeneous; also the reaction time and energy are consumed.Thus,some auxiliary methods were proposed to overcome these shortages.
Kosova et al.[42]synthesized highly dispersed stoichiometric and nonstoichiometric LixMn2O4spinel starting from different manganese(MnO2,Mn2O3,MnO) and lithium (LiOH,LiOH·H2O,Li2CO3) compounds by a mechanochemical method.It was shown that a strong effect of temperature and lithium content on the composition and lattice constant of the final products was found.Another auxiliary method is the microwave combustion method,which was developed in recent years.By this method,the reaction time and preparation efficiency have been greatly optimized.Cui et al.[43]prepared LiMn2O4by rheological-phase-assisted microwave synthesis method,which significantly shortened the reaction time.As a result,the powders prepared by this method at 750 °C are pure spinel LiMn2O4with regular shapes and uniform distribution,which exhibit higher capacity and much better reversibility than the sample prepared by the traditional solid-state reaction.
4.1.2.Soft-chemical synthesis methods
The soft chemical methods are aimed to improve the uneven mixing of solid-phase synthesis methods.Typically,in these methods,atomic mixtures of raw materials are prepared by dissolving the soluble lithium compounds and manganese compounds in aqueous solutions.Several most commonly used soft-chemical synthesis methods for preparing LIS precursors are introduced in this section.
4.1.2.1.Sol-gel method.The sol-gel method is a typical soft-chemical method.It is usually used to prepare nanoparticles with good chemical homogeneity,high purity,and homogeneous phase distribution.This method is to disperse raw materials in the sol and then form a continuous three-dimensional network gel through agglomeration.Then aerogels can be obtained by heating the aerogel under proper conditions.By this method,starting materials are mixed at an atomic scale in a multi-component system,so perfect crystallization precursors can be obtained.
Sun et al.[44]prepared spinel LiMn2O4powders by a sol-gel method using an aqueous solution of metal acetates containing PAA as a chelating agent.They proposed that the sol-gel method required much lower calcination temperature and shorter calcination time than the conventional solid-state reaction.Seyedahmadian et al.[45]prepared spinel LiMn2O4by sol-gel using citric acid as a chelating agent.They investigated the influence of different parameters such as pH conditions,solvent,the molar ratio of citric acid to total metal ions,calcination temperature,starting material on the structure,morphology,and purity of this oxide.The XRD pattern (Fig.7) suggested that the optimum pH and molar ratio of a chelating agent to total metal ions are 4 ≤pH ≤6 and 1.0,respectively.Chu et al.[46]prepared spinel Li1.33Mn1.67O4by a facile sol-gel process.They dissolved a certain stoichiometric ratio of LiOH·2H2O and Mn (CH3COO)2·4H2O in deionized water,followed by adding citric acid to the mixed solution at 75 °C under water bath with constant stirring,then add ammonia to adjust pH to 6.5,and finally dry the gel at 100 °C in a vacuum.
4.1.2.2.Hydrothermal method.The hydrothermal method has the advantages of evenly mixing of raw materials,simple operation,and low equipment cost.Moreover,new compounds could be created by this method due to its unique homogeneous nucleation mechanism[47].
Li1.6Mn1.6O4was first prepared by Chitrakar et al.[9]by calcining orthorhombic LiMnO2which was synthesized by hydrothermal method.In this process,a certain amount of γ-MnOOH was mixed with LiOH solution in a Teflon-lined stainless steel vessel and autoclaved at 120°C for 1 day.The product was filtered,washed,and dried at 60 °C.The corresponding ion-sieve (MnO2·0.5H2O) yielded the maximum lithium uptake value (72.8 mg g-1) in theory.
On the other hand,different morphologies including nanorods,nanowires,cubic shape,and spheres could be obtained by changing the hydrothermal conditions.For instance,Xiao et al.[24]prepared LiMn2O4with diameter less than 0.2 μm by hydrothermal method(Fig.8a).Zhang et al.[48]prepared Li4Mn5O12by a mild hydrothermal method using Li-birnessite ultrafine fiber as the precursor.The SEM image (Fig.8b) of the obtained powders shows the particles are cubicshaped whose average size is about 40-50 nm.It is well known that the nano-adsorbents with specific morphologies have a great diverse impact on the adsorption behaviors.Generally,for LMO-type LIS,the smaller the particle size,the higher the adsorption capacity.
Different from conventional hydrothermal method,the microwave hydrothermal method could solve the problem of non-uniform temperature and long reaction time due to its unique heating mechanism.Chitrakar et al.[49]achieved semi-crystalline orthorhombic LiMnO2by microwave hydrothermal using γ-MnOOH and LiOH at 120 °C.The reaction time was only 30 min,which was significantly reduced compared with the traditional hydrothermal day.Moreover,the sample obtained in this process is needle-like(Fig.10a),which is different from the cubic shape (Fig.10b) obtained by conventional hydrothermal method at the same temperature.It suggested that a direct interaction between γ-MnOOH and the microwaves,in addition to the rapid heating of the LiOH solution.
4.1.2.3.Molten-salt synthesis method.The molten-salt synthesis method was mainly used to obtain ceramic material[50].High purity products can be obtained by this method.In the last few years,some researchers reported that using molten-salt synthesis method to prepare LMO-type LIS precursors [51-54].In this method,the low melting point molten salts act as both the reactant and the solvent.
Yang et al.[51]prepared single crystals of precursors by using molten-salt synthesis method.They proposed that the octahedral LiMn2O4single crystals and orthorhombic LiMnO2crystals with tubeand rod-like shapes could be obtained by use of different shaped containers to control the exposure to the atmosphere (Fig.9).Helan et al.[53]prepared lithium manganese oxide powders by a molten salt synthesis method using a eutectic mixture of LiCl and MnO2salt at 900 °C in an open atmosphere.The final compound possesses excellent physical properties,implying the synthesis process is economically viable which can be extended for the large scale preparation of this material.
In general,the molten-salt synthesis method has the advantages of good adaptability to high concentration reactants,simple operation and high purity of products.However,a certain degree of safety risks in the process limits its large-scale industrial application.
4.1.2.4.Other methods.In addition to the methods mentioned above,there are some less commonly used soft-chemical methods.A summary of these is as following.
Wu et al.[55]prepared spine LiMn2O4by a spray-drying method.The obtained particles are fine,narrowly distributed,and well crystallized,as shown in Fig.11.Naghash and Lee [56]prepared LiMn2O4by co-precipitation method.In this process,stearic acid in tetramethylammonium hydroxide (TMAH) is used to co-precipitate lithium and manganese in stoichiometric proportions from MnSO4and LiNO3solutions.Sinha et al.[57]prepared submicron size particles of LiMn2O4by the microemulsion method in a quaternary microemulsion medium.They proposed that the samples from the above reaction heated at 800 and 900 °C are found to possess a pure spinel phase with particle size<200 nm.
4.2.Heat treatment of o-LiMnO2
Li1.67Mn1.67O4is the precursor of MnO2·0.5H2O,which has the largest lithium uptake capacity(72.8 mg g-1)of LMO-type LIS in theory.Moreover,the chemical stability may be sufficiently high because it contains only tetravalent manganese.However,it can be obtained only by limited methods:heat treatment of o-LiMnO2precursor at an appropriate temperature in air.
Chitrakar et al.[9]prepared Li1.67Mn1.67O4by heating o-LiMnO2at 450 °C in air.They proposed that the particle shape became clear with heating,but particle growth by calcination did not take place.In this process,the DTA-TG curves of o-LiMnO2(Fig.12) showed an 8.5%weight gain,which agrees well with the theoretical weight increase by the oxidation of trivalent manganese oxide to tetravalent,as shown in equation (5).Xiao et al.[24]obtained Li1.67Mn1.67O4by heating o-LiMnO2at 350 °C under an air atmosphere.In their work,they studied the effect of calcination time.They proposed that the loss ratio of Mn2+ions decreased from 1.8% to 1.1% when the calcination time was increased from 12 to 72 h (i.e.,the chemical stability was improved by increasing the calcination time from 12 to 72 h).
4.3.Acid treatment
Typically,ion-sieve is derived from precursors by acid treatment.Regular acid treatment causes the removal of nearly all the lithium while the spine structure is retained.The reaction in this process is as follow:
However,the dissolution of manganese in acid treatment is a problem that can not be ignored.Shen et al.[35]synthesized λ-MnO2with a manganese dissolution rate of 4%in 1-2 mol L-1HNO3solution.Xiao et al.[21]synthesized MnO2·0.3H2O with a manganese dissolution rate of 2% in 0.5 mol L-1HCl solution.Wang et al.[23]prepared MnO2·0.5H2O with a manganese dissolution rate of 3%.The dissolution of manganese will not only lead to the destruction of the spinel structure,thus reducing the life of adsorbent,but also damage the environment.Recently,for investigating the mechanism of manganese dissolution,Gao et al.[58]prepared MnO2·0.5H2O by stirring 0.1 g Li1.67Mn1.67O4in 100 mL of HCl solution with a concentration ranging from 0.01 mol L-1to 0.5 mol L-1for less than 1 min at 40 °C.They proposed the mechanism of manganese dissolution:an electron of trivalent manganese in the bulk phase transfers to the surface and is captured by tetravalent manganese within the acidic environment.Then,tetravalent manganese is converted to bivalent manganese after acquiring sufficient electrons,and dissolution occurs simultaneously.Unfortunately,little research has been done on the effects of different acids on the dissolution of manganese.
4.4.Doping modifications
Due to the Jahn-Teller effect induced by Mn3+,the octahedral MnO6structure is seriously distorted,which leads to the reduction of LMO stability and the decrease of lithium-ion intercalation efficiency[59,60].Moreover,it causes a large amount of manganese dissolution in industrial operation,which leads to serious water pollution.Therefore,some researchers proposed doping modifications to replace Mn3+with other metal ions.
Generally,divalent metals are the most studied elements in doping modifications.Feng et al.[61]studied the Li+extraction reactions in LiZn0.5Mn1.5O4spinel.They found that the Li+extraction and insertion proceeded by ion-exchange type mechanisms.The structure of this metal-doping material is shown in Fig.13.Chitrakar et al.[62]first prepared spinel-type lithium antimony manganese oxide by aging the precipitates that were obtained by reaction of a mixed aqueous solution of manganese(II) and antimony(V) chlorides with (LiOH+H2O2) solution,followed by hydrothermal treatment at 120 °C.The results showed that the exchange capacity for Li+of the synthesized samples Li1.16Sb(V)0.29Mn(III)0.77Mn(IV)0.77O4reached 5.6 mmol g-1,and the affinity order was K < Na«Li.In the following research,Ma et al.[63]obtained series Li-Sb-Mn composite oxides by conventional solid state reaction.Among these samples,the acid treated spinel Li-Sb-Mn composite oxide with Sb/Mn molar ratio of 0.05 had a high Li+adsorption capacity of 33.23 mg g-1in lithium solution.They proposed that the Sb/Mn molar ratio of these Li-Sb-Mn composite oxides should be a crucial factor in determining their structure and Li+extraction and adsorption properties.Tian et al.[64]synthesized magnesium(II)doped spinel lithium manganese oxide by soft chemical method.The adsorption capacity of this adsorbent reached 37.4 mg g-1,and the adsorption capacity remained above 95% after four cycles.Chitrakar et al.[65]investigated the impact of magnesium-doped manganese oxide on the dissolution of manganese during acid treatment,they proposed that the magnesium-doped sample greatly inhibited the dissolution of manganese.Furthermore,regeneration study showed that the lithium ions can be desorbed with HCl solution,and the efficiency of adsorptive capacity of the sample did not decrease even after 10 cycles.Ma et al.[66]prepared series spinel LiMxMn2−xO4(M=Ni,Al,Ti; 0 ≤x ≤1) and carried out a study of their stability and Li+extraction and adsorption properties in aqueous solution.LiAl0.5Mn1.5O4exhibited a relatively high Li extraction ratio and relatively low Mn and Al extraction ratios during acid treatment,and in the subsequent adsorption experiment it showed a better Li+adsorption performance.However,due to the significant cell contraction or expansion,Li-Ni0.5Mn1.5O4and LiTi0.5Mn1.5O4did not show satisfying Li+extraction and adsorption properties.Chitrakar et al.[67]obtained iron-doped lithium manganese oxides Li1.33FexMn1.67−xO4by calcination of carbonates of Li,Mn,and nitrate of Fe in air.The result indicated that the amount of Mn extracted in HCl solution decreased with increasing of Fe/Mn ratio.Among the adsorbents studied,the adsorbent with Fe/Mn ratio of 0.1 obtained from calcination of the precursor at 450°C showed the highest lithium extractability with HCl solution.Moreover,the adsorbent can be easily regenerated with HCl solution with 90% lithium extraction.It still needs further study about the mechanism of doping modification on the adsorbent regeneration ability.
5.Forming methods of LMO
Typically,LMO is present in powder form; it can hardly be used directly in industrial application because a huge amount of powder adsorbent is not easy to handle in the aqueous solution including its recovery problem after Li+adsorption.More seriously,in powder form,it leads to high energy consumption in the column operation,limiting its industrial application.Therefore,it is necessary to find methods of LMO forming.Granulation,membrane formation,and foaming are conventionally introduced to overcome this shortage.
5.1.Granulation
Granulation technology is the most commonly used technology in nano-particle forming.LMO powder forms microspheres of various sizes through the bonding action of organic polymers,which makes ion sieve have good mechanical strength and can adapt to industrial column operation.However,due to the coverage of active sites,the adsorption capacity of ion-sieves decreases after granulation,which is the biggest problem faced by granulation.
Xiao et al.[68]prepared a spherical PVC-MnO2ion-sieve of 2.0-3.5 mm diameter by anti-solvent method,where the precursor was the Li4Mn5O12powder,the binder was poly (vinyl chloride),and the solvent was N-methyl-2-pyrrolidone.According to the calculation results of the mass transfer coefficient (kf) and pore diffusion coefficient(Dp),the adsorption process of lithium is controlled by the intraparticle diffusion mechanism.Moreover,this kind of spherical adsorbent had a high capacity (3.38 mmol g-1),excellent selective,and superior regeneration performance.It indicated that the spherical PVC−MnO2ion sieve has a good potential in industrial application for lithium extraction from brine or seawater.
On the other hand,their research group also obtained granulated polyacrylamide (PAM)-MnO2ion-sieve with 0.3-0.7 mm diameter by the inverse suspension polymerization method using Li4Mn5O12as the precursor,acrylamide as the binder,N,N'-methylenebisacrylamide(MBA) as the cross-linker,and ammonium persulfate (APS) as the initiator (Fig.14)20.The experiment showed that the maximum lithium equilibrium adsorption capacity is up to 2.68 mmol g-1at 303 K.Furthermore,in the packed column,the Li+loading amount of PAM-MnO2ion-sieve remained almost constant after the 30 cyclic adsorption-desorption experiments (see Fig.15).
In the study by Hong et al.[69].Adsorbent was immobilized on alpha-alumina beads (AABs) to recover lithium effectively.Their method was to soak AABs in Li/Mn acetate solution (Li,Mn ratio 1),then calcined the sample,dried it and acid treatment,and then prepared spinel-type manganese hydroxide fixed on AAB.This composite material showed excellent Li+adsorption (8.87 mg g-1) and less than 2%Li+adsorption capacity loss after 15 repeated use in real seawater.These findings indicated that this granulated adsorbent has good potential for the recovery of Li+from seawater.
In recent year,Hong et al.[70]prepared a highly porous,surfacemaximized LMO/Al2O3composite by using low-temperature calcined mesoporous alumina.All of the samples exhibited large surface area owing to the mesoporous character of γ-Al2O3.This material exhibited similar Li+uptakes compared to the corresponding powder due to their highly expanded surface area and porous structure.However,an excessive amount of Al2O3decreased the crystallinity of the LMO spinel structure,resulting in manganese dissolution during the regeneration process.
5.2.Membrane formation
Membrane forming technology is another widely used forming technology.Compared with granular adsorbents,membrane adsorbents have the advantage that they can easily construct adsorption modules by stacking or winding membranes.Therefore,membrane adsorbents are suitable for continuous operation and industrial applications.However,this process is costly and complicated.On the other hand,both membrane forming method and granulation need a large number of organic,which not only increases the cost but also causes environmental pollution.
Umeno et al.[71]prepared a membrane-type adsorbent of spineltype manganese oxide by a solvent exchanging method using poly(vinyl chloride) as the binder,N,N-dimethylformamide (DMF) as the solvent.They investigated the membrane precursor with different initial PVC concentration and LMO content.The result showed that the adsorption rate correlates only to LMO content and the outer surface area plays an essential role in controlling the adsorption rate,regardless of the adsorbent form,granular or membrane.Moreover,they designed a new type of adsorption cell for obtaining a parallel seawater flow along with the membrane-type adsorbents,which is advantageous for the effective lithium recovery from seawater utilizing natural flow.
Zhu et al.[72]obtained a series of PVC-Li1.67Mn1.67O4by the solvent exchange method.In this process,the precursor was the Li1.67Mn1.67O4,the binder was the PVC,and the solvent was the N,Ndimethyl acetamide (DMAc).The adsorption capacity depended on the preparation conditions.They proposed that the membrane prepared with a concentration of 10 wt% PVC and 15 wt% Li1.67Mn1.67O4in DMAc and liquid film thickness of 0.30 mm is optimum for adsorption.The adsorption cycle experiment indicated that this membrane-type adsorbent could be effectively regenerated and reused for lithium enrichment without significant adsorption capability loss.The process diagram is shown in Fig.16.
5.3.Foaming
It is not a new conception to attach adsorbent on the support surface.Adding LMO into foam support to prepare composite material should be a more feasible method and has a similar effect.Foam-type lithium adsorbents have been developed these years.Usually,they present superior mechanical property,excellent lithium selectivity,and good regeneration performance.However,these absorbents show a significantly lower adsorption capacity compared to the powder.More seriously,the material will disintegrate gradually with repeated adsorptions because of its looser internal skeleton.
Ma et al.[73]prepared an LMO-foam by a polyurethane template method using the oxygen-containing pitch as the support and crosslink.The result showed that the foam-type adsorbent had a homogeneous three-dimensionally interpenetrating network.SEM images of LMO foam are shown in Fig.17.It exhibited considerable lithium adsorption capacities of 1.49 mg g-1in brine.This makes the LMO-foam a promising inorganic lithium adsorbent.However,the combination of the pitch support and the nano-particles became loose after several adsorption cycles,and the numerous environmentally hazardous substances generated in the manufacturing process.Thus,further studies should emphasize optimizing its preparation and application methodology,especially its mechanical repeatability.
Han et al.[74]obtained millimeter-sized spherical ion-sieve foams(SIFs) from LMO by a composite process of foaming,drop-in-oil,and agar gelation.The experimental results showed that the prepared SIFs possessed a hierarchical trimodal pore structure after acid treatment.SEM images of the cross-sectional area (inner surface) of the SIFs are shown in Fig.18.The maximum lithium adsorption capacities of the adsorbent could reach 3.4 mg g-1in seawater.Moreover,after five adsorption cycles,the lithium uptake amount remained over 95%.It suggested that the foam-type LMO could be a promising candidate as an environmentally friendly and semi-permanent lithium adsorbent.
Nisola et al.[75]fabricated a flexible LMO/PVA composite foam with hierarchical porosity composed of macro and mesopores by a devised method of combined surfactant blending,cryo-desiccation and chemical cross-linking.The corresponding preparation schematic diagram of this material is shown in Fig.19.In this work,PVA acted as binder and support; this accessible material with high hydrophilicity improved the kinetic properties of composite foam adsorbent.Compared with the corresponding powder,the LIS/PVA foams produced minimal reductions in Li+capacity due to its high porosity,excellent surface area,and superior LMO loading (250 wt%).Therefore,they proposed that this material,which does not require extra energy-intensive processes,could be used practically for Li+mining from secondary sources.However,like most foam materials,the lower mechanical strength of the LMO/PVA foam is still the main factor limiting its large-scale industrial application.
5.4.Other methods
In addition to the three methods mentioned above,there are some less commonly used methods for LMO forming.We summarized some of these methods in this section.
Composite nanofibers could resolve the persistent limitations existing in currently available LMO-type LIS due to the unique structural characteristics of nanofibers,including high specific surface area,good morphology,high porosity,and unique mechanical strength [76-78].Park et al.[79]prepared a composite PAN nanofiber with ion-sieve powder.As is shown in Fig.20,this composite material has stronger mechanical strength and longer service life compared with currently available LMO composites.
Non-woven package method is an interesting LMO forming method proposed by Chung et al.[80]The schematic diagram is shown in Fig.21.In this method,the inorganic adsorbent powder was wrapped in PSF/non-woven fabric composite membrane to form a “tea bag”.The proposed system has the advantage of direct application in the sea without using pressurized flow system.Thus,they indicated that it could be the most promising system for lithium uptake from seawater.However,it would be a big challenge to avoid the leakage of ion-sieve particles.
Recently,Kim et al.[81]prepared magnetite-LMO composite adsorbents(M-LMOs)for reducing the difficulty of separation by growing magnetite on LMO.This method has the advantages of non-toxicity,low cost,and facile synthesis.The results confirmed that the Li+adsorption capacity was 1.2 mg g-1in concentrated seawater and it can be easily separated from the liquid by an external magnetic field.However,the growth of magnetite will destruct LMO,which is also a critical problem to be solved in future work.
6.Concluding remarks
As a new,efficient,green and economical adsorbent,LMO-type LIS has great development potential and application prospects.Additionally,compared to other conventional methods,the adsorption process has excellent safety,reliability,and high effi-ciency.It is no doubt that the development of LMO-type LIS will effectively alleviate the market pressure for lithium resource across the world.
Nanostructured LMO precursors have been synthesized by the soft-chemical method,which is of considerable significance to improve the selectivity,adsorption capacity and adsorption efficiency of the adsorbent.Li1.67Mn1.67O4has the highest theoretical adsorption capacity among LMO-type LIS,so we should focus on this kind of material in future studies.Furthermore,based on convention methods,we need to try to integrate the advantages and disadvantages of various methods for composite synthesis or develop other new technologies to synthesize precursors with higher Li/Mn molar ratio.
On the other hand,fundamental research is urgently needed to better understand the adsorption/desorption behavior,including adsorption kinetics,isothermal adsorption performance,manganese dissolution and lithium intercalation/de-intercalation mechanism of LIS.Currently,another big challenge in converting LMO-type LIS from laboratory to large-scale industrial applications is the forming method.We should optimize the forming method to resolve the problem of reduced adsorption capacity and uptake efficiency compared to raw powder.
Declaration of competing interest
There are no conflicts of interest.
Acknowledgements
We thank the funding support from National Natural Science Foundation of China Project (grant No.21677098,No.51375253,No.51703116 and No.51775296) and Shanghai government(18230742500 and TP2016034).We also acknowledge the support of this work from Research Program of State Key Laboratory of Tribology Tsinghua University (SKLT2018C06).
 Progress in Natural Science:Materials International2020年2期
Progress in Natural Science:Materials International2020年2期
- Progress in Natural Science:Materials International的其它文章
- Hollow bismuth ferrite combined graphene as advanced anode material for sodium-ion batteries
- Magnetically separable and visible light-active Ag/NiCo2O4 nanorods prepared by a simple route for superior photodegradation of atrazine in water
- Solid electrolyte composite Li4P2O7-Li3PO4 for lithium ion battery
- Wearable supercapacitor self-charged by P(VDF-TrFE) piezoelectric separator
- Enhanced superconducting performance in solution-derived YBCO-BaZrO3 composite film
- FeCo-based hybrid MOF derived active species for effective oxygen evolution
