A Pilot Test for A One-year Cognitive Training Intervention in Elderly Adults with Mild Cognitive Impairment
MENDOZA-HOLGADO Cristina, LOPEZ-ESPUELA Fidel, MORAN Jose Maria,RONCERO-MARTIN Raul, LAVADO-GARCÍA Jesús, ALIAGA Ignacio,PUERTO-PAREJO Luis Manuel, LEAL-HERNANDEZ Olga, VERA Vicente, and PEDRERA-CANAL Maria
In Spain, the proportion of elderly individuals has increased to 19.1%. By 2068, the proportion of elderly individuals is estimated to increase to 29.4%given the largest birth cohorts in Spain’s history(Instituto Nacional de Estadística, INE). This challenge needs a supportive plan, as economic and caring problems may occur because of age-related health problems, including cognitive decline and dementia[1]. Mild cognitive impairment (MCI)represents a transitional state between healthy aging and very mild Alzheimer’s disease[2]. Several studies in Spain reported a prevalence of MCI ranging from 14.5% (range, 12.4%–16.8%) to 17.6%(range, 14.3%–20.9%) among people aged > 65 years, and its prevalence increases with age[3]. Thus,older adults with MCI have high risk for disability in daily activities and require support in the form of caregiver assistance, community resources, and long-term care[4]. The original Mayo criteria for MCI focus on memory impairment with the relative preservation of other cognitive domains and essentially intact activities of daily living (ADL);however, these criteria have been expanded[2].Although ADL are largely preserved, some difficulties can be present in the early stage of cognitive decline;the most common issues in daily functioning are related to appointment scheduling/attendance,transportation, and financial management[5]. In addition, people with MCI need more time to cope with instrumental ADL.
Currently, no pharmacological treatments are recommended for MCI, so the focus for treatment is increasingly being shifted to the preclinical stage,and an increased effort is exerted to establish the efficacy of nonpharmacological interventions[6]. In particular, cognition-based interventions are increasingly being recognized as important adjunct methods and, in some cases, as alternative to pharmacological treatments for individuals with dementia and those at risk for dementia[7]. Most of the previous studies on cognitive training analyzed people with mild to moderate dementia. In fact,several reviews were conducted on MCI and cognitive training[5].
This prospective study aimed to assess the effect of a cognitive intervention on the cognitive status of people with cognitive impairment. As regards the protection of rights and research, the Clinical Research Ethics Committee of the CHUC approved this study. All participants provided written informed consent.
This study was outlined inPlan de Atención al Deterioro Cognitivo(PIDEX) and was developed byConsejería de Sanidad y Políticas Sociales de Extremadura(Spain). In this project, the participants were enrolled in a cognitive intervention for at least 1 year that was conducted by an occupational therapist. All assessments were performed at baseline and immediately after the intervention(posttest).
The sample consisted of 36 patients aged 53 years. All patients had essentially normal ADL performance and were living independently in the community. All participants met the following inclusion criteria: (1) a formal diagnosis of cognitive impairment, (2) Global Deterioration Scale (GDS)score between 3 and 4, and (3) a communitydwelling living status. The exclusion criteria were as follows: (1) psychiatric disorders, (2) presence of sensorial impairments that would hinder the sessions, and (3) moderate to severe anosognosia.The assessments were completed before and after the intervention.
A general interview questionnaire was developed by the main researcher to collect sociodemographic characteristics of the subjects (age, sex, level of education), medical history, and current pharmacological treatment data. Cognitive status was assessed with the Spanish version of the Montreal Cognitive Assessment (MoCA)[8], which is a screening instrument used to assess global cognitive function, and GDS, which is a brief clinical rating of dementia severity. The Charlson Comorbidity Index(CCI) was used as a method to quantify the number of chronic disorders and their severity. Moreover,Lawton and Brody scale was used to assess the abilities to perform instrumental ADL or complex tasks, and Barthel index was used to assess basic ADL.
The interventions were administered on an individual basis for 45 min and were conducted by an occupational therapist. All subjects participated in a cognitive intervention session once or twice per week for a total of 12 months. The intervention focused on different aspects. First, a semistructured occupational interview was carried out to identify the participant’s personal goals relevant to everyday life and preserved skills. The cognitive intervention included cognitive training, which typically involved tasks designed to reflect particular cognitive functions, such as memory, attention, or executive function. Furthermore, restoration or compensation techniques were performed according to personal needs. External aid strategies were offered to compensate for memory difficulties, and internal strategies for approaching learning and remembering were practiced to learn new information. The participants applied these strategies to daily life situations, and the families took part in the interventions, which could contribute to the implementation of the strategies.Environmental adaptations were used to enhance the performance of the participants in daily life. The subjects were encouraged to develop healthy habits and routines according to their daily experiences and personal cognitive failures.
The descriptive analysis was performed by calculating the percentage of categorical variables and de mean together with the standard deviation of distribution in continuous variable (age). The Chi-Square test or Fischer exact test was used to examine the between-group differences in categorical variables. The Mann-WhitneyUtest was used with continuous variable. The analysis of paired group at baseline, 6 months and 12 months was carried out with Wilcoxon test (MoCA Scores and Lawton and Brody) and the McNemar test for categorical variables. The Mann-WhitneyUtest was carried out with unpaired group (womenvs.men).Post-Hoc analysis was performance with Connover’s Pos Hoc Test. Statistical significance was established whenP< 0.05. Analyses were performed using JASP 0.9.0.1 software.
The average participant age was (71.3 ± 7.2)years. The female and male patients were (72.15 ±7.64) and (70.38 ± 6.83) years of age, respectively(P= 0.271). Most of the participants completed high school (36.1%). Two participants were unschooled(5.6%), and three obtained a university degree(8.3%). No statistically significant differences were found in the distribution of the educational level between the female and male patients (P> 0.05 for all categories) (Table 1). In addition, no differences were noted in the marital status between sexes, with the majority being married in both groups (Table 1).No differences were observed in the type of MCI or in the diagnosis (Table 1) between sexes. As regards the GDS, 83.3% of the participants had MCI, while the remaining patients had mild dementia (Table 1).No differences were found in the GDS score between sexes (P= 0.05). The comorbidities were similarly distributed between sexes, except for diabetes mellitus which was present in 56.3% (n= 9) of the male patients (P= 0.037). Overall, the Charlson Comorbidity Index (CCI) was 3.69 (1.00). No statistically significant differences were found between female and male patients in terms of CCI[3.55 (0.88)vs.3.87 (1.14);P= 0.481]. Statistically significant differences were noted between the baseline and follow-up MoCA scores at 6 and 12 months (Table 2). However, no statistically significant differences were observed between 6 and 12 months (P= 0.148) in the MoCA score. A timedependent effect was not observed in female patients (P= 0.118), while an increase in the MoCA score was observed in male patients from baseline to 6 months (P= 0.016) and to 12 months (P< 0.001)(Table 2). In addition, no statistically significant differences were observed in the MoCA score between 6 and 12 months among male patients (P=0.169) (Table 2). Low to moderate effect sizes(Cohen’s d) were observed for the total sample from baseline to 12 months [d = 0.29 (95% confidence interval (CI): 0.09–0.50)] and in male patients between baseline and 12 months [d = 0.36 (95%CI:0.07–0.68)]. No statistically significant differences were observed from baseline to 12 months in either the Barthel index (data not shown) or Lawton and Brody index (P> 0.05) (Table 2).
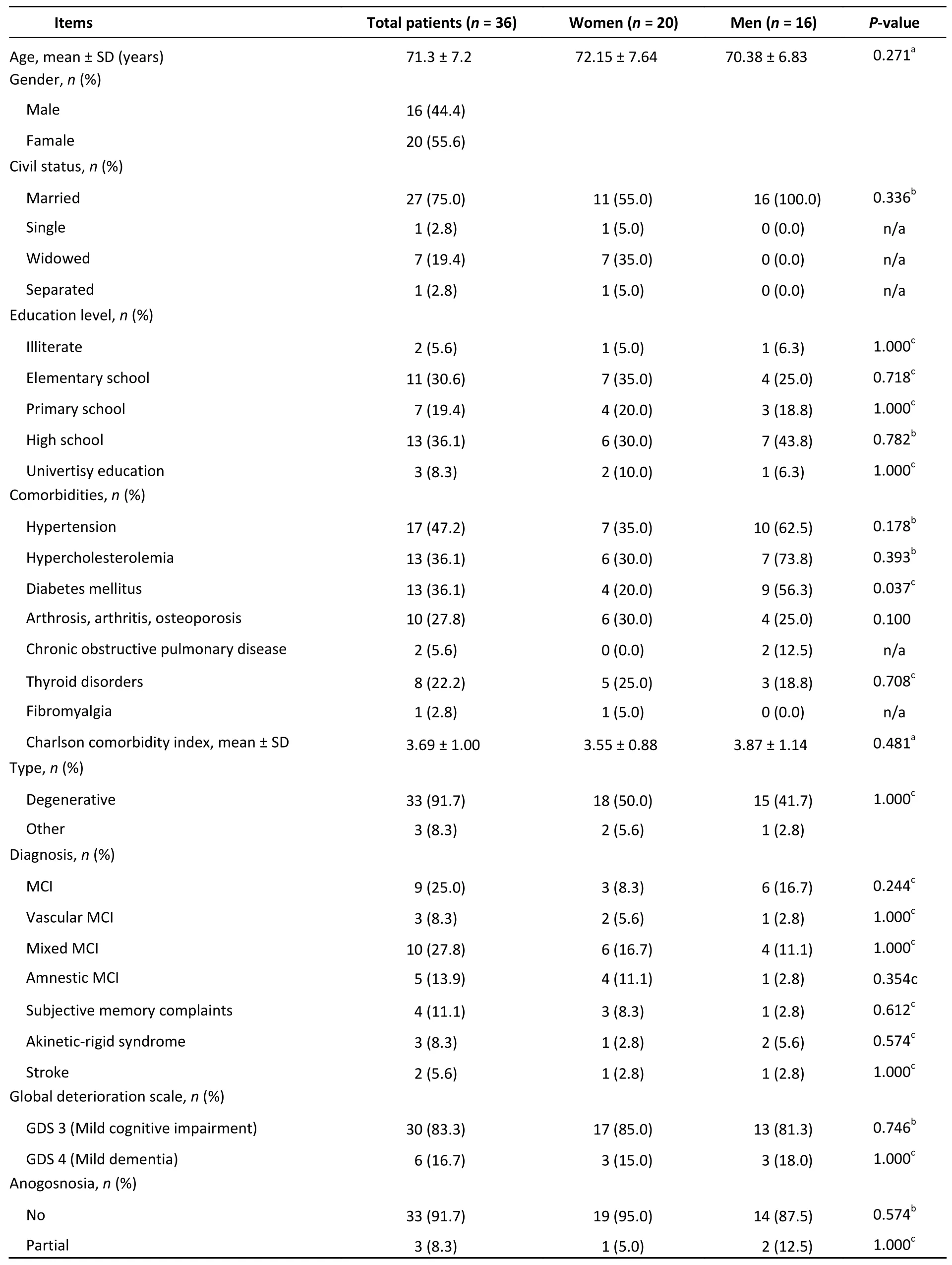
Table 1. Demographic and clinical characteristics of patients
In all patients, statistically significant differences were observed between baseline and 12-month MoCA subtest scores for visuospatial/executive function (P= 0.001), language (P= 0.012), and abstraction (P= 0.011). For the visuospatial/executive function score, a significant increase was observed from baseline to 6 months, and the score remained similar at the 12-month follow-up (P=0.001) (Table 3). For abstraction, a significant increase was observed from baseline to 12 months(P= 0.003), while for language, an increase in MoCA subtest score was observed from baseline to 6 months (P= 0.024). Then, the subscore returned to the baseline level at 12 months (P= 1.00). In the subgroup analysis, statistically significant results were observed among male patients in terms of language [at 6 months (P= 0.003)], and the score returned to the baseline level (P= 1.00). As regards naming and abstraction, increases in scores were observed at 12 months compared with the baseline(P< 0.05 for both) (Table 3). No statistically significant results were observed for the MoCA subtest scores among female patients (P> 0.05).
In the total sample, multiple linear regression analysis showed that the intermediate MoCA score(B= 0.950,P< 0.001) was associated with the final MoCA score. Participant’s age, baseline MoCA score,CCI, sex, and baseline and final Lawton and Brody scores were not predictors of the final MoCA score in the studied sample (P> 0.005 for all).
A cognitive intervention demonstrated feasibility with improvement on important outcomes, such as the MoCA score, which is a very credible measurement tool of cognitive decline in elderly people with MCI and can effectively reflect changes in an individual’s overall cognitive function before and after cognitive training[8]. Cognitive training is considered a promising way to delay cognitive function decline. Unlike experimental studies conducted in controlled clinical settings, this study used quantitative and qualitative evaluation approaches that considered real-life factors influencing MCI. Elderly adults with MCI participatedin this training program, which may stimulate brain activity and promote cognitive ability, as the participants are required to use cognition to complete all tasks independently.
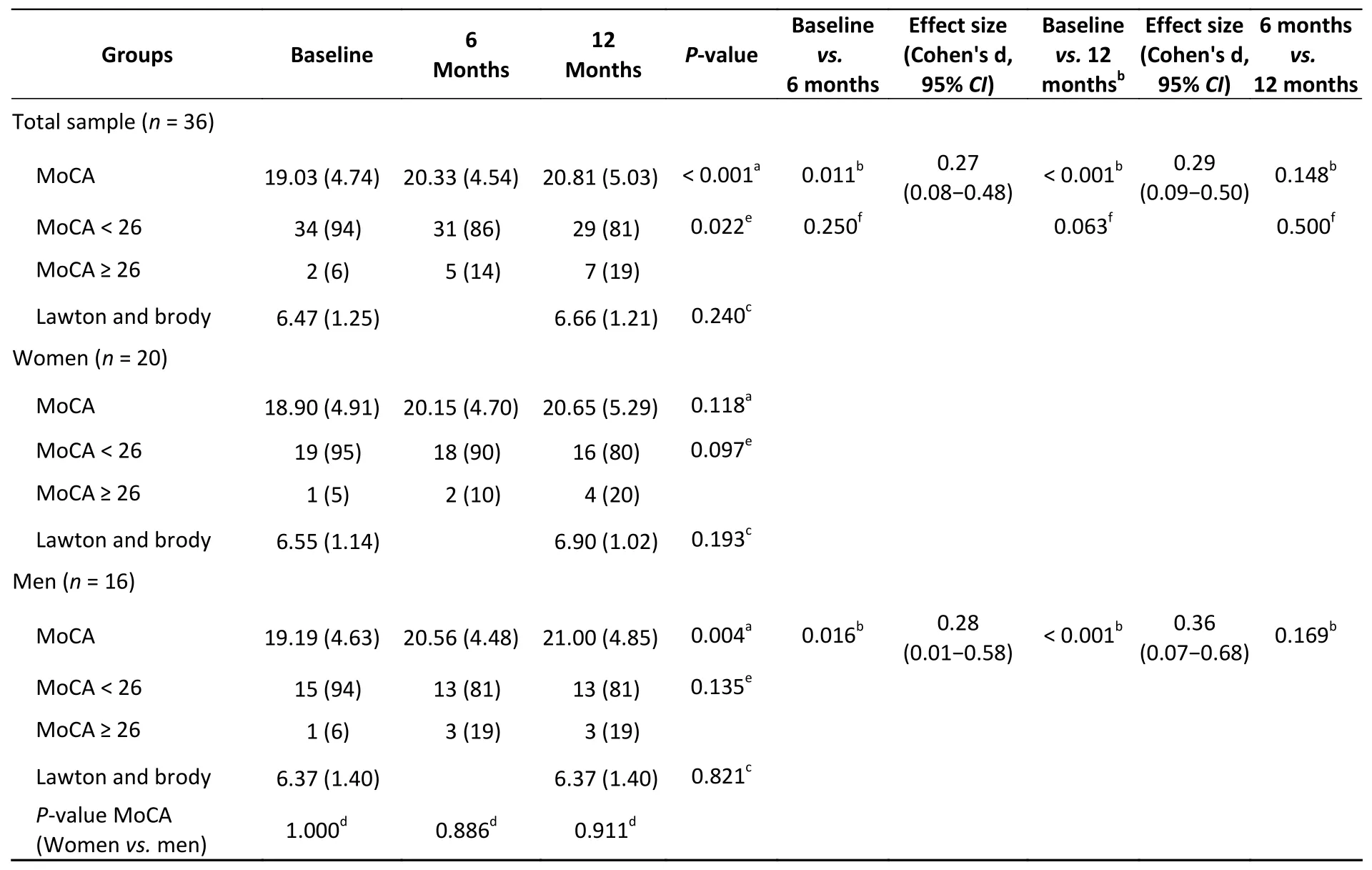
Table 2. Scores in MoCA at baseline, 6 months and 12 months and lawton and brody at baseline and 12 months
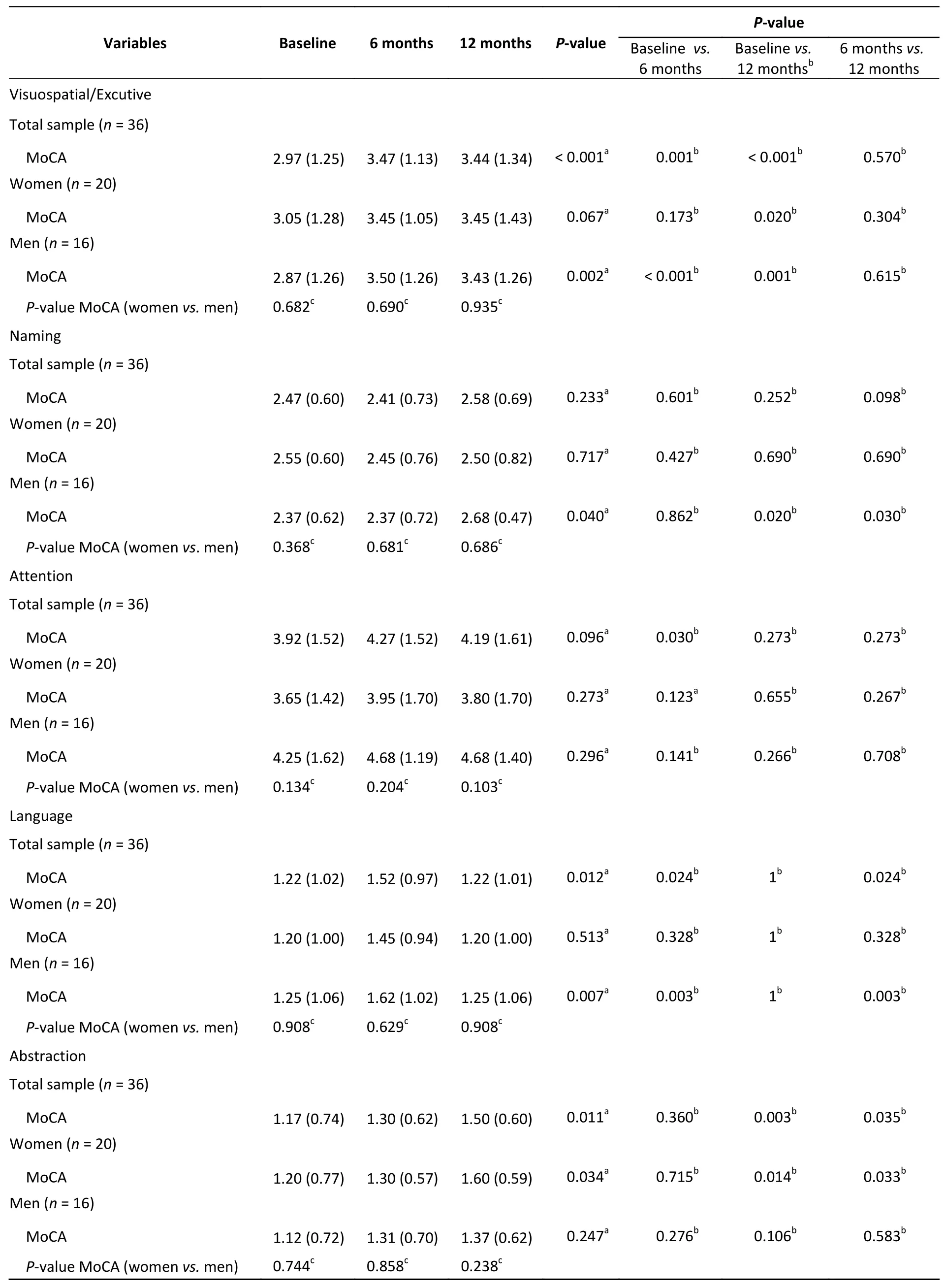
Table 3. Analysis of MoCA scores
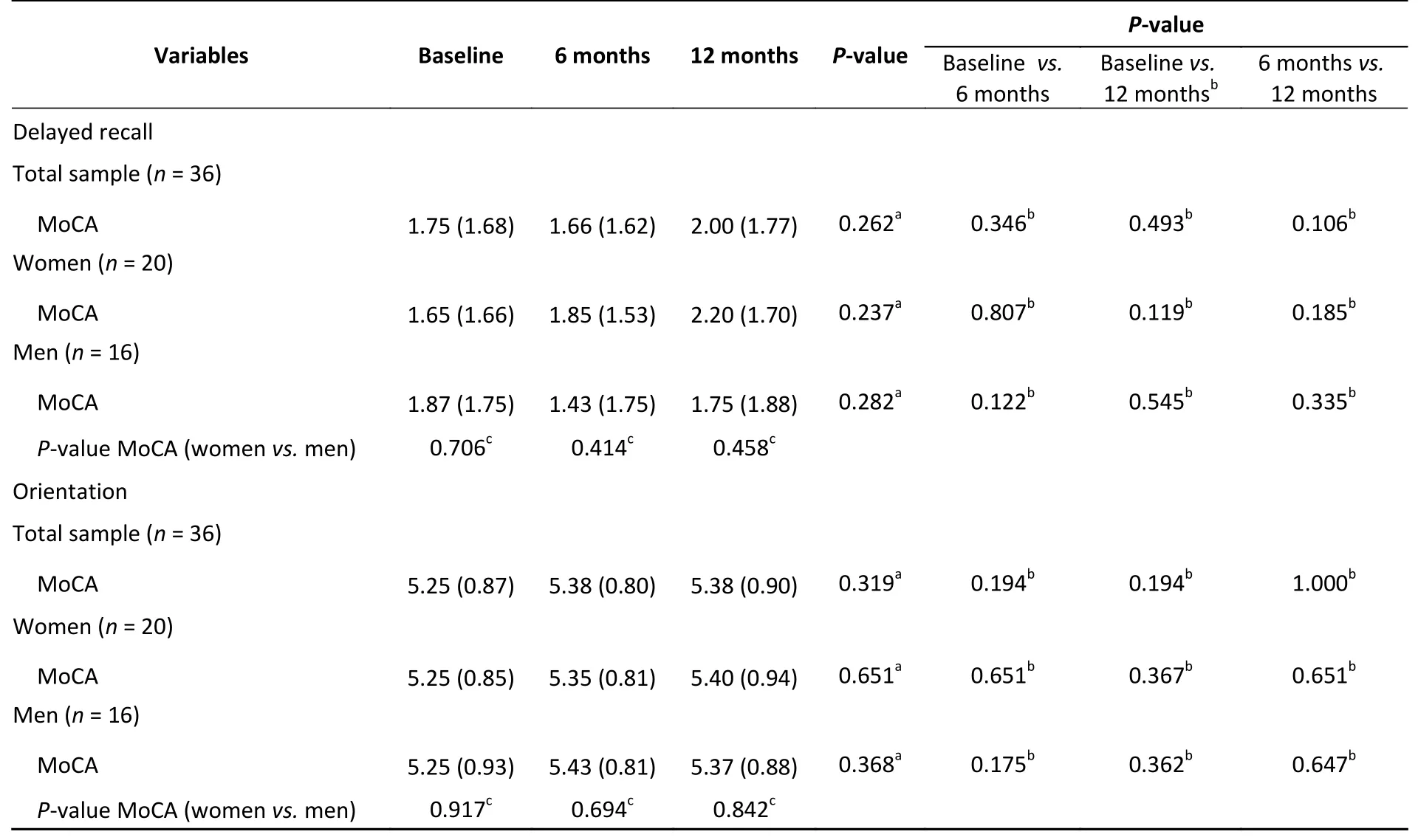
Continued
The items in the MoCA scale may be difficult for some participants to understand, particularly for participants with a low level of education and limited verbal skills[1]. In this study, the participants were> 53 years old, and the mean age was (71.3 ± 7.2)years. Decline in bodily functions, especially in the nervous and perception systems, greatly influences cognitive abilities and the ability to perform ADL.Our results from the overall sample analysis and subgroup analysis demonstrate that cognitive training improved the cognitive ability of patients with MCI, particularly among male patients.Improvements were observed mainly between the baseline and metaphase (6 months), and greater improvements were observed in male patients.
Although the level of education has been suggested to be a key factor affecting cognitive abilities, and an association between fewer years of education and faster decline in cognitive ability with age[9]have been observed, the educational level was similar between male and female patients, so the MoCA test scores were not influenced by the educational level. We did not observe differences in the subtest scores between the male and female patients at any time point. This observation confirms the multiple linear regression results, that is, sex was neither a positive nor a negative predictor of the final MoCA score in the studied population. The small sample size in this pilot study may be responsible for the partially positive results related to male sex; thus, additional studies with larger sample sizes and control groups are warranted to clarify the role of sex on the MoCA score.
Cognitive training involves guided practice on a set of standard tasks that are designed to reflect particular cognitive functions, such as memory and executive function. Overall, our results confirm those of other studies, indicating that cognitive training for patients with MCI can improve cognition[1], and support the hypothesis that continuous cognitive training in the elderly population can help stimulate cerebral function and delay degeneration[10].
In this study, follow-up evaluations were performed at 6 and 12 months, and we aimed to detect whether improvements remained over time.No significant improvements were observed after 1–2 cognitive training sessions per week for 6 months, so we concluded that the benefits of cognitive training were evident up to 6 months of training. For a cognitive training program, the length of time needed for the program is crucial. The cognitive training program used in this study could effectively delay the decline in overall cognitive ability in elderly people with MCI for up to 6 months,and this conclusion was supported by the result that the MoCA score tended to be stable from 6 months to 12 months.
This study has some limitations. The sample and design characteristics limited the generalizability of the results of this pilot study. In addition, generalizability of the findings is limited by the intervention parameters and outcomes of interest. Although this was a small-scale pilot study that was not intended to produce generalizable results, the findings indicate that cognitive training for elderly patients with MCI is promising. The next step is to use our preliminary sample size calculations and study design for a randomized controlled trial to determine the efficacy of cognitive intervention.Future research studies should involve a larger sample of patients with MCI to test the efficacy of cognitive training compared to a control condition.
In conclusion, this study demonstrates improvement in the global cognitive performance after a cognitive intervention for elderly people with MCI, according to objective experimental cognitive measures. In addition, we observed that benefits were mainly evident after 6 months of training compared with the baseline and tended to remain stable from 6 months to 12 months. Our findings support the common belief that continuous cognitive training can help delay the onset of disability and dementia. We encourage researches to perform additional studies, particularly longitudinal studies, that involve larger samples of participants and well-controlled randomized designs.
&These authors contributed equally to this work.
#Correspondence should be addressed to MORAN Jose Maria, PhD, E-mail: jmmorang@unex.es
Biographical notes of the first authors: MENDOZAHOLGADO Cristina, female, born in 1985, MS, PhD Candidate, majoring in mild cognitive impairment and neurodegenerative disease; LOPEZ-ESPUELA Fidel, male,born in 1974, PhD, majoring in metabolic bone disease.
Received: March 18, 2019;Accepted: August 15, 2019
 Biomedical and Environmental Sciences2020年10期
Biomedical and Environmental Sciences2020年10期
- Biomedical and Environmental Sciences的其它文章
- Application Research on Gated Recurrent Unit Deep Learning Prediction and Graded Early Warning of Emergency Department Visits Based on Meteorological Environmental Data*
- Safflower Yellow Compounds Alleviate Okadaic Acid-lnduced lmpairment of Neurite Outgrowth in Differentiated SH-SY5Y Cells*
- Concentration of Selected Elements in the Tissues of the Knee Joint*
- Antineoplastic Drug Handling: Higher Risk for Healthcare Workers in Tunisia than in France?
- Recent Transmission Analysis of 66 MDR-TB Strains Using the 15-locus MlRU-VNTR Method*
- Multidimensional Analysis of Risk Factors Associated with Breast Cancer in Beijing, China: A Case-Control Study*
