Diagnostic and prognostic role of long noncoding RNA MALAT1 in breast cancer:a meta-analysis
Xue-Feng Jiang,Yi-Gui Lai,Qiang Wang,Ye-Jian Hu,Fang-Hua Yang,Hui-Jie Fan,2
1Department of Traditional Chinese Medicine,Yangjiang People’s Hospital,Yangjiang 529500,China.2College of Traditional Chinese Medicine,Southern Medical University,Guangzhou 510515,China.
Abstract
Keywords:Diagnostic,Prognostic,MALAT1,Breast cancer
Background
Cancer is expected to become the main cause of death and the most challenging stumbling block to human life extension in the 21st century [1].According to the GLOBOCAN 2018 estimated,there are about 18.1 million new cancer cases and 9.6 million deaths worldwide.Among females,breast cancer (BC) was the most frequently diagnosed cancer as well as the leading cause of cancer death.There would be 2,088,849 newly diagnosed cases and 626,679 deaths of female BC that year[2].
Currently,breast biopsy is the most reliable procedure for diagnosis,and mammography is diffusely utilized in the screening and detection of BC.Whereas these methods are defective in invasive and radioactive,which hinder women’s health-seeking as well as early diagnosis.In addition,clinical pathological features,e.g.,solid tumor size,lymph node status,histological grade,etc,are not subtle enough to predict the trend of BC.It is urgently needed to identify novel noninvasive biomarkers for early diagnosis and prognosis of BC patients who are receiving increasing critical public health concerns.
Mammalian Genome Sequencing projects has demonstrated that at least 98%of the human genome is transcribed into non-coding RNAs [3,4].Decades of RNA biology studies and innovations in RNA-sequencing technology,revolutionizing our understanding of lncRNA,that has emerged as the result of an important regulator of almost all aspects of biology [5,6].Originally,lncRNAs were elucidated primarily as having nuclear function and only until lately,where studies showed that most lncRNAs present in the cytoplasm,and in particular in ribosome complexes,having a potential function in post transcriptional gene regulation,protein and transcript trafficking and shuttling [7,8].Meanwhile,lncRNAs have also been shown to play critical roles in different processes of tumor development such as tumorigenesis,invasion,metastasis,and drug resistance[9].
Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) is a highly abundant nucleus-restricted RNA with a length of 8.7 kb on chromosome 11q13 [10].There is growing evidence that MALATI has extensive biological functions through multiple mechanisms in different tissue contexts,such as regulate cell cycle and promote gene expression and regulating splicing of mRNAs [12].MALAT1 regulates the pre-mRNA processing of p53 and B-MYB to promote cell proliferation [13,14].Since MALAT1 was found to be a prognostic marker for lung cancer metastasis,it has been associated with several human tumor entities.It may be related to clinical parameters and may control the proliferation,apoptosis,migration or invasion of tumor cells[15].
Recently,numerous studies have reported that MALAT1 may be a promising biomarker [16–19].However,the results of the MALAT1 study were still controversial due to uncontrolled factors such as different sample sources,different disease statuses and different detection methods.Although several published meta-analyses have evaluated inconsistent results for MALAT1,there are still limitations to the obtained findings.Most meta-analysis merely focused on the value of MALAT1 as diagnosis or prognosis marker [20–22].In addition,many researchers ignored the heterogeneity of different lncRNAs or different cancer types and merged MALAT1 with other lncRNAs to obtain the value of lncRNAs on BC [21],while some investigators examined the significance of MALAT1 to most cancers,including BC [20,22].Meanwhile,some of them were conducted using relatively few studies [20–22].These studies discussed too little of our concerns.Thus,we conducted a more integrative meta-analysis based on all relevant reported studies to explore the diagnostic and prognostic value of MALAT1 in BC,which was intended to provide the fresh biomarker for cancer.
Methods
Search strategy
Two researchers searched the electronic databases(PubMed,MEDLINE,EMBASE,Web of Science,CNKI,VIP and Wanfang).The following search strategies were applied for literature retrieval:(breast cancer OR breast tumor OR breast tumour OR breast carcinoma OR breast neoplasm) AND (Metastasis Associated Lung Adenocarcinoma Transcript 1 OR long noncoding MALAT1 OR MALAT1 OR Nuclear-Enriched Abundant Transcript 2 OR NEAT2).Additionally,the reference catalogues of the included literature were checked and relevant studies were collected [23].The latest search was updated until December 5,2019.
Study selection criteria
The inclusion criteria were as follows:(1) clinical studies in respect to the diagnostic and prognostic function of MALAT1 in BC;(2) patients were histopathological diagnosed with BC;(3) studies were designed as case-control studies;(4) studies embodied ample data for calculating the rates of true positive(TP),false positive (FP),false negative (FN),and true negative(TN)for diagnostic studies or odds ratio(OR)and hazard ratio (HR) with corresponding 95 %confidence interval (CI) for prognostic studies;and (5)the study population was larger than 20.The exclusion criteria were:(1) meetings,letters,reviews,case reports,abstracts,animal and in vitro studies;(2)incomplete data;(3)duplicate publications.
Data extraction
Two researchers extracted data from eligible articles.The information was collected as follows.(1)Fundamental details containing:the first author,publication year,nation,race,pathologic type,sample size and specimen source,test methods and reference genes.(2) Diagnostic results including:area under curve (AUC),sensitivity,specificity,cut-off value,and diagnostic 4-fold contingency table (TP,FP,FN,TN).(3) Prognostic consequences containing:time of follow-up,cut-off value and HR estimates with 95%CIs for overall survival (OS),recurrence free survival(RFS),metastasis-free survival (MFS),disease specific survival (DSS),disease free survival (DFS),and other clinical parameters such as age,solid tumor size,tumor node metastasis (TNM) stage,lymph node metastasis(LNM),estrogen receptor (ER) and progesterone receptor(PR)status[24].
Quality assessment
Quality assessment of diagnostic accuracy studies(QUADAS)-2 was used to assess the quality and the risk of bias of eligible diagnostic studies [25].For prognostic studies,newcastle-ottawa scale (NOS) was used to examine the methodological quality[26].

Figure1 Flow diagram of the study selection proces
Statistical analysis
All these analyses were conducted using Meta-Disc 1.4 and Stata 15.0.With respect to the diagnostic meta-analysis,a bivariate model was utilized to calculate the pooled sensitivity,specificity,the positive likelihood ratio (PLR),the negative likelihood ratio(NLR) and the diagnostic OR (DOR) with their corresponding 95%CI.The summary receiver operator characteristic (SROC) curve was plotted and the pooled AUC value was computed.Heterogeneity among studies was estimated using the Cochran’s Q statistic andI2tests.P<0.05 orI2>50%were defined to have heterogeneity.Deek’s funnel-plot was utilized to determine the potential publication bias.For the prognostic meta-analysis,all of the ORs and their 95%CIs to calculate were combined to the association of MALAT1 with clinical features of BC.As for survival rates,HRs with corresponding 95% CIs were used.WhenI2value was more than 50%,which indicated a notable heterogeneity,the random-effects model was selected;else,the fixed-effects model was applied[27].Subgroup analysis and sensitivity analysis were further carried on to detect the source of heterogeneity.Finally,Egger’s linear regression test was used to determine publication bias.P<0.05 was considered significant.
Results
Identification of the included studies
The flow diagram of this study was shown in Figure1.Totally,365 articles were picked out by mining databases and manual searching,of which 112 were duplicates.Following application of the inclusion and exclusion criteria,19 articles were finally enrolled in the meta-analysis[18,19,28–44].For the most part of these articles were published in the last three years.9 articles explored the diagnostic value of BC [19,28–35],9 articles described the clinicopathological parameters of BC [18,19,36–39,41–43],and 10 articles investigated the survival outcomes of BC [18,19,36–42,44].
Diagnostic meta-analysis of MALAT1 in breast cancer
Study features and quality evaluation.About diagnostic value,totally 9 articles [19,28–35] were analyzed,covering 10 studies,involving 744 patients and 526 controls.All studies were from China except one from Egyptian.There were 7 studies [19,30–35]of BC and 3 study [28,29] of triple negative breast cancer.The sample sizes included from 60 to 200.Besides,the sample types were plasma in 2 studies,serum in 6 studies [30,35],tissue in 1 study [32],and plasma exosomal in 1 study [33].The MALAT1 expression was used quantitative real time PCR(qRT-PCR) methods and was normalized to GAPDH and β-actin.The characteristic of each study was presented in Table1.Figure2 summed up the ratios of risk of bias by QUADAS-2 checklist,and all enrolled studies had high score(≥4)(Table1).

Table1 Main characteristics of the eligible diagnostic studies.

Figure2 Study quality and bias assessment was conducted by QUADAS-2
Diagnostic accuracy of MALAT1 in breast cancer.There was significant heterogeneity between studies for theI2values in sensitivity (88.86,P≤0.001),specificity (76.21,P≤0.001),PLR (64.92,P≤0.001)and NLR (86.80,P≤0.001).Random effect model was selected for next analysis.Forest plots and the results of the sensitivity and specificity of MALAT1 were shown in Figure3.The combined sensitivity and specificity with 95%CIs of MALAT1 in BC were 0.81(95% CI:0.71–0.88),and 0.77 (95% CI:0.68–0.84).The overall pooled results for PLR,NLR and DOR were 3.52 (95% CI:2.39–5.17),0.25 (95% CI:0.15–0.41),and 14.09 (95% CI:6.34–31.31),respectively (Figure4 and Figure5).Moreover,the combined AUC value was predicted to be calculated to be 0.85 (95% CI:0.82–0.88) from the SROC curve(Figure6),evidencing that MALAT1 had lofty diagnostic accuracy for BC.
Analysis diagnostic threshold effect.Two main reasons for heterogeneity of diagnostic tests are threshold and non-threshold effect.Spearman’s correlation coefficient was selected to explore heterogeneity from threshold effect.Spearman correlation coefficient was 0.055 with aP-value of 0.881(P>0.05),implying that the threshold effect has no heterogeneity.In addition,the result (I2>50%)showed that the existence of heterogeneity from non-threshold effect among these studies.
Subgroup analysis.In this inquiry,subgroup analyses were organized to explore the origins of heterogeneity according to race,BC subtype and specimen.As shown in Table2,we found that AUC values of the studies were 0.85 for Asian populations versus 0.89 for Caucasian ones.When stratified by specimens,the pooled sensitivity,specificity,and AUC were 0.84(95% CI:0.70–0.93),0.81 (95% CI:0.70–0.88) and 0.89 for serum–versus 0.73 (95% CI:0.66–0.79),0.83(95%CI:0.76–0.89),and 0.90 for plasma-,which were superior to tissue-.The subgroup analysis of BC subtypes showed the diagnostic effect of MALAT1 in BC was highter than that in TNBC (AUC:0.81 versus 0.85).Sensitivity and meta-regression analysis.As shown in Figure7,there was no study on exceeding the upper or lower limits of CI,indicating that the picked out studies had lofty homogeneity.In order toexplain the source of heterogeneity,meta-regression analysis was conducted depending on race (P=0.82),pathologic type (P=0.88),specimen (P=0.60)(Table2).The results showed that there was no significantly heterogeneous among these factors.
Publication bias.By Deek’s funnel plot asymmetry test,the publication bias was assessed evaluated.TheP-value of 0.19 and the funnel plot symmetry(Figure8)showed not evident publication bias in this analysis.
Clinical utility and index test of MALAT1.As shown in Figure9,when MALAT1 was tested in all individuals with a pretest probability of BC of 50%,a positive result improved the post-test probability suffered BC to 78%,while a negative result dropped the post-test probability to 20%.All of the results indicated that MALAT1 was the diagnosis biomarker for BC.
Prognostic meta-analysis of MALAT1 in breast cancer
Study features and quality evaluation.Totally 3,943 patients from 11 articles [18,19,36–44] covering 19 cohort studies were embodied to assess prognostic value.The chief features of the enrolled studies are presented in Table3.The survival outcomes were recoded including 18 studies for OS,RFS,MFS,DSS and DFS,10 of which were univariate analysis and 8 of which were multivariate analysis.Additionally,1,693 patients from 9 studies [18,19,36–39,41–43]explained clinical parameters including age,tumor size,TNM stage,LNM,ER and PR status.The patients’ethnic backgrounds were classified as Asian or Caucasian.Most studies enrolled more than 100 samples.Among all these studies,14 studies were about BC and 5 studies were about TNBC.Detect MALAT level by way of qRT-PCR and in situ hybridization.The quality of these studies were high(Table3).
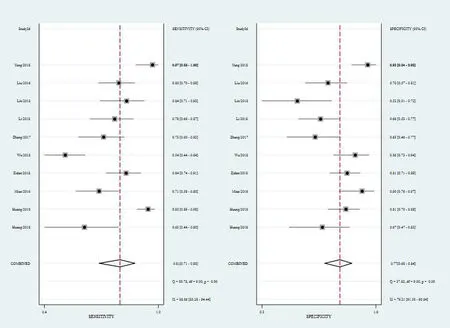
Figure3 Forest plots of sensitivity and specificity for metastasis associated MALAT1 in breast cancer

Figure4 Forest plots of PLR and NLR for MALAT1 in breast cancer

Figure5 Forest plots of DOR for MALAT1 in breast cancer
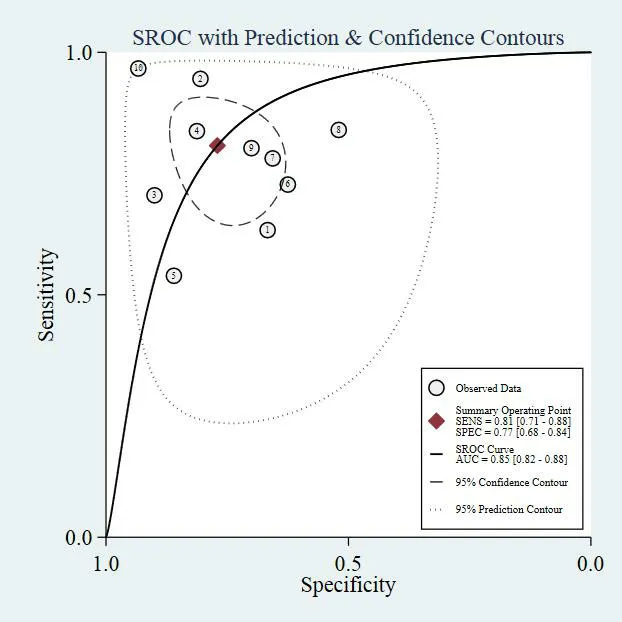
Figure6 SROC curve for MALAT1 in breast cancer

Table2 Subgroup analysis of MALAT1 in diagnosis of breast cancer

Figure7 Sensitivity analysis for the overall pooled study for outliers
Connection of MALAT1 expression with clinicopathological parameters of breast cancer.The pooled consequences were shown in Table4,and the significant association was presented in Figure10.The increased expression level of MALAT1 was obviously related to positive PR conditions (OR=1.48,95% CI:1.18–1.86,P≤0.001).Nevertheless,there were no significant correlation between MALAT1 expression and age,tumor size,TNM stage,lymph node metastasis or ER status (age:P=0.51,tumor size:P=0.14,TNM stage:P=0.70,lymph node metastasis:P= 0.80 and ER:P=0.07).The up-regulated MALAT1 expression level was associated with positive ER and PR conditions for BC((OR=1.83,95%CI:1.26–2.65,P≤0.001) and (OR =.49,95% CI:1.13–1.98,P=0.01)).In TNBC,MALAT1expression was correlated with lymph node metastasis (OR=4.53,95% CI:1.21–16.96,P=0.03)(Figure11 and Figure12).

Figure8 Deeks’funnel plots for the assessment of publication bias
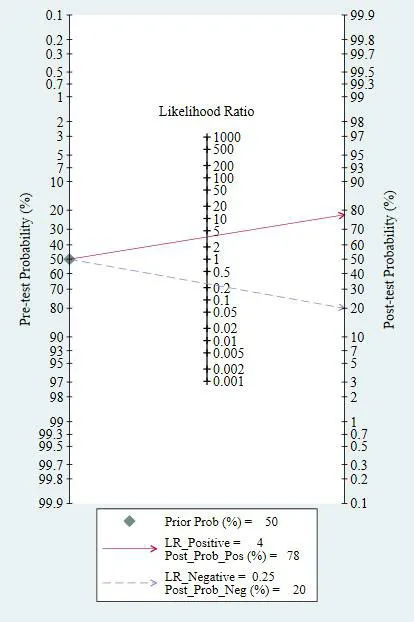
Figure9 Fagan’s nomogram for MALAT1 in the diagnosis of breast cancer

Table3 Main characteristics of the eligible prognostic studies

Table3 Main characteristics of the eligible prognostic studies(continued)

Table4 Relationship between MALAT1 and clinicopathological characteristic in breast cancer
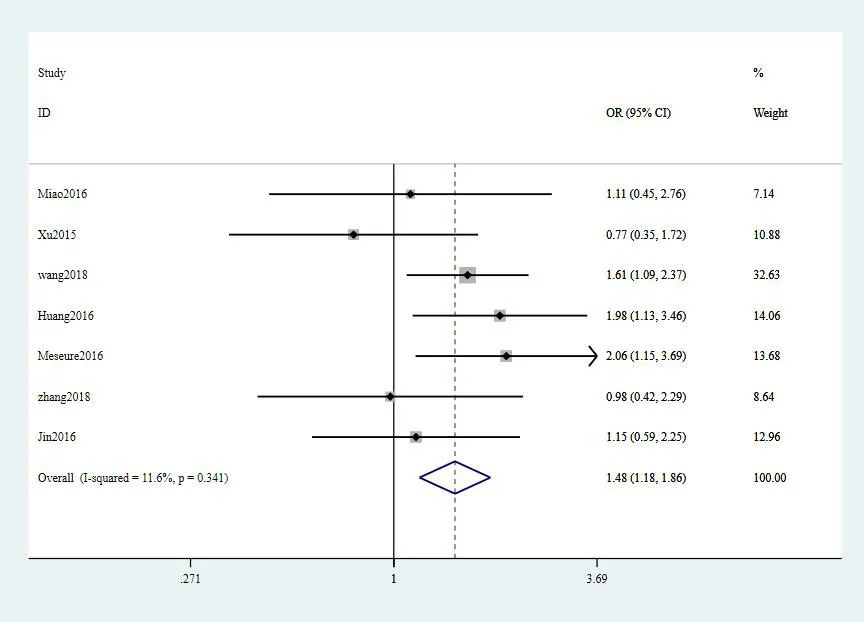
Figure10 Reationship between MALAT1 and PR status in breast cancer
Connection of MALAT1 expression with survival outcomes of breast cancer.Totally 10 articles[18,19,36–42,44] comprising 3,974 patients concentrated on assessing the association between MALAT1 expression and survival outcomes(Table5).Overall,patients with the up-regulated MALAT1 had a higher risk of poor OS (univariate:(HR=2.11,95% CI:1.51–2.97,P≤0.001) and multivariate (HR=2.51,95% CI:1.32-4.77,P=0.01)).As shown in Figure13 and Figure14,he up-regulated MALAT1 had shorter MFS(HR= 081,95% CI:0.67–0.99,P=0.04),DSS (HR =2.40,95% CI:1.43–4.03,P≤0.001) and DFS (HR =2.36,95% CI:1.04–5.38,P=0.01) in univariate analysis.Unlike subgroup of univariate analysis,elevated expression of MALAT1 was related to poor RFS (HR=2.57,95% CI:1.74–3.79,P≤0.001) and DSS (HR=2.49,95% CI:1.48–4.19,P≤0.001) in multivariate analysis.In addition,subgroup analysis of race,tumor type and specimen were not carried on because of the small number of enrolled studies.
Sensitivity analysis and publication bias.The results of Egger’s test revealed no publication bias for OS (P= 0.790) and RFS (P=0.103).In addition,the sensitivity analysis showed that the pooled HR for MALAT1 was associated with OS and RFS.
Discussion
MALAT1 is the first discovered and most widely investigated lncRNA,which caused cell phenotypic changes such as tumor cell viability,invasiveness and migration [46–48].Researches demonstrated that siRNA-mediated knockdown of MALAT1 significantly impaired BC cell viability,proliferation,migration,and invasion,as well as in vivo tumor growth in mice [49–51].Arun reported that in the PyMT-mouse mammary tumor model,knock-down of MALAT1 leads to slow tumor growth,and reduced cystic tumors differentiation and metastasis [52].Mechanistically,MALAT1 might promote development and migration of BC cells through XBP1-HIF-1α pathway and Her-2 pathway [53].Knock-down of MALAT1 inhibited splicing genes expression involved in differentiation and tumorigenic pathways [52].In addition,it was demonstrated that MALAT1 can modulate migration and invasion of BC cells by targeting miR-448 and miR-1 as competing endogenous RNA[54,55].

Figure11 Reationship between MALAT1 and clinicopathological characteristic in breast cancer
In the diagnositic meta-analysis,the pooled sensitivity and specificity were calculated 0.77 (95%CI:0.68–0.84) and 0.81 (95% CI:0.71–0.88),separately.The SROC curve was drawn and the AUC was computed to be 0.85 (95% CI:0.82–0.88),which again showed MALAT1 is a promising biomarker for diagnosing BC.We performed subgroup analysis according to race,BC subtype and specimen,and found that of MALAT1 in caucasian populations was higher than that in Asians.Additionally,the analysis in regards to BC subtype marked that MALAT1 in BC was higher than that in TNBC.In addition,circulating MALAT1 (plasma or serum) had plainly more excellent overall diagnostic accuracy than tissue ones.

Figure12 Reationship between MALAT1 and clinicopathological characteristic in triple negative breast cancer
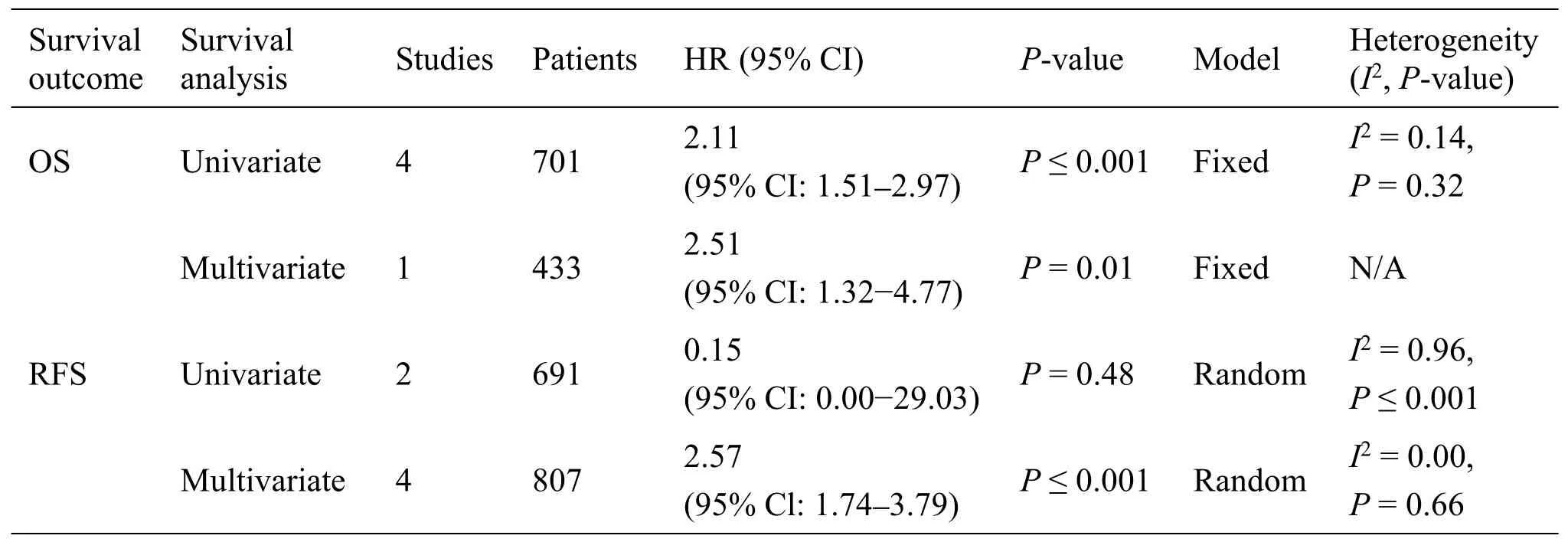
Table5 Reationship between MALAT1 expression and survival outcomes in breast cancer

Table5 Reationship between MALAT1 expression and survival outcomes in breast cancer(continued)

Figure13 The association between MALAT1 expression and survival outcomes in breast cancer in univariate analysis

Figure14 Reationship between MALAT1 and survival outcomes in multivariate analysis
On the other hand,we also methodically looked back all published literature on the clinical and prognostic value of MALAT1 in BC.We found that over-expression of MALAT1 was significantly related to positive PR status (OR=1.48,95% CI:1.18–1.86).The result of subgroup analysis expressed that the up-regulated MALAT1 expression level was associated with lymph node metastasis for TNBC.However,MALAT1 expression was independent of patient age,tumor size,TNM stage,LNM and ER status.These results indicated that MALAT1 may not be significantly correlated with the clinicopathologic features of BC.As for survival outcomes,the study indicated patients with over-expression of MALAT1 had a disappointing OS in univariate and multivariate models.In addition,the high level of MALAT1 had shorter RFS,MFS,DSS and DFS.We have faith that MALAT1 may be a helpful biomarker.
Recently,several systematic reviews have published on the application value of lncrnas including MALAT1 in tumor diagnosis and prognosis [20–22].Compared with previous meta-analysis,our study has several important advantages.First,we conducted diagnostic and prognostic meta-analysis for estimating the clinical roles of MALAT1 in BC patients for the first time.Second,we performed comprehensive and specific subgroup analysis including diagnosis,clinicopathological parameters and survival outcomes.Third,the diagnosis results are basically consistent with previous studies [21].The prognostic meta-analysis expressed that the association between the high level of MALAT1 and RFS,MFS,DSS and DFS.However,MALAT1 might not be significantly correlated with the clinicopathologic features of BC.Several interesting results obtained in our study may provide a direction for future experimental research.
Conclusion
In conclusion,this study confirmed that circulating MALAT1 is a promising diagnostic biomarker for BC detection,while tissue MALAT1 is a useful biomarker for predicting BC survival.The credibility of these correlations is difficult to pinpoint due to many uncontrollable factors.Before the clinical use of MALAT1 as diagnostic and prognostic marker,more large-scale studies are authorized to identify the diagnostic and prognostic value of MALAT1 in BC.

