醋甲唑胺治疗正常压力脑积水患者5例分析
杨琼 滑蓉蓉 刘春艳


[摘要] 目的 研究醋甲唑胺(MTZ)对正常压力脑积水(NPH)患者治疗的有效性及安全性,探讨其作用机制,丰富NPH的治疗手段,拓宽MTZ应用新途径。 方法 收集中国医科大学航空总医院2019—2020年4例特发性正常压力脑积水(iNPH)和1例继发性正常压力脑积水(sNPH)患者。iNPH患者均符合2005年国际iNPH指南诊断标准,5例患者均完善单次腰穿放液试验阳性,且因各种原因不适宜或拒绝手术治疗,在征得患者及家属同意后,给予口服MTZ 25~50 mg,2次/d進行治疗。评价患者口服MTZ前后步态评分、认知评定、膀胱功能测定、头MRI检查、iNPH分级评分。口服MTZ后iNPH评分改善≥1分定义为有效,并密切观察药物不良反应,评估MTZ对NPH的有效性及安全性。 结果 5例患者中4例有效,轻度改善1例,明显改善3例(2例iNPH和1例sNPH患者),表现为步态、认知或膀胱功能三方面改善。5例患者均未发现明确的MTZ相关的不良反应。 结论 MTZ可能成为潜在的治疗NPH的药物之一,但仍需大样本随机对照试验进一步证实。
[关键词] 醋甲唑胺;乙酰唑胺;正常压力脑积水;腰穿放液试验
[中图分类号] R742.7 [文献标识码] A [文章编号] 1673-7210(2020)09(b)-0053-05
[Abstract] Objective To study the effectiveness and safety of Methazolamide (MTZ) in the treatment of patients with normal pressure hydrocephalus (NPH), to explore its mechanism of action, to enrich NPH treatment methods, and to broaden new ways of MTZ application. Methods A total of four cases of idiopathic normal pressure hydrocephalus (iNPH) and one case of secondary normal pressure hydrocephalus (sNPH) were collected from 2019 to 2020 in Aviation General Hospital, China Medical University. All iNPH patients met the diagnostic criteria of the 2005 International iNPH Guidelines. The five patients were all tested positive for a single lumbar puncture and were not suitable for or refused surgical treatment for various reasons. After obtaining the consent of the patient and their family members, they were given oral MTZ 25-50 mg, two times per day for treatment. The gait score, cognitive assessment, bladder function measurement, head MRI examination, iNPH grading score before and after oral MTZ were evaluated. After oral administration of MTZ, an improvement in iNPH score is greater than or equal to one point was defined as effective, and adverse drug reactions could be closely observed to evaluate the effectiveness and safety of MTZ on NPH. Results Among the five patients, four case were effective, including one case was slightly improved, and three cases were improved significantly (two patients with iNPH and one patient with sNPH), showed improvements in gait, cognition or bladder function. None of the five patients found clear MTZ-related adverse reactions. Conclusion MTZ may become one of the potential drugs for the treatment of NPH, but it still needs to be further confirmed by large randomized controlled trials.
[Key words] Methazolamide; Acetazolamide; Normal pressure hydrocephalus; Cerebrospinal fluid tap test
1965年Adams和Hakim首先提出正常压力脑积水(NPH)综合征的概念[1],其临床表现为认知障碍、行走困难及尿失禁三联征,伴有脑室扩大,脑脊液(cerebrospinal fluid,CSF)压力正常。NPH临床分为常继发于明确原因的继发性正常压力脑积水(sNPH)和无明确病因的持发性正常压力脑积水(iNPH)。目前国际指南及共识一致推荐脑脊液分流手术是NPH的唯一确切有效治疗措施,早期手术可明显改善患者病情及预后[2-3]。但临床实践中iNPH患者多具有高龄、常伴有心脑血管疾病、骨关节疾病、合并神经系统变性疾病等特点,外科手术在临床应用中具有一定的局限性,因此探讨NPH非手术治疗势在必行。乙酰唑胺(AZA)相对于其他药物依据较多,应用广泛[4-7],醋甲唑胺(MTZ)与AZA结构类似,具有脂溶性高、易透过血脑屏障等优点[8]。因此,MTZ有可能成为治疗NPH的有效手段之一。本研究旨在研究口服MTZ在NPH患者中的有效性及安全性,拓宽对NPH发病和进展机制的认识。
1 资料与方法
1.1 一般资料
收集中国医科大学航空总医院(以下简称“我院”)2019—2020年门诊及住院的临床诊断为iNPH患者4例,sNPH患者1例。纳入标准:4例iNPH患者均符合2005年国际iNPH指南诊断标准[9],5例患者均完善单次腰穿放液试验为阳性(一次性放出CSF 30 mL),经临床医师评估外科手术风险较大且患者及家属因各种原因坚持拒绝手术;患者本人或法定代表签署知情同意书。排除标准:不能配合MRI;严重失语、视听障碍等不能配合相关评估;严重肾功能不全、肝硬化、闭角型青光眼、磺胺过敏等禁止服用MTZ的患者。本研究通过我院医学伦理审查委员会批准。
1.2 方法
患者评估基线资料后口服MTZ 25 mg,2次/d,1周后依据患者耐受情况增加至50 mg,维持2次/d(剂量依据MTZ说明书),且常规给予氯化钾缓释片补钾治疗。
1.3 观察指标及检测方法
1.3.1 认知功能评测 包括简易精神状态检查(mini-mental state examination,MMSE)和蒙特利尔认知评估量表(Montreal cognitive assessment,MoCA)。任意一项评分增加3分或>10%为有效[10]。
1.3.2 步态分析 ①10 m行走试验:选择固定10 m无障碍直线距离行走,要求患者按照平常步速或辅助工具行走,由2名受过培训的神经内科医师进行评估,另有1名医师录像,评估步态、步数及时间,行走3次取平均值,计算其各自分值及总分。步数及时间单项改善>20%或2个参数均改善>10%为阳性[11]。②起立-步行计时测验(timed up-and-go test,TUG):受试者从有扶背的椅子上站起,步行3 m,转身180°,返回坐下所需的时间,行走3次取平均值。所需时间缩短10%或绝对值缩短>5 s为阳性[10,12]。
1.3.3 膀胱功能 膀胱日记,记录放液前后72 h内排尿次数、尿急次数、遗尿次数。
1.3.4 头MRI 包括常规MRI序列、海马MRI,计算Evans′指数、颞角、胼胝体角,评价蛛网膜下腔不成比例扩大的脑积水(disproportionately enlarged subarachnoid space hydrocephalus,DESH)征,作为辅助诊断。
1.3.5 iNPH综合评分 随访过程中由2名有经验的神经内科医师进行iNPH综合评分[13],iNPH综合评分改善≥1分定义为有效。
1.3.6 不良反应监测 检测患者血常规、肝肾功能、电解质、血气等指标。
2 结果
2.1 纳入患者基本情况描述
纳入患者年龄67~86岁,病程10~120个月。具体情况见表1。
2.2 iNPH患者口服MTZ基线及口服MTZ后临床评估
患者口服MTZ基线及口服MTZ后进行步态、认知和膀胱功能的评估见表2。患者编号与表1对应同一患者,因患者5为sNPH患者,未进行上述评估,但临床上步态、认知及膀胱功能均有明显所改善。
2.3 患者分析
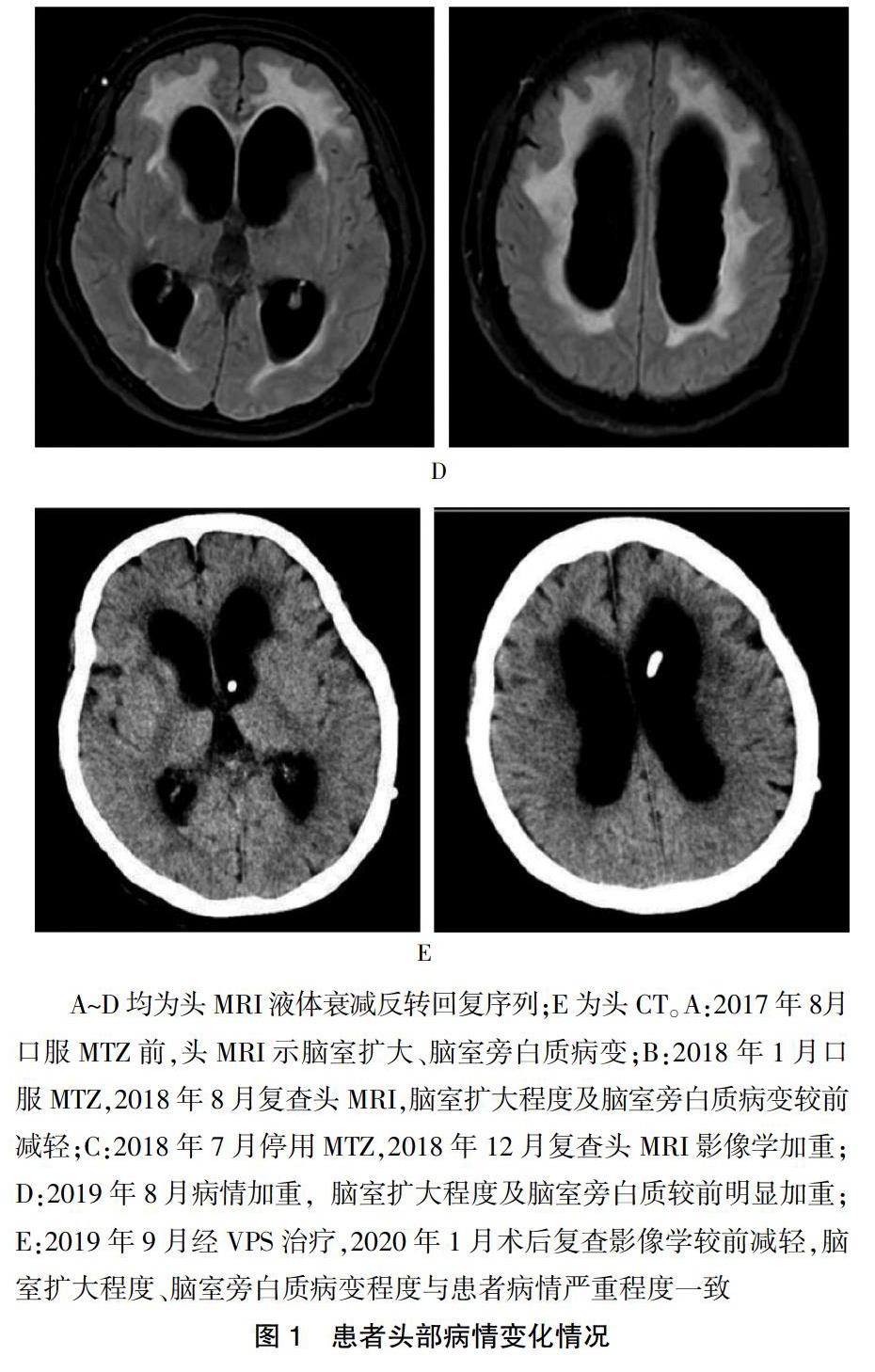
上述5例中患者3分別于口服MTZ后23 d和154 d评估均无明显改善。患者3单次脑脊液放液试验为阳性,但仅3 m折返试验阳性,改善率为11.1%;而患者2及患者4口服MTZ 1~3个月后均明显改善,患者2放液前搀扶下不能行走,放液后6 h可在搀扶下行走。患者4放液前及放液后6 h使用助步器行走,放液后48 h及72 h自己行走。患者5为系统性红斑狼疮(SLE)继发性脑积水,主因“行走不稳5年,记忆力下降、二便失禁2年余”就诊,曾多次应用激素冲击及免疫抑制剂治疗,无明显效果。患者病情严重时呈嗜睡、缄默、二便失禁、长期卧床状态,口服MTZ 50 mg(2次/d)病情逐渐减轻,停用MTZ后即缓慢逐渐加重,后行脑室-腹腔分流术(VPS)治疗症状再度明显减轻。患者病情变化与头MRI脑积水严重程度、脑室旁白质病变严重程度一致。见图1。
3 讨论
本研究推测结论如下:无论iNPH或者sNPH,口服MTZ均可能有效;MTZ起效时间多为2周至1个月,逐渐起效,停用后患者病情逐渐加重;脑脊液放液试验改善程度可能与口服MTZ效果有一定相关性;上述患者在50 mg,2次/d剂量内无一例出现明确的MTZ相关的不良反应,这在临床经验性的应用MTZ时也得到证实。同时,本研究存在较多局限性:纳入患者例数较少,应进一步完善RCT研究评估MTZ的有效性及安全性;部分患者影像学未进行定量研究,如比较头MRI脑室旁白质病变体积或血流变化客观评价MTZ的疗效;iNPH的自然病程均为逐渐加重,但短期内可有波动,不除外患者本身病情波动,应延长其随访时间。
AZA和MTZ均為碳酸酐酶抑制剂。1953年,AZA开始被作为利尿剂应用于临床。多项研究证实AZA可以减少CSF的产生[14-15],从而应用于iNPH和sNPH,且在影像学上有一定改善[16-21]。MTZ化学结构类似AZA(在氮原子上多1个甲基),药理作用及作用机制与AZA基本相同,又因MTZ的结构设计减少了电离作用,其减少房水形成和穿透血-房水和血-脑屏障的功能亦较AZA强[22]。但国内外均无MTZ在NPH的相关研究。另有研究发现[22-24],MTZ尚能通过抑制线粒体caspase-3及ROS释放、抑制细胞色素C(cytochrome C)及Aβ介导的线粒体损伤起到神经元及胶质保护作用。iNPH病理机制不明,常合并脑血管疾病、神经变性疾病,发病原因可能涉及多个机制。鉴于MTZ较强的透过血脑屏障的能力,在神经元及胶质细胞变性、神经缺血缺氧损伤中的潜在保护作用,MTZ可能成为潜在的有效治疗NPH的药物之一。
除此之外,MTZ在其他领域的应用及本篇研究证实其相对安全,不良反应大多发生在治疗的早期,包括感觉异常,尤其是四肢末端的麻木感等,也可能会出现代谢性酸中毒和电解质紊乱,当减少或停止本品治疗后这种现象都会减退。因此应当定期检测血常规、肝肾功能、电解质、尿常规、血气分析。其中对磺胺少见的严重反应,包括Stevens-Johnson综合征及中毒性表皮坏死松解症,与HLA基因型有关,口服MTZ前一定要询问是否有磺胺过敏史,必要时进行上述基因型检测[25]。
综上所述,较多NPH患者难以进行手术治疗,推测MTZ可能比AZA具有更广阔的应用前景,本研究有望为NPH提供更简便安全易行的治疗方案,但仍需进一步大规模随机对照研究。
[参考文献]
[1] Hakim S,Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics [J]. J Neurol Sci,1965,2(4):307-327.
[2] Torsnes L,Blafjelldal V,Poulsen FR. Treatment and clinical outcome in patients with idiopathic normal pressure hydrocephalus--a systematic review [J]. Dan Med J,2014, 61(10):A4911.
[3] Williams MA,Malm J. Diagnosis and Treatment of Idiopathic Normal Pressure Hydrocephalus [J]. Continuum (Minneap Minn),2016,22(2 Dementia):579-599.
[4] Carrion E,Hertzog JH,Medlock MD,et al. Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts [J]. Arch Dis Child,2001,84(1):68-71.
[5] Davson H,Luck CP. The effect of acetazoleamide on the chemical composition of the aqueous humour and cerebrospinal fluid of some mammalian species and on the rate of turnover of 24Na in these fluids [J]. J Physiol,1957,137(2):279-293.
[6] Vogh BP. The relation of choroid plexus carbonic anhydrase activity to cerebrospinal fluid formation: study of three inhibitors in cat with extrapolation to man [J]. J Pharmacol Exp Ther,1980,213(2):321-331.
[7] Aimard G,Vighetto A,Gabet JY,et al. Acetazolamide:an alternative to shunting in normal pressure hydrocephalus? Preliminary results [J]. Rev Neurol (Paris),1990,146(6/7):437-439.
[8] Swenson ER. Pharmacology of acute mountain sickness:old drugs and newer thinking [J]. J Appl Physiol(1985),2016, 120(2):204-215.
[9] Relkin N,Marmarou A,Klinge P,et al. Diagnosing idiopathic normal-pressure hydrocephalus [J]. Neurosurgery,2005,57(3 Suppl):S4-S16.
[10] Ishikawa M,Hashimoto M,Mori E,et al. The value of the cerebrospinal fluid tap test for predicting shunt effectiveness in idiopathic normal pressure hydrocephalus [J]. Fluids Barriers CNS,2012,9(1):1.
[11] Boon AJ,Tans JT,Delwel EJ,et al. Dutch normal pressure hydrocephalus study:baseline characteristics with emphasis on clinical findings [J]. Eur J Neurol,1997,4(1):39-47.
[12] Yamada S,Ishikawa M,Miyajima M,et al. Timed up and go test at tap test and shunt surgery in idiopathic normal pressure hydrocephalus [J]. Neurol Clin Pract,2017,7(2):98-108.
[13] Kubo Y,Kazui H,Yoshida T,et al. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus [J]. Dement Geriatr Cogn Disord,2008,25(1):37-45.
[14] Maren TH. Carbonic anhydrase:chemistry,physiology,and inhibition [J]. Physiol Rev,1967,47(4):595-781.
[15] Birzis L,Carter CH,and Maren TH Effects of acetazolamide on CSF pressure and electrolytes in hydrocephalus [J]. Neurology,1958,8(7):522-528.
[16] Miyake H,Ohta T,Kajimoto Y,et al. Diamox(R) challenge test to decide indications for cerebrospinal fluid shunting in normal pressure hydrocephalus [J]. Acta Neurochir (Wien),1999,141(11):1187-1193.
[17] Alperin N,Oliu CJ,Bagci AM,et al. Low-dose acetazolamide reverses periventricular white matter hyperintensities in iNPH [J]. Neurology,2014,82(15):1347-1351.
[18] Ivkovic M,Reiss-Zimmermann M,Katzen H,et al. MRI assessment of the effects of acetazolamide and external lumbar drainage in idiopathic normal pressure hydrocephalus [J]. Fluids Barriers CNS,2015,12:9.
[19] Gao F,Zheng M,Hua Y,et al. Acetazolamide Attenuates Thrombin-Induced Hydrocephalus [J]. Acta Neurochir Suppl,2016,121:373-377.
[20] Visudhiphan P,Chiemchanya S. Hydrocephalus in tuberculous meningitis in children:treatment with acetazolamide and repeated lumbar puncture [J]. J Pediatr,1979,95(4):657-660.
[21] Ameli PA,Madan M,Chigurupati S,et al. Effect of acetazolamide on aquaporin-1 and fluid flow in cultured choroid plexus [J]. Acta Neurochir Suppl,2012,113:59-64.
[22] Wang X,Figueroa BE,Stavrovskaya IG,et al. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury [J]. Stroke,2009,40(5):1877-1885.
[23] Wang X,Zhu S,Pei Z,et al. Inhibitors of cytochrome C release with therapeutic potential for Huntington′s disease [J]. J Neurosci,2008,28(38):9473-9485.
[24] Fossati S,Giannoni P,Solesio ME,et al. The carbonic anhydrase inhibitor methazolamide prevents amyloid beta-induced mitochondrial dysfunction and caspase activation protecting neuronal and glial cells in vitro and in the mouse brain [J]. Neurobiol Dis,2016,86:29-40.
[25] Tangamornsuksan W,Lohitnavy M. Association between HLA-B*5901 and methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: a systematic review and meta-analysis [J]. Pharmacogenomics J,2019,19(3):286-294.
(收稿日期:2020-06-02)

