Efficacy and safety of Shen Zhi Ling oral liquid (a Chinese patent) for Alzheimer's disease:A meta-analysis of randomized controlled trials
Shiqi Chen,Chunxiang Liu,Hui Wang,Chengyu Li,Hongcai Shang,*
1 Key Laboratory of Chinese Medicine of Ministry of Education and Beijing, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine,Beijing 100700,China
2 Evidence-based medicine center, Tianjin University of Traditional Chinese Medicine, Tianjin 300193,China
Abstract
Keywords:Shen Zhi Ling oral liquid,Traditional Chinese medicine,Alzheimer's disease,Systematic review,Meta-analysis
Background
Alzheimer's Disease (AD) is a chronic progressive neurodegenerative disorder characterized by dramatical impairments of the cognitive ability and even the personality [1].According to the World Alzheimer Report 2016, there were 47.8 million people worldwide living with AD until 2015, and it is estimated that the number will soar to 131.5 million by 2050 [2].Elderly AD patients in the severe stage lose the ability to take care of themselves, causing large socioeconomic burden and serious mental and economic burden on their families [3].Currently, the main therapeutic medications are cholinesterase inhibitors (CHEIs) andN-methyl-D-aspartate receptor(NMDA-R), including donepezil, rivastigmine,galantamine, tacrine (CHEIs) and memantine(NMDA-R).These drugs can temporarily treat its symptoms, and at present no treatment can eventually cease or reverse the inexorably dysfunctional process[3].These drugs have also displayed some common side effects such as nausea, vomit and anorexia, and tacrine may cause liver damage [4, 5].Therefore, the management of AD patients in the early stage should be stressed and the efficacy and safety of the medication should also be emphasized.
Formerly named Shen Gui Jian Nao oral liquid,Nao Rui Kang oral liquid, Yang Xin Jian Nao oral liquid or Tiao Xin preparation, Shen Zhi Ling oral liquid (SZL) is the first Chinese patent drug approved by the former China Food and Drug Administration(CFDA) for the treatment of mild-to-moderate AD.The approval number is Z20120010 and the patent number is ZL03129657·2.Professor Lin Shuimiao from Shanghai University of Traditional Chinese medicine took advantage of the theory of the heart controlling mental activities and developed SZL on the basis of two formulas (Kai Xin powder and Ling Ren Bu Wang formula) recorded in the ancient medical book of Bei Ji Qian Jin Yao Fang.SZL consists of the following 10 different traditional Chinese herbs:Codonopsis pilosula(Franch.) Nannf.(Codonopsis Radix, dang shen),Cinnamomum cassiaPresl (Cinnamomi Ramulus, gui zhi),Paeonia lactifloraPall.(Paeoniae Radix Alba, bai shao),Glycyrrhiza uralensisFisch.(Glycyrrhizae Radix Et Rhizoma Praeparata Cum Melle, zhi gan cao),Poria cocos(Schw.) Wolf (Poria, fu ling),Zingiber officinaleRosc.(Zingiberis Rhizoma, gan jiang),Polygala tenuifolia Willd.(Polygalae Radix,yuan zhi),Acorus tatarinowiiSchott(Acori Tatarinowii Rhizoma,shi chang pu),Fossilia Ossia Mastodi(Fossilizid,Long Gu) andOstrea gigasThunberg (Ostreae Concha, mu li).According to the Chinese medicine theory, SZL can supplement qi, warm yang, resolve phlegm and sooth the nerves, and it is beneficial to AD patients with heart-qi deficiency syndrome.The characteristic symptoms of these patients are amnesia,palpitation, lack of energy, dizziness and insomnia.Previous clinical study has confirmed that SZL improved cognitive function and the daily living ability of AD patients to a certain extent[6].However,the efficacy and safety of SZL has not been systematically evaluated so far.Therefore, in this study we carried out a systematic review of randomized controlled trials to assess its efficacy and safety for AD and hope to provide evidence for further research and development.
Methods
Criteria for considering studies for this review
Types of studiesRandomized controlled trials (RCTs)with no limit of publishing language.
Types of participantsParticipants who were diagnosed with AD according to one of the internationally recognized diagnostic criteria for AD were included in the analysis.These criteria are shown as follows: the diagnostic guidelines of AD from the National Institute on Aging(NIA)and the Alzheimer's Association (AA) workgroups in 2011 [7], the diagnosis of mild cognitive impairment (MCI) due to AD from NIA-AA in 2011 [8], clinical diagnosis of the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (ADRDA)in 1984 [9], the revision of the NINCDS–ADRDA criteria in 2007[10].Age and race were not limited.The degree of AD was not restricted as well.There should exist no significant difference(P>0.05)for the baseline between the experiment group and the control group.
Types of interventionsThe treatment of the experiment group was SZL without other nootropic drugs.The control group was required to take CHEIs or NMDA-R or other recommendations including donepezil,tacrine,galantamine,rivastigmine,memantine and huperzine[4,11].Basic treatment(BT)comprises drugs of reducing blood pressure, reducing blood glucose,anticoagulant therapy and anti-infection therapy.BT can be applied simultaneously in both 2 groups when necessary.
Types of outcome measuresThe primary outcomes were scores of the Mini Mental State Examination(MMSE) [12, 13] and the Activities of Daily Living scale (ADL) [14, 15] for post-treatment AD patients.The secondary outcomes were other neuropsychological scale scores including Rapid Verbal Retrieve (RVR), Digit Span (DS), Fuld Object-Memory Evaluation (FOM) [16] and adverse reactions or events after treatment.
Information sources and search strategy
The search was applied to 7 databases including PubMed, the Cochrane Library, Embase, Web of science, China National Knowledge Infrastructure(CNKI), Wanfang Database and Chinese Science and Technology Journal Database (VIP) (publishing time was ranged from the inception through September 20,2020).The retrieval strategy used the following general terms individually or combined:“Shenzhiling”, “Shen-zhi-ling”, “Shen Zhi Ling oral liquid”, “Shen Gui Jian Nao oral liquid”, “Yang Xin Jian Nao oral liquid”, “Nao Rui Kang oral liquid”,“Tiao Xin”,“Tiao Xin fang”,“Tiao Xin recipe”,“Tiao Xin prescription”,“cognitive impairment”,“Alzheimer's Disease”, “Dementia”.For instance, the detailed search strategy of CNKI was shown as follows: SU = ('Shen Zhi Ling' + 'Shen Zhi Ling oral liquid'+'Tiao Xin fang'+'Shen Gui Jian Nao'+'Shen Gui Jian Nao oral liquid' + 'Yang Xin Jian Nao' +'Yang Xin Jian Nao oral liquid' + 'Nao Rui Kang oral liquid' + 'Nao Rui Kang') AND SU = ('cognitive impairment'+'CI'+'AD'+'Alzheimer'+'Dementia'+'Alzheimer's disease').
Study selection and data extraction
Two investigators (SQC and CXL) independently carried out literature search using predetermined criterion in the NoteExpress 3.2 software.At first,duplications were found in all databases and removed from the original searching results.Secondly, we eliminated the obviously irrelevant studies after reading the titles and abstracts.Finally, the full-texts were screened to find out the related studies, and the unqualified studies were excluded.The process of study selection was cross-checked by 2 researchers.Any disagreement was discussed and resolved in a consensus meeting with the corresponding author(HCS).
Two authors (HW and CYL) independently extracted data from the contained literatures employing a standardized sheet prepared for this review.The extraction data included study title, year of research and publication, name of the first author,source of patients, age and gender of participants,diagnostic criteria, disease type, sample size, baseline,treatment duration, interventions in the treatment and control groups,outcome indicators,etc.
Assessment of risk of bias in individual studies
Two authors independently assessed the risk of bias using assessment tools provided by the handbook of Cochrane Collaboration to evaluate the methodological quality of included studies, involving blinding of outcomes assessment (i.e., detection bias),blinding of participants and personnel (i.e.,performance bias), random sequence generation (i.e.,selection bias), allocation concealment (i.e., selection bias), incomplete outcomes data (i.e., attrition bias),selective reporting (i.e., reporting bias) and other biases.Disagreements were resolved by consensus with the corresponding author.
Summary measures and data analysis
Statistical analysis
Review Manager 5.3 software made by the Cochrane Collaboration was used to conduct data analysis.Dichotomous data were calculated as the risk ratios(RR) and the 95% confidence interval (CI), and continuous outcomes were expressed as weighted mean difference (WMD).Pvalue<0.05 was considered statistically significant.
Heterogeneity assessment
The heterogeneity of included studies was analyzed with the χ2test.If I2<50%, then we took it as a small heterogeneity among studies, and applied the fixed effects model for data analysis.If statistical heterogeneity was detected as I2≥50%, then we measured the sources of heterogeneity.Subgroup analysis was performed when clinical heterogeneity exists, such as the type of AD, treatment course, dose of medicine and so on.If heterogeneity was still significant, we considered the random effects model or perform only descriptive analysis.
Sensitivity analysis
By comparing the pooled statistics before and after excluding studies of great weight and low quality,differences can be found from other studies.We can also conduct sensitivity analyses to explore the stability of the results if necessary.
Results
Study selection
A total of 275 articles were retrieved from 7 literature databases.After removing 146 duplicates, 129 potentially relevant articles remained for next assessment.After the evaluation of titles and abstracts,we excluded 94 articles.Of the 35 remaining literatures, we further excluded 31 articles after investigating the full text.Finally, we included four studies for the meta-analysis.A flow chart (Figure 1)showed the search process and study selection.
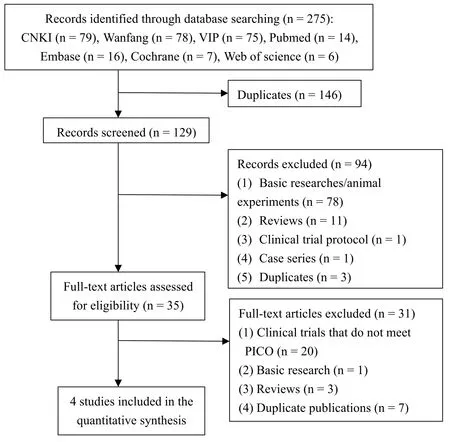
Figure 1 Flow chart of searching and screening studies.
Study characteristics
We initially identified 275 records, and four RCTs were finally included, covering 217 participants [6,17-19].All the studies were published in Chinese and were conducted in China.They were published from 2000 to 2017, the duration of SZL treatment ranged from 12 to 24 weeks, and the sample size of the studies varied from 40 to 69.The disease diagnoses of the studies were AD or MCI due to AD.Further details in the characteristics of the included studies were shown in Table 1.
Risk of bias and methodological quality
In accordance with the assessment tools provided by the handbook of Cochrane Collaboration,the included studies displayed the methodological bias (Figure 2).All the included studies [6, 17-19] were described as“randomized” studies; among them, one study [18]stated that they used the “randomized block design”.However, the random sequence generation and the allocation concealment were not clear in all of the studies.In addition, 2 studies [17, 19] mentioned the application of blinding, but the process of blinding was not described in detail.The incomplete outcome data was not mentioned in all included articles as well.Although all studies did not provide any protocol,they had reported our expected outcome indicators, and we couldn't distinguish the selective reporting bias.The results showed that the methodological quality of the four literatures included in this meta-analysis was generally low.
Outcome measures
MMSE scoreIn total, four studies[6, 17-19]reported the MMSE score and were included in a meta-analysis.The result showed no statistically significant difference in the comparison of the experiment groups and comparison groups [MD = 0.05, 95% CI (-0.77,0.88),P= 0.90>0.05,I2= 46%] (Figure 3).Due to moderate heterogeneity among groups, the fixed effects model was applied to combine the outcomes.As indicated by the results, SZL performed no better than other nootropic drugs in improving the MMSE score.
ADL scoreThree studies[6,18,19]reported the ADL score and were included in a meta-analysis.The result also indicated no statistically significant difference between the experiment and comparison groups[MD=0.18,95%CI(-0.21,0.58),P=0.36>0.05,I2=0%](Figure 4).Considering low heterogeneity among groups,we also used the fixed effects model to combine the outcomes.According to the result,SZL was no better than other nootropic drugs in improving the ADL score.
RVR scoreA total of 3 studies[6,18,19]reported the RVR score and were included in a meta-analysis.No statistically significant difference between the experiment and comparison groups[MD =-1.24,95%CI (-2.70, 0.22),P= 0.10>0.05,I2= 0%] (Figure 5)was found in the results.Still in a low heterogeneity,we applied the fixed effects model for combining the results.As shown in Figure 5, SZL performed no better than other nootropic drugs in improving the RVR score.
DS scoreThree studies[6,18,19]reported the DS score.In the meta-analysis.The result showed that there was no statistically significant difference between the experiment and comparison groups[MD=0.07,95%CI(-0.45,0.60),P=0.79>0.05,I2=0%](Figure 6).Because of the low heterogeneity,we used the fixed effects model to analyze the results.The result also showed that SZL performed no better than other nootropic drugs in improving the DS score.
FOM scoreAltogether 3 studies[6,17,18]described the FOM score and were included in a meta-analysis.The result also indicated that no statistically significant difference between the experiment and comparison groups[MD=-0.37,95%CI(-1.39,0.65),P=0.47>0.05,I2=0%](Figure 7).Considering the low heterogeneity,we applied the fixed effects model to combine the outcomes.The outcome also revealed that SZL was no better than other nootropic drugs in improving the FOM score.
Adverse eventsNo adverse events were reported in the included studies.
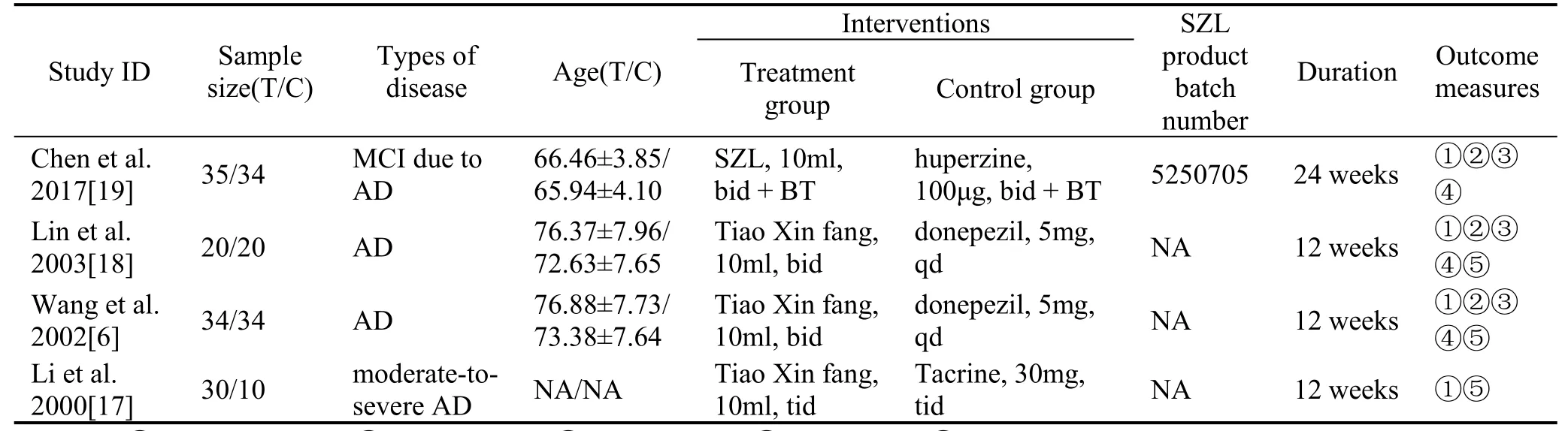
Table 1 Characteristics of contained studies
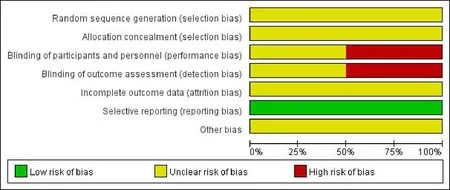
Figure 2 Risk of bias graph.
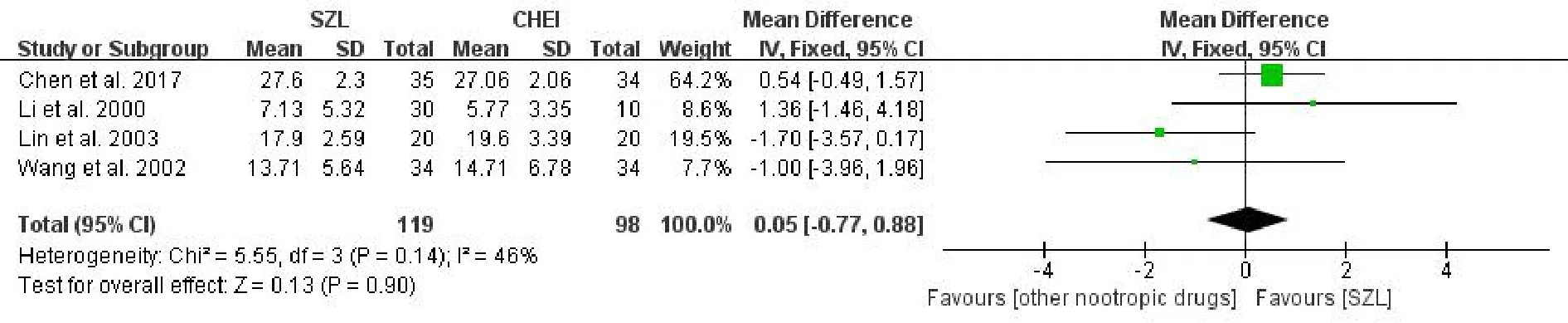
Figure 3 Meta-analysis forest of MMSE score.
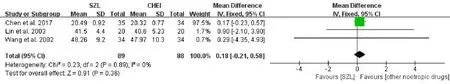
Figure 4 Meta-analysis forest of ADL score.
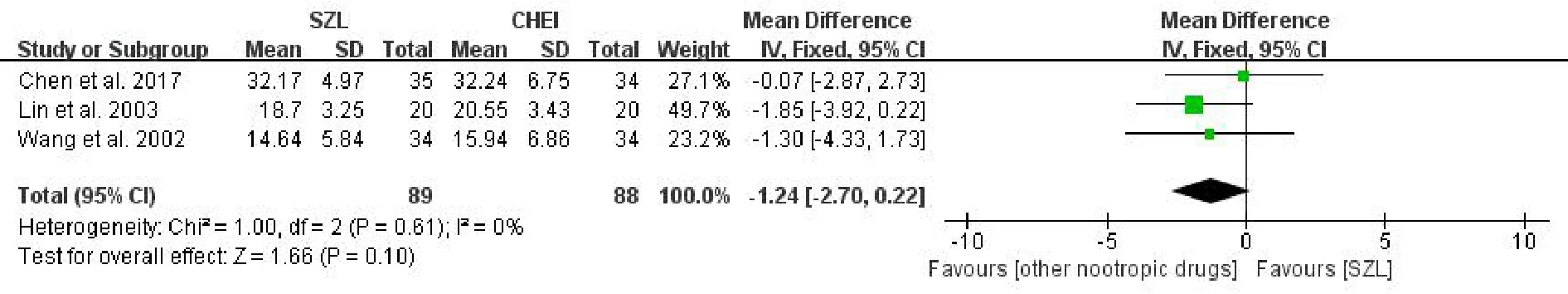
Figure 5 Meta-analysis forest of RVR score.
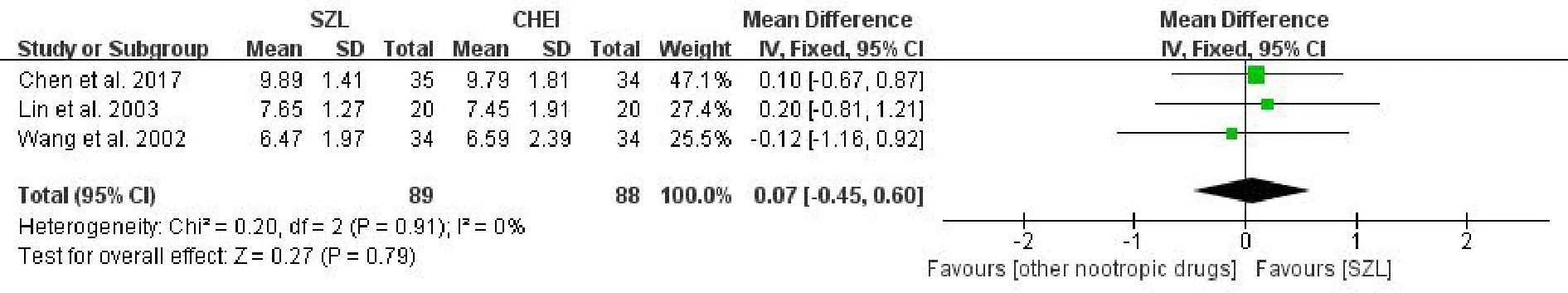
Figure 6 Meta-analysis forest of DS score.
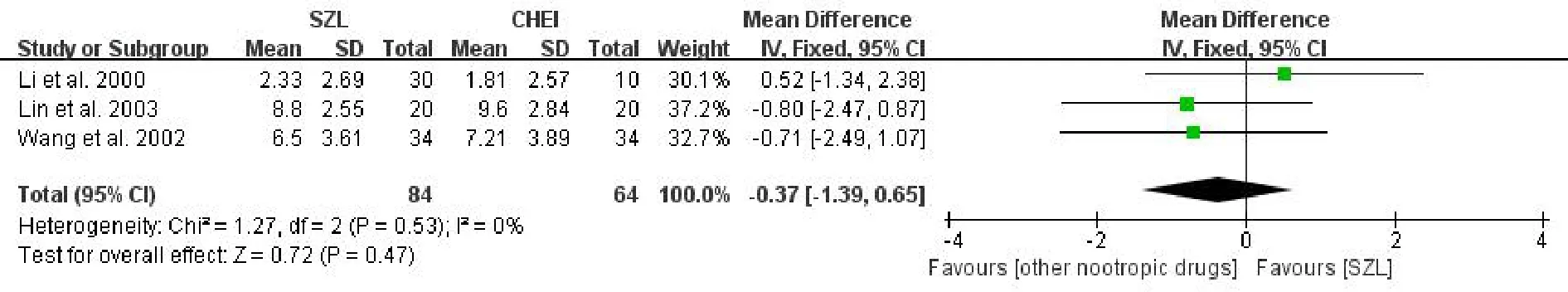
Figure 7 Meta-analysis forest of FOM score.
Heterogeneity analysis
The statistical heterogeneity of all meta-analyses in ADL score,RVR score,DS score and FOM score was 0%, showing almost no heterogeneity in the results.As indicated in the MMSE score,there existed 46%in the heterogeneity analysis, and it was less than 50%,so we still employed the fixed effects model and took it as a relatively small heterogeneity.We considered that the clinical heterogeneity came from 2 sources:one was the patients in different stages of AD,including mild, moderate and severe AD, the other could be the different nootropic drugs adopted in the comparison groups.Besides these, the methodological quality of all included studies was generally low.Due to the results of the low heterogeneity and the limitation of the amount of included studies,subgroup analysis and sensitivity analysis were no longer performed.
Discussion
AD, a common type of dementia, is a degenerative disease of the neurological system and has a higher incidence in elderly people.It contains complexly pathological hypotheses including the apoptosis of cholinergic neuron, amyloidβ-protein toxicity, tau protein phosphorylation,the insulin signaling pathway disorders, the energy metabolism dysfunction, the neuroinflammation and so on [20, 21].At present, the etiology of AD has not been fully illuminated, and all the approved western drugs can only slow down rather than reverse AD progression [20].SZL, a formula of 10 Chinese herbs, has been used to treat mild-to-moderate AD patients with heart-qi deficiency in China.It has also been demonstrated that SZL can inhibit the excessive accumulation of amyloidβ-protein [22] and the phosphorylation of tau protein, regulate multiple neurotransmitters, and improve the energy metabolism in the basic researches[23].
In our study, the results showed no significant difference (P >0.05) between SZL and other nootropic drugs in MMSE score, ADL score, RVR score, DS score and FOM score, indicating no advantages for the sole SZL therapy.The negative results could be explained by three reasons: (1) The included four studies involved different stages of AD patients, and the application of SZL may be more beneficial to AD patients in the early stage; (2) We should not ignore the diagnosis and treatment from the traditional Chinese theory, and the formula of SZL is effectively used for AD patients with the reduction of heart-qi, characterized as amnesia, palpitation, lack of energy,dizziness and insomnia;(3)The auxiliary SZL plus other nootropic drugs may play a better role than the sole SZL therapy in treating AD patients.The following trials could take advantages of the shortages into further experimental design.
The present study has several limitations.First, the amount and the sample size of the included studies were small, and the severity of AD differed among studies, which influenced the precision of the therapeutic effect evaluation.Second,the methodological quality of the included studies was low, and the year of publication was relatively early,causing difficulties in tracing the data information.Therefore, RCTs with rigorous design, large sample and multi-center are still needed to confirm the efficacy and safety of SZL in the treatment of mild-to-moderate AD in order to provide the evidence of high quality for its clinical application.
Conclusion
In a word, our analysis found that the sole SZL, as a Chinese patent medicine,might be no better than other nootropic drugs for improving the MMSE score,ADL score, RVR score, DS score and FOM score for AD patients.According to its specification, it may benefit mild-to-moderate AD patients with heart-qi deficiency syndrome.However,no relative studies recruited such specific patients.No serious adverse events were found as well.Given the low quality of the included trials and the involved AD patients,we may be unable to draw any conclusion about the efficacy and safety of SZL.Moreover, rigorously designed, multi-center,large-scale trials are still warranted to further identify the efficacy and safety of SZL.
Contributions
HCS designed the research and supervised the whole process.SQC and CXL conducted the literature searching and selection.HW and CYL extracted and analysed the data.SQC and HW assessed the risk of bias.SQC drafted the manuscript with the help of CXL and CYL.HCS detected mistakes in the entire process.All authors have approved the final manuscript for submission.
 TMR Modern Herbal Medicine2020年4期
TMR Modern Herbal Medicine2020年4期
- TMR Modern Herbal Medicine的其它文章
- Network-pharmacology and molecular docking-based investigation of mechanism of Sophora flavescens on cancer and inflammation
- Effects of Ginkgo biloba extract on diabetic retinopathy: a meta-analysis and systematic review
- Exploring the mechanism of Xiaopi Hewei capsule in treating functional dyspepsia based on network pharmacology
- A new sesquiterpenoidal glucoside from the roots of Paeonia lactiflora
- Efficacy and safety of the combination of Liushen capsules and Arbidol in the treatment of COVID-19:protocol for a randomized,multi-center pilot study
