乳癌病人术前外周血SII和ELR与预后的关系
金丽涛 李福年
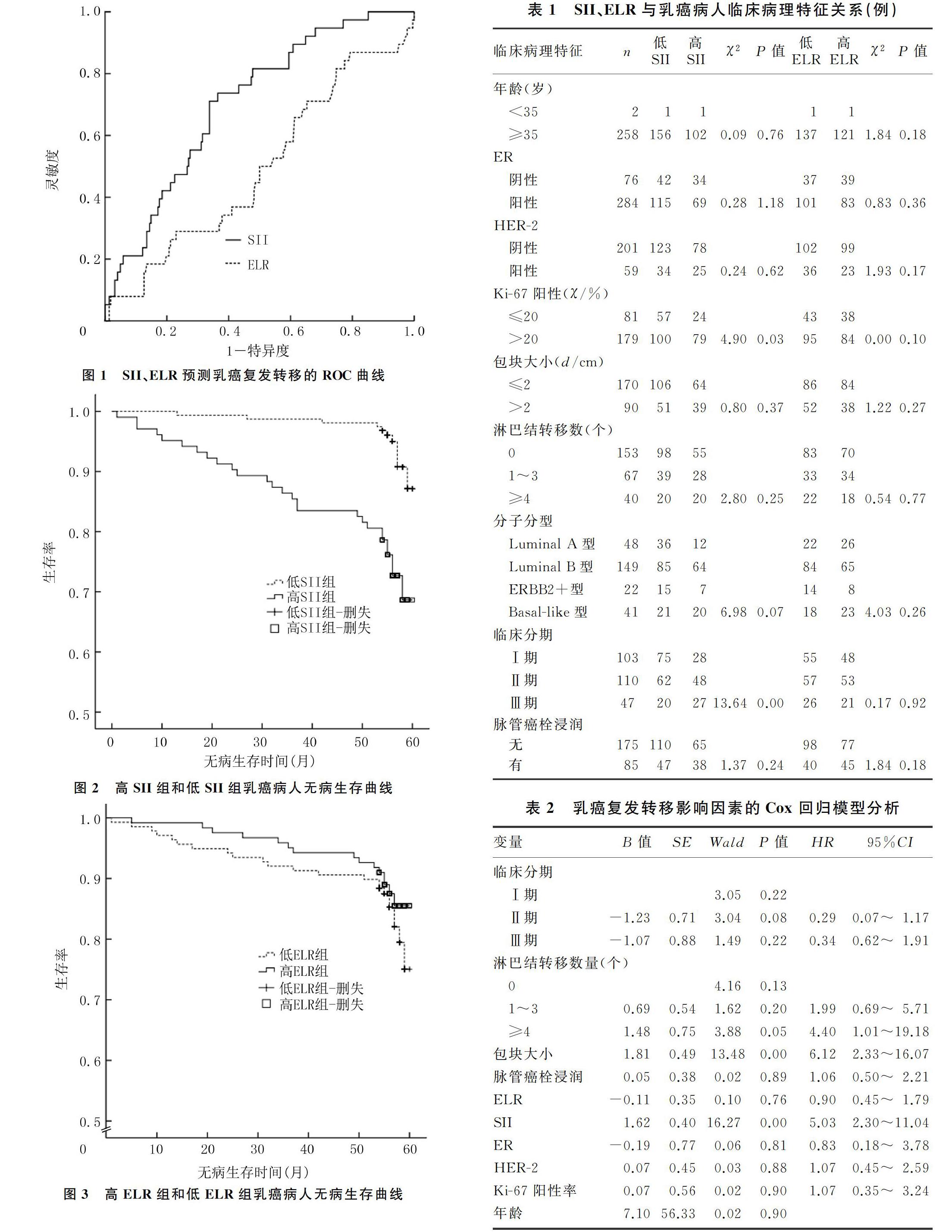
[摘要] 目的 探討乳癌病人术前外周血系统免疫炎症指数(SII)、嗜酸性粒细胞-淋巴细胞比率(ELR)与预后的关系。方法 回顾性分析2013—2014年我院治疗的260例女性乳癌病人的临床病理资料,采用受试者工作特征(ROC)曲线确定SII、ELR最佳截断值,将病人分为高SII和低SII组、高ELR和低ELR组,比较各组临床病理特征,采用Kaplan-Meier法绘制病人生存曲线,Log-rank检验分析生存差异,Cox回归模型对乳癌病人预后影响因素进行分析。结果 SII水平高低与临床分期(TNM)、Ki-67阳性率密切相关(χ2=13.64、4.90,P<0.05),ELR水平高低与各临床病理特征无相关性(P>0.05)。乳癌病人5年无病生存率为85.40%,高SII组和低SII组5年无病生存率分别为73.80%和93.00%,差异有统计学意义(χ2=20.84,P<0.05);高ELR组和低ELR组的5年无病生存率分别为87.70%和83.30%,差异无统计学意义(χ2=0.96,P>0.05)。Cox回归分析显示,SII(HR=5.03,95%CI=2.30~11.04,P<0.05)、包块大小(HR=6.12,95%CI=2.33~16.07,P<0.05)、淋巴结转移数量(HR=4.40,95%CI=1.01~19.18,P<0.05)对乳癌病人复发转移影响有统计学意义。结论 术前SII可作为预测乳癌预后的独立因素,高SII值提示预后不良;而ELR不能作为预测乳癌预后的因素。
[关键词] 乳房肿瘤;系统免疫炎症指数;嗜酸性粒细胞-淋巴细胞比率;预后
[中图分类号] R737.9 [文献标志码] A [文章编号] 2096-5532(2020)05-0571-05
doi:10.11712/jms.2096-5532.2020.56.129 [开放科学(资源服务)标识码(OSID)]
[ABSTRACT] Objective To investigate the association of preoperative systemic immune-inflammation index (SII) and eosinophil-lymphocyte ratio (ELR) in peripheral blood with the prognosis of breast cancer patients. Methods A retrospective analysis was performed for the clinicopathological data of 260 female patients with breast cancer who underwent treatment in our hospital from 2013 to 2014. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off values of SII and ELR, and then the patients were divided into high and low SII groups and high and low ELR groups. The clinicopathological features were compared between groups. The Kaplan-Meier method was used to plot survival curves, the Log-rank test was used for survival difference analysis, and the Cox regression model was used to investigate the influencing factors for the prognosis of patients with breast cancer. Results The level of SII was closely associated with clinical stage (TNM) and positive rate of Ki-67 (χ2=13.64,4.90;P<0.05), while there was no correlation between the level of ELR and clinicopathological features (P>0.05). The 5 year disease-free survival rate was 85.40% for all 260 breast cancer patients, and there was a significant difference in 5 year disease-free survival rate between the high SII group and the low SII group (73.80% vs 93.00%,χ2=20.84,P<0.05), while there was no significant difference in 5 year disease-free survival rate between the high ELR group and the low ELR group (87.70% vs 83.30%,χ2=0.96,P>0.05). The Cox regression analysis showed that SII (HR=5.03,95%CI=2.30-11.04,P<0.05), tumor size (HR=6.12,95%CI=2.33-16.07,P<0.05), and number of lymph node metastases (HR=4.40,95%CI=1.01-19.18,P<0.05) had significant influence on recurrence and metastasis in patients with breast cancer. Conclusion Preoperative SII can be used as an independent predictive factor for the prognosis of breast cancer, and high SII value indicates poor prognosis, while ELR cannot be used as a predictive factor for the prognosis of breast cancer.
SII、ELR是基于中性粒細胞、淋巴细胞、血小板计数的综合指标,SII、ELR影响恶性肿瘤病人的预后与外周血中性粒细胞、血小板、淋巴细胞等指标的变化有关。近年研究发现,中性粒细胞与肿瘤之间存在密切关系,中性粒细胞具有肿瘤促进作用。其肿瘤促进作用主要表现在以下方面:①肿瘤细胞异位分泌粒系集落刺激因子导致中性粒细胞数量增加,增多的中性粒细胞分泌大量血管内皮生长因子,为肿瘤细胞生长和增殖提供有利条件[10-11];②中性粒细胞释放中性粒细胞弹性蛋白酶,其进入肿瘤细胞内涵体直接诱导肿瘤细胞增殖[12];③中性粒细胞在TGF-β刺激下释放一氧化氮合成酶或精氨酸酶,抑制CD8+T淋巴细胞抗肿瘤反应,促进肿瘤增殖、转移[13-15]。
有研究显示,血小板不仅参与机体生理性凝血过程,还参与了肿瘤的生长与扩散,血小板对肿瘤生长、扩散的影响主要表现在以下方面[16-17]:肿瘤细胞可以通过直接接触或释放ADP、凝血酶、TXA2或肿瘤相关蛋白酶等刺激血小板活化,活化的血小板能释放溶血磷脂酸,溶血磷脂酸会增强肿瘤细胞的侵袭性和血管通透性;同时,血小板通过血小板衍生的TGF-β下调NK细胞活化的免疫受体自然杀伤细胞活化受体2D(NKG2D)表达,抑制NK细胞活性,促进肿瘤生长、增殖[18-21]。晚期恶性肿瘤病人常伴血小板增多。
淋巴细胞是机体细胞免疫的主要成分,在肿瘤免疫监视中发挥巨大作用,肿瘤浸润性淋巴细胞减少,相应免疫应答激活减少,机体抗肿瘤作用下降,增加肿瘤转移和复发风险[22]。恶性肿瘤促进了炎症反应,同时持续的机体炎症状态为恶性肿瘤进展提供了适宜的微环境[23-24]。基于上述机制,较高的SII促进肿瘤血管生成、侵袭和转移,从而导致乳癌病人预后较差。
本研究结果显示,ELR与乳癌病人预后无相关性,可能与嗜酸性粒细胞对肿瘤进展发挥双重作用有关。有研究表明,嗜酸性粒细胞能够诱导各种肿瘤细胞死亡,其机制是嗜酸性粒细胞具有与细胞毒性T淋巴细胞相同的受体和递质,因而能发挥抗肿瘤的作用。又有研究表明,嗜酸性粒细胞可能通过合成多种促血管生成因子(如血管内皮生长因子、成纤维细胞生长因子-2和IL-8)促进肿瘤生长[25-29]。
本文研究分析了SII、ELR与乳癌病人临床病理特征的关系,结果显示,SII与乳癌病人的Ki-67阳性率和临床分期相关,提示SII参与了肿瘤的发生发展;而ELR与各临床病理特征无相关性。本研究进一步分析显示,术前ELR与乳癌病人无病生存率无相关性,但SII与乳癌病人的无病生存率有显著相关关系。这与ZHANG等[30]的研究结果相似。HUANG等[9]对458例宫颈癌病人回顾性分析发现,SII是宫颈癌的独立不良预后因素,高水平SII的宫颈癌病人无病生存率较低且肿瘤复发或转移的概率较高。以上结果说明SII是预测乳癌无病生存率的独立因素,可以作为评估乳癌病人预后依据之一,高SII值提示预后不良。
本研究将肿瘤临床分期、淋巴结转移数量、SII、ELR、HER-2、ER、Ki-67、年龄等纳入Cox回归模型进行多因素分析,其结果显示,高SII、包块大小>2 cm、淋巴结转移数量≥4为影响乳癌病人预后的独立危险因素,而在包块大小、淋巴结转移数量一定的情形下,高SII病人复发风险是低SII者的5.03倍;在SII、淋巴结转移数量一定情形下,包块大小>2 cm病人复发风险是包块大小≤2 cm者的6.12倍;若SII、包块大小一定,淋巴结转移数量≥4者的复发风险是淋巴结转移数量<4病人的4.40倍。
本研究采用ROC曲线对SII、ELR预测复发风险的诊断价值进行分析,AUC越大,其诊断价值越高。结果显示,SII的AUC为0.71,灵敏度和特异度分别为0.71和0.66,SII的AUC>0.7,表明SII对乳癌病人预后具有较好的预测价值;而ELR的AUC为0.49,其灵敏度和特异度分别为0.63和0.48,ELR的AUC<0.7,表明ELR对乳癌病人预后的预测价值较差。
综上所述,术前外周血SII是乳癌术后病人预后评估指标之一,术前高SII病人更容易发生复发和转移,SII为乳癌病人预后的影响因素。而ELR不能作为预测乳癌预后的指标。本研究存在一定局限性:首先,本文研究对象均来自同一机构,存在选择偏倚;其次,本研究是回顾性分析,尚需前瞻性临床试验进一步验证。
[参考文献]
[1] SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2018[J]. CA: A Cancer Journal for Clinicians, 2018,68(1):7-30.
[2] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: A Cancer Journal for Clinicians, 2018,68(6):394-424.
[3] GUO W, CAI S H, ZHANG F, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer[J]. Thorac Cancer, 2019,10(4):761-768.
[4] SHI H T, JIANG Y Q, CAO H G, et al. Nomogram based on systemic immune-inflammation index to predict overall survi-val in gastric cancer patients[J]. Disease Markers, 2018, 2018:1-11.
[5] ZHOU Z Q, PANG S, YU X C, et al. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer[J]. Current Medical Science, 2018,38(5):798-808.
[6] COFFELT S B, DE VISSER K E. Inflammation lights the way to metastasis[J]. Nature, 2014,507(7490):48-49.
[7] GRIVENNIKOV S I, GRETEN F R, KARIN M. Immunity, inflammation, and cancer[J]. Cell, 2010,140(6):883-899.
[8] JOMRICH G, GRUBER E S, WINKLER D, et al. Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection[J]. Journal of Gastrointestinal Surgery, 2020,24(3):610-618.
[9] HUANG H P, LIU Q, ZHU L X, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer[J]. Scientific Reports, 2019,9:3284.
[10] ETHIER J L, DESAUTELS D, TEMPLETON A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis[J]. Breast Can-cer Research, 2017,19:2.
[11] ELYASINIA F, KERAMATI M R, AHMADI F, et al.Neutrophil-lymphocyte ratio in different stages of breast cancer[J]. Acta Medica Iranica, 2017,55(4):228-232.
[12] HOUGHTON A M, RZYMKIEWICZ D M, JI H B, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth[J]. Nat Med, 2010,16(2):219-223.
[13] FRIDLENDER Z G, SUN J, KIM S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN[J]. Cancer Cell, 2009,16(3):183-194.
[14] BODOGAI M, MORITOH K, LEE-CHANG C, et al. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells[J]. Cancer Research, 2015,75(17):3456-3465.
[15] OCANA A, NIETO-JIMNEZ C, PANDIELLA A, et al. Neutrophils in cancer:prognostic role and therapeutic strategies[J]. Molecular Cancer, 2017,16:137.
[16] PLANTUREUX L, CRESCENCE L, DIGNAT-GEORGE F, et al. Effects of platelets on cancer progression[J]. Thrombosis Research, 2018,164:S40-S47.
[17] OLSSON A K, CEDERVALL J. The pro-inflammatory role of platelets in cancer[J]. Platelets, 2018,29(6):569-573.
[18] BASTIDA E, ORDINAS A, GIARDINA S L, et al. Differentiation of platelet-aggregating effects of human tumor cell lines based on inhibition studies with apyrase, hirudin, and phospholipase[J]. Cancer Res,1982,42(11):4348-4352.
[19] PINTO S, GORI L, GALLO O, et al. Increased thromboxane A2 production at primary tumor site in metastasizing squamous cell carcinoma of the larynx[J]. Prostaglandins, Leukotrienes and Essential Fatty Acids, 1993,49(1):527-530.
[20] GRIGNANI G, PACCHIARINI L, RICETTI M M, et al. Mechanisms of platelet activation by cultured human cancer cells and cells freshly isolated from tumor tissues[J]. Invasion Metastasis,1989,9(5):298-309.
[21] ZUCCHELLA M, DEZZA L, PACCHIARINI L, et al. Human tumor cells cultured “in vitro” activate platelet function by producing ADP or thrombin[J]. Haematologica,1900,74(6):541-545.
[22] DUMITRU C A, LANG S, BRANDAU S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression[J]. Se-minars in Cancer Biology, 2013,23(3):141-148.
[23] FRANCESCONE R, HOU V, GRIVENNIKOV S I. Micro-biome, inflammation, and cancer[J]. The Cancer Journal, 2014,20(3):181-189.
[24] DIAKOS C I, CHARLES K A, MCMILLAN D C, et al. Cancer-related inflammation and treatment effectiveness[J]. Lancet Oncol, 2014,15(11):e493-e503.
[25] SAKKAL S, MILLER S, APOSTOLOPOULOS V, et al. Eosinophils in cancer:favourable or unfavourable[J]? Current Medicinal Chemistry, 2016,23(7):650-666.
[26] MOREIRA A, LEISGANG W, SCHULER G, et al. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy[J]. Immunotherapy, 2017,9(2):115-121.
[27] DAVIS B P, ROTHENBERG M E. Eosinophils and cancer[J]. Cancer Immunology Research, 2014,2(1):1-8.
[28] RIGONI A, COLOMBO M P, PUCILLO C. Mast cells, basophils and eosinophils: from allergy to cancer[J]. Seminars in Immunology, 2018,35:29-34.
[29] HARBAUM L, POLLHEIMER M J, KORNPRAT P, et al. Peritumoral eosinophils predict recurrence in colorectal cancer[J]. Modern Pathology, 2015,28(3):403-413.
[30] ZHANG K, HUA Y Q, WANG D, et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer[J]. Journal of Translational Medicine, 2019,17:30.
(本文編辑 黄建乡)

