Features of extrahepatic metastasis after radiofrequency ablation for hepatocellular carcinoma
Jae H Yoon, Young J Goo, Chae Jun Lim, Sung K Choi, Sung B Cho, Sang S Shin, Chung H Jun
Abstract
Key words: Hepatocellular carcinoma; Metastasis; Radiofrequency ablation; Surveillance; Risk factor
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related death[1]. Unlike other cancers that cause distant metastasis during progression, HCC has unique characteristics of loco-regional progression with an increase in size, intrahepatic metastasis, and vascular invasion. Therefore, current guidelines in Korea suggest follow-up surveillance after curative HCC treatment using abdominal imaging focusing on the liver and tumor marker such as serum alpha-fetoprotein (AFP) level[2]. Although enrolled subjects of previous studies represented all stages of HCC, the incidence of extrahepatic metastasis (EHM) of HCC ranged from 14%-37% and the presence of EHM was associated with poor clinical outcomes[3]. The reported median survival duration of patients with EHM is less than 6 (range, 4.9-5.9) mo, and the 1-year survival rate was reported as 24.9%[3,4]. Natsuizakaet al[3]suggested that the lung was the most common site of metastasis, and screening tests for lung metastases, as well as abdominal imaging, should be performed periodically for HCC patients. Lamet al[5]reported that when EHM is identified early and surgical resection is possible, the prognosis may be improved. Therefore, early diagnosis of EHM of HCC may help improve the prognosis.
Radiofrequency ablation (RFA) for the treatment of small HCC lesions (tumor diameter < 3 cm) has shown favorable clinical outcomes with less invasiveness and a low complication rate. RFA is recommended as one of the first-line treatment modalities for the management of HCC along with liver transplantation and surgery[6,7]. However, the recurrence-free survival (RFS) varies substantially (3.2%-38.2%) between studies, and cumulative extrahepatic recurrence rates were reported as 19.1% and 38.2% at 5 and 10 years, respectively[8,9]. Hence, careful monitoring for intra- and extrahepatic HCC recurrence after RFA is essential, but there is a lack of data regarding the effect of post-treatment surveillance on the prognosis of recurred HCC[10]. Moreover, few studies have been conducted on individualized post-treatment surveillance for EHM after RFA according to risk factors[11].
Therefore, we aimed to assess the characteristics and risk factors of EHM among patients who had undergone RFA as initial treatment for HCC and to investigate the appropriate surveillance tool for EHM detection.
MATERIALS AND METHODS
Patients
From January 2008 to December 2017, 1421 patients who underwent RFA for hepatic tumors at 2 tertiary hospitals were assessed. The inclusion criteria for this study were age ≥ 18 years, a diagnosis of HCC, and treatment-naivety. After excluding patients with hepatic tumors other than HCC or a history of HCC treatment, 661 patients were finally enrolled (Figure 1). Baseline clinical and tumor characteristics, complications of RFA, the status of recurrence, RFS, and rescue treatment methods for HCC recurrence were assessed retrospectively. The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-2019-203). The research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
The indications of RFA for HCC in our institutes were as follows: (1) Ineligible for surgical resection/liver transplantation or patient refusal for surgery; (2) Single nodular HCC < 5 cm in maximum diameter or multinodular HCC (3 in number, each < 3 cm in maximum diameter); (3) No EHM or vascular invasion; and (4) Prothrombin time ratio > 50% (international normalized ratio < 1.7).
Baseline staging and work-up
HCC was diagnosed according to the guidelines proposed by the Korean Liver Cancer Study Group and the National Cancer Center[12]. HCC was staged at diagnosis according to the modified Union for International Cancer Control (mUICC) staging system[13]and the Barcelona Clinic Liver Cancer (BCLC) classification system[14]. The initial tumor size was based on planning ultrasonography performed before RFA, and ablation size was measuredviacomputed tomography (CT) scan performed immediately after RFA. All measurements were reviewed by board-certified radiologists who were experts in abdominal imaging.
Abdominal CT or magnetic resonance imaging (MRI) and AFP measurements were routinely performed at 1 month after RFA and followed-up at intervals of 3-6 mo. Positron emission tomography (PET)-CT, bone scanning, spinal MRI, and chest CT were also performed in cases of clinically suspected metastasis or as intermittent routine follow-up based on the decision of the treating physician.
Diagnosis of extrahepatic metastasis
The time of EHM diagnosis was defined as the date on which EHM was detectedviaimaging study for the first time. Most cases of EHM were diagnosed during routine follow-up studies while few patients were diagnosed during the evaluation of new symptoms or significant AFP/serial AFP elevation without definite intrahepatic lesions. An increase in AFP > 15 ng/mL compared to the previous value within less than 6 months was considered significant; this was regarded as a feasible cutoff point for the prediction of long-term outcome among patients with HCC[15]. An AFP level that showed an increasing tendency more than twice was considered as serial elevation of AFP.
Statistical analysis
The data are expressed as means ± standard deviations or medians with ranges. Univariate analyses were performed using the chi-squared test or Student’st-test, and univariate and multivariate analyses were performedvialogistic regression, as appropriate. Variables withPvalues ≤ 0.05 in the univariate analysis were included in the multivariate logistic regression analysis. All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, United States). All analyses items withP< 0.05 were considered statistically significant.

Figure 1 Flowchart indicating the method of patient enrollment. HCC: Hepatocellular carcinoma; RFA: Radiofrequency ablation.
The statistical methods of this study were reviewed by Ja Young Baek from Biomedical Research Institute, Hwasun Chonnam National University Hospital.
RESULTS
Baseline characteristics of enrolled patients
We identified 661 patients who underwent RFA as initial treatment for newly diagnosed HCC, and EHM occurred among 44 patients during the study period. We compared the baseline characteristics between the groups with and without EHM among the enrolled patients (Table 1). The mean age was 66.9 years, and 75.2% of the patients were male. The serum albumin level was lower among patients with EHM (4.3 mg/dLvs3.9 mg/dL,P= 0.015). A higher proportion of patients in the EHM group had initial BCLC stage A and mUICC stage II disease compared to patients in the group without EHM who had a higher prevalence of BCLC stage 0 and mUICC stage I disease. There were no differences in tumor size and numbers between the 2 groups; however, the ratio of the ablation zone to tumor size was smaller in the group with EHM (2.07vs1.57,P= 0.001). There were no significant differences in the initial HCC recurrence site and mUICC tumor stage. The median follow-up duration of the enrolled patients was 1204 d.
Characteristics of first recurrence following RFA
Among 661 enrolled patients, 289 (43.7%) developed recurrent lesions without EHM, and 44 (6.7%) were diagnosed with EHM during the follow-up period. The median AFP level was higher at diagnosis of EHM than before the diagnosis of EHM in 79.0% of patients. The 10-year cumulative rates of HCC recurrence and EHM were 92.7% and 33.7%, respectively (Figure 2). Among the 661 patients, the median time to the detection of first HCC recurrence after RFA was 1.75 years, and the median duration to the development of EHM was 2.68 years. In addition, 68.2% of patients developed EHM within 2 years after the first recurrence regardless of RFS, and 75.0% of patients developed EHM within 5 years of the first recurrence (Figure 3). The most common site of initial recurrence was intrahepatic; initial extrahepatic recurrence occurred in only 1.2% (8/661) of patients. Most cases of EHM occurred after multiple intrahepatic recurrences, and the HCC stages at first recurrence are shown in Table 2. In addition, the peritoneum was the most common site of first EHM among 8 patients with EHM (5/8, 62.5%) followed by the lymph nodes (3/8, 37.5%). There was no case of pulmonary metastasis as the first recurrence in this study. Transcatheter arterial chemoembolization and RFA were mostly used as rescue treatment modalities (45.9%and 36.9%, respectively) for recurrent HCC (Table 2).

Table 1 Baseline characteristics of enrolled patients
Clinical features of patients with extrahepatic metastasis
Forty-four patients with EHM were assessed for clinical features related to metastasis (Table 3). The location of metastasis was distributed evenly in the lymph nodes, bone, lung, and peritoneum. In 68.2% of patients, abdominal CT was used for the diagnosis of EHM (12 patients with lymph node, 11 patients with peritoneum, 6 patients with lung, and 5 patients with bone metastasis including 3 patients with multiple EHM), and 3 (6.8%) patients were diagnosedviachest X-rays during routine follow-upexaminations. Three patients (6.8%) were diagnosedviaabdominal MRI: 1 patient during HCC surveillance and 2 patients while evaluating elevated tumor marker levels without definite HCC recurrence on abdominal CT. Spine MRI was used for the diagnosis of spinal metastasis among 3 patients who presented with new-onset back pain. Five patients (11.4%) underwent PET-CT for re-staging of HCC without signs of intrahepatic recurrence, and EHM was detected in the lung, lymph nodes, and bone.In detail, 9 patients (20.5%) had multiple sites of EHM at first diagnosis. Among 13 patients with pulmonary metastasis, 7 (53.8%) were diagnosedviaabdominal CT, 3 (23.1%)viachest X-ray, and 3 (23.1%)viachest CT.

Table 2 Characteristics of patients with first recurrence following radiofrequency ablation (n = 333)
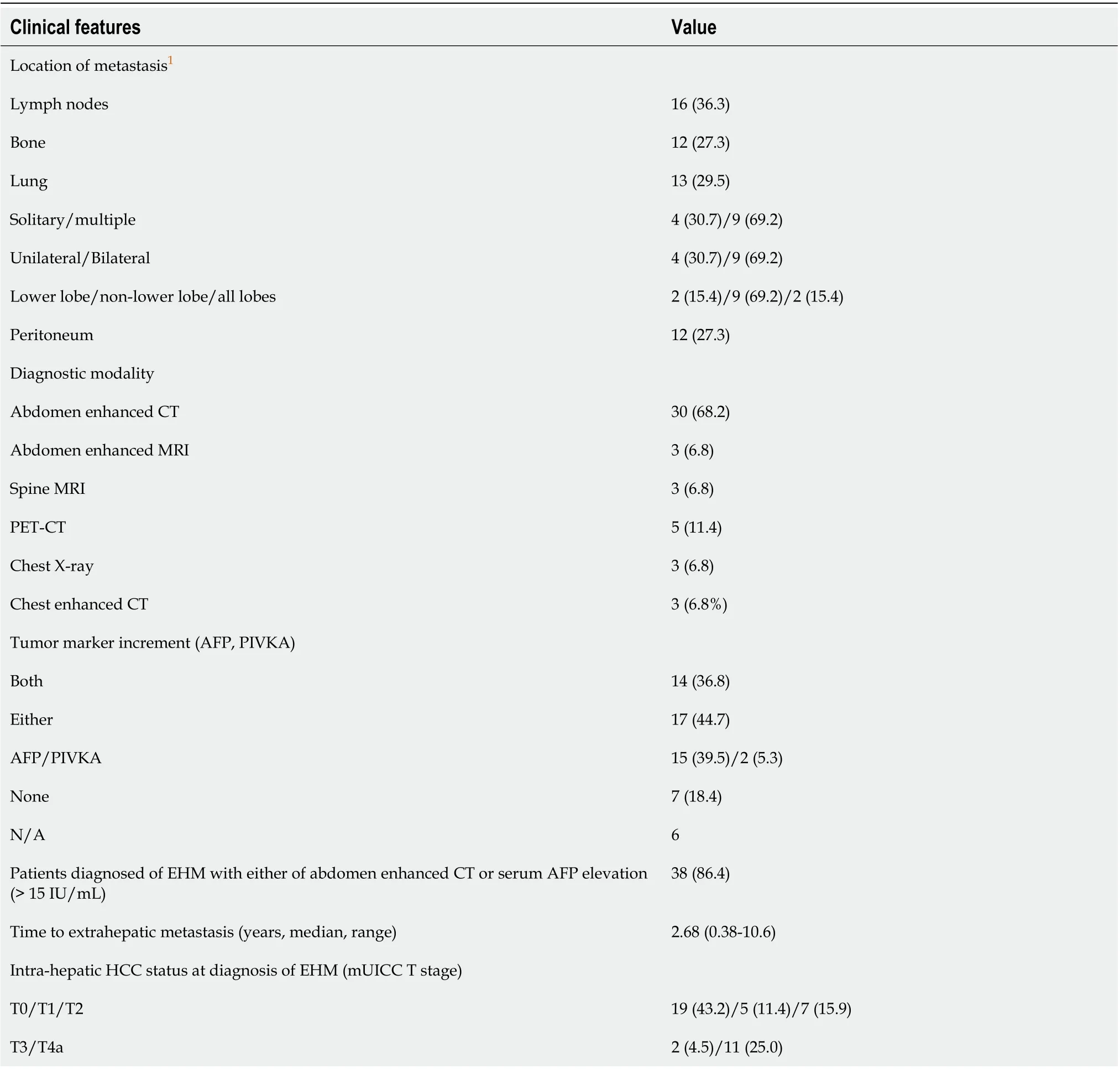
Table 3 Clinical features of patients with extrahepatic metastasis (n = 44), n (%)
Thirty-one patients (31/38, 81.5%) had increased tumor marker levels at the time of diagnosis of EHM. A diagnosis of EHM was made in most patients (86.4%) based on either contrast-enhanced CT findings or elevated serum AFP levels. Intrahepatic tumor status categorized according to the mUICC T stage at the time of EHM diagnosis was assessed; 43.2% of patients had no findings of intrahepatic HCC remnant tumor (T0), and 25.0% of patients had stage T4a disease.
Factors associated with extrahepatic metastasis
Multiple factors that might be relevant to EHM were assessed (Table 4). In the univariate analysis, more advanced stage, the presence of intrahepatic recurrence, serum alkaline phosphatase level > 97 U/L, first RFS within less than 2 years, ablation zone/tumor size > 2, and AFP level > 400 IU/mL at first HCC recurrence wereassociated with a high risk of EHM. Among these factors, a first HCC RFS < 2 years [odds ratio (OR), 2.44;P= 0.019], ablation zone/tumor size < 2 (OR, 3.33;P= 0.01), and AFP > 400 IU/mL at first recurrence (OR, 3.35;P= 0.01) were identified as significant factors in the multivariate analysis. We stratified the patients who had recurrence after RFA by numbers of risk factors related to EHM occurrence, and in proportion to the numbers of risk factors, the cumulative occurrence of EHM showed an increase (Figure 4).

Table 4 Univariate and multivariate analysis of factors associated with extrahepatic metastasis

Figure 2 Recurrence curves of 661 patients with hepatocellular carcinoma treated with radiofrequency ablation. A: Cumulative rates of recurrence. The 1-, 3-, 5-, 8-, and 10-year cumulative rates of recurrence were 15.1%, 43.8%, 62.5%, 77.9%, and 92.7%, respectively; B: Cumulative rates of extrahepatic metastasis. The 1-, 3-, 5-, 8-, and 10-year cumulative rates of extrahepatic metastasis were 1.0%, 2.9%, 8.1%, 15.7%, and 33.7%, respectively.
DISCUSSION
Although RFA is one of the most effective curative treatment modalities for HCC along with surgical resection and liver transplantation, our study and previous studies showed that there is a substantial possibility of EHM even if intrahepatic lesions are stable[8,16,17]. Most other studies showed that the most common initial site of recurrence is intrahepatic and that most cases of EHM occur after multiple intrahepatic recurrences with several treatments[8]. This study focused on the characteristics and risk factors of extrahepatic recurrence after RFA and whether regular surveillance for EHM is needed. The results are of particular interest because data on the pattern of EHM in HCC after RFA and the role of regular surveillance for EHM are limited, and there have been no reports regarding surveillance methods for EHM in HCC.

Figure 3 Timelines of recurrence of extrahepatic metastasis after radiofrequency ablation for hepatocellular carcinoma. RFA: Radiofrequency ablation; EHM: Extrahepatic metastasis; RFS: Recurrence-free survival.

Figure 4 Cumulative occurrence of extrahepatic metastasis in patients with recurrence of hepatocellular carcinoma after radiofrequency ablation: Stratified by numbers of risk factors.
In the present study, 289 patients experienced tumor recurrence without EHM, and 44 experienced EHM during a median follow-up period of 1204 d. The 1-, 3-, 5-, 8-, and 10-year cumulative rates of HCC recurrence and EHM development were concordant with the results of previous studies[8,9], but only 1.2% of the enrolled patients presented with EHM as the first recurrence of HCC. The median time to the detection of EHM was 2.68 years, and 68.2% of patients developed EHM within 2 years after the first recurrence regardless of RFS. Furthermore, 75% of patients developed EHM within 5 years of the first recurrence (Figure 3).
Other studies that reported the locations of EHM showed predominance in the lung (39%-54%), lymph nodes (34%-40%), and bone (25%-39%)[3,11,18]. In the present study, the location of EHM was relatively evenly distributed among the intra-abdominal lymph nodes (36.3%), bone (25.0%), lung (29.5%), and peritoneum (27.3%). The proportions of extrahepatic metastatic sites in our study may have differed from those identified in previous studies as our population comprised post-RFA patients who had early-stage disease, while the other studies included patients with diverse stages of HCC ranging from early to terminal. The median duration of peritoneal seeding was 883 d (range: 138–3878); however, in 3 patients, the time from RFA to EHM was less than 1 year, suggesting the possibility that peritoneal dissemination occurredviaRFA in some patients. There was no case of pulmonary metastasis as the first recurrence in this study. Most pulmonary metastases occurred after intrahepatic recurrences.
The spread pattern of lung metastasis at the initial diagnosis of recurrent HCC is important because of the possible value of metastectomy, which was reported to yield favorable outcomes in some studies[19,20]. We found that at the time of HCC recurrence 69.2% of patients had multiple lung metastatic lesions, and 69.2% had bilateral lung metastasis for which metastectomy was not indicated. Thus, the option of metastectomy after RFA may be of limited value in the group we studied.
Regarding the detection method, EHM was diagnosedviaabdominal imaging (CT/MRI) in most patients (75.0%). The backbone of surveillance after RFA for HCC is abdominal imaging, and only 14/661 patients (2.1%) required other diagnostic modalities such as PET-CT, spine MRI, chest CT, or chest X-ray. Considering the small proportion of diagnostic modalities other than abdominal imaging and the low incidence (1.2%) of EHM as the first recurrence of HCC, the need for regular surveillance tools other than abdominal imaging may not be very high. In particular, 38/44 patients (86.4%) with EHM had either positive abdominal CT scan results or serum AFP elevation, which we currently use as surveillance tools for HCC after RFA in clinical practice. Considering the high cost of spine MRI, PET-CT, and chest CT in general, the cost-effectiveness of routine surveillance using these modalities may be high however, an individualized approach in accordance with each country's reimbursement policy is needed.
A correlation between serum PIVKA-II levels and EHM was reported previously[21,22]. In our study, 31/38 patients (81.5%) had increasing tumor marker levels (serum AFP or protein induced by vitamin K absence-II [PIVKA-II]), and serum AFP levels were elevated in 29/38 (76.3%) which showed correlation with the development of EHM, as reported in other studies[23]. Although the PIVKA-II level was only assessed in 24 patients, 16 (66.7%) showed elevated serum levels at EHM development. Regarding the pattern of HCC recurrence, the most common initial site of recurrence was intrahepatic. Although the rate of extrahepatic initial recurrences in our population was only 1.2%, 43.2% of the patients had no sign of intrahepatic HCC at the time of diagnosis of EHM. This implies that even if loco-regionally managed HCC lesions are stable, close surveillance for possible EHM is warranted. In particular, when tumor marker levels increase without definite aggravation of previous HCC lesions, additional examination for EHM development should be considered. Refaatet al[17]reported that among 65 patients who underwent loco-regional therapy for HCC and had elevated serum AFP levels, 10 (15.4%) had EHM without intrahepatic tumor recurrence. In addition, Chenet al[16]reported that among 26 patients who had elevated serum AFP levels without findings of recurrence on conventional imaging studies, 8 (30.8%) experienced EHM.
Recurrence of HCC after curative treatment is reported to occur mostly within 2 years[24]; therefore, guidelines suggest surveillance for HCC recurrence within a short interval of 2-6 mo until the second year after treatment[25-27]. In the present study, the 1-, 3-, 5-, 8-, and 10-year cumulative rates of HCC recurrence were 15.1%, 43.8%, 62.5%, 77.9%, and 92.7% respectively, and those of EHM development were 1.0%, 2.9%, 8.1%, 15.7%, and 33.7%, respectively. Our results also showed a 1.75-year median time to first recurrence after RFA, a median time to the development of EHM of 2.68 years regardless of RFS, and 75.0% of patients experiencing EHM within 5 years after RFA. Thus, we suggest that it is prudent to pay attention to possible EHM occurrence for at least 5 years after RFA, and patients who experience tumor recurrence may require close observation for the development of EHM, particularly within 2 years after the first recurrence (Figure 3).
In the multivariate analysis of potential risk factors for EHM, first RFS < 2 years, ablation zone/tumor size < 2, and serum AFP level > 400 IU/mL at first recurrence were factors relevant to EHM development. Some studies have suggested that early recurrence is associated with vascular invasion, initial tumor staging, and poor prognosis, and our findings are consistent with these reports[28,29]. Other studies have assessed the risk of local tumor progression in relation to insufficiently ablated margins after RFA in liver malignancies, a minimal margin of < 2-5 mm being reported as an independent factor for local tumor progression[30-32]. Our study showed that ablation zone/tumor size was associated with the risk of EHM development. Most patients (97.9%) had minimal margins of > 5 mm, and the margin length between the tumor and ablation zones showed no relevance in the occurrence of EHM. Some studies have reported that higher AFP levels are associated with increased recurrence following liver transplantation for HCC, as well as worse disease-free survival and overall survival[33,34]. By stratifying patients according to the number of risk factors associated with EHM, the cumulative occurrence of EHM showed an increasing trend related to the number (≥ 2) of risk factors (Figure 4). Therefore, we suggest close surveillance for EHM after RFA, especially in these high-risk patients.
Our study had some limitations. First, this was a retrospective study based on medical records. Thus, there was no uniform post-treatment or surveillance schedule, and the surveillance modality used for each patient was at the physician’s discretion. Second, until the development of EHM, patients underwent different treatment modalities for local HCC recurrence depending on the tumor and patient’s status. There may be diverse statuses regarding tumor stage, liver reserve function, and patients’ physical performance status. However, we tried to overcome these limitations by using a considerable number of patients with a long-term follow-up duration in multiple tertiary centers.
In conclusion, EHM as the first recurrence after RFA was rare, but cumulative EHM occurred frequently following multiple intrahepatic recurrences. Thus, optimal surveillance for EHM after RFA for HCC is essential according to stratified risk factors (RFS < 2 years, ablation zone/tumor size < 2, and AFP level > 400 IU/mL) related to EHM, and combined contrast-enhanced abdominal CT and serum AFP level have been found useful for this purpose.
ARTICLE HIGHLIGHTS

 World Journal of Gastroenterology2020年32期
World Journal of Gastroenterology2020年32期
- World Journal of Gastroenterology的其它文章
- Emergency department targeted screening for hepatitis C does not improve linkage to care
- Dual targeting of Polo-like kinase 1 and baculoviral inhibitor of apoptosis repeat-containing 5 in TP53-mutated hepatocellular carcinoma
- New advances in radiomics of gastrointestinal stromal tumors
- Current status of Helicobacter pylori eradication and risk factors for eradication failure
- Development of a novel score for the diagnosis of bacterial infection in patients with acute-on-chronic liver failure
- Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease
