Optimize Operation of Partial Denitrification System for Simultaneous Treatment of Low C/N Municipal and Nitrate Wastewaters
Chunxue BI Deshuang YU
Abstract In order to realize the simultaneous treatment of low C/N municipal and nitrate (NO-3-N) wastewaters, a sequencing batch reactor (SBR) was used to optimize the partial denitrification (PD), which the influent substrate and the anoxic reaction time were appropriately controlled. The carbon and nitrogen removal and the characteristic parameters of PD during long-term operation were studied. Experimental results showed that the PD showed stable characteristics of nitrogen and carbon removal and NO-2-N accumulation after an adaptation of 20 d with municipal wastewater used. The anoxic reaction time was extended from 50 to 70 min with the initial COD/NO-3-N decreased from 3.0 to about 2.5. When the influent NO-3-N was 117.93 mg/L, the effluent NO-2-N and NAR were 23.10 mg/L and 82.26%, respectively, and the nitrogen and carbon removal rate reached 91.76% and 65.70%, respectively. The effluent NO-2-N/NH+4-N meantime reached 1.17-1.22. Moreover, the cumulative concentration of NO-2-N and the system NAR increased linearly with the consumption of NO-3-N and COD, and the change trend was highly significant within 0-20 min, and gradually flattened.
Key words Partial denitrification; Municipal wastewater; Nitrate wastewater; Nitrogen and carbon removal; Nitrite accumulation
Received: February 7, 2020Accepted: April 5, 2020
Chunxue BI (1994-), female, P. R. China, master, devoted to research about water pollution control.
*Corresponding author. E-mail: ye.ling@foxmail.com.
Previous research discovered that the partial denitrification process could provide the electron acceptor NO-2-N for the anaerobic ammonia oxidation (Anammox)[1]. Therefore, the partial denitrification process that converts NO-3-N to nitrite (NO-2-N) has attracted widespread attention, which has provided the possibility for the development of partial denitrification (PD) technology.
Recently, Ge et al. [2] explored the effects of different C/N conditions on NO-2-N accumulation performance. And Cao et al. [3] proved that the NO-2-N accumulation rate (NAR) during partial denitrification process under different C/N conditions could be stable up to about 80%. Besides, Shi et al. [4] explored the effects of different pH on the partial denitrification process. It was found the pH value could affect denitrification enzyme activity, and found that reasonable control of the system pH was also a relevant factor to promote NO-2-N accumulation performance. Related research[5] alsoproved the reliability of PD to provide NO-2-N for Anammox.
This study aims at evaluating the feasibility of achieving PD using organic matters in municipal wastewater expecting to produce stable NO-2-N for Anammox. The effects of different influent substrate and anoxic time on PD performance were investigated during 93 d long-term operation. Moreover, the characteristic parameters of PD were researched. It could be expected to provide theoretical support for the combination of PD and Anammox in the simultaneous treatment of actual municipal and NO-3-N wastewaters.
Material and Methods
Experimental device and operation
The experimental device was carried out a working volume of 4 L sequencing batch reactor (SBR) with the height of 30 cm and the inner diameter of 15 cm. The cantilever stirrer was used to keep the contents completely mixed. The PD operated under anoxic conditions and ran 4-5 cycles per day, including: feeding 10 min , anoxic reaction 50-70 min, settling 10 min and decanting 10 min.
The seeding sludge and wastewater
The seeding sludge was taken from a stable exogenous partial denitrification (EPD) system. It was long-term acclimated with sodium acetate as the sole carbon source. The MLSS after inoculation was 2 800 mg/L. The municipal wastewater was taken from in the Maidao WWTPs (Qingdao, China). The main characteristics were: COD 137.2-244.5 mg/L, NH+4-N 41.2-67.1 mg/L, NO-2-N<1.0 mg/L, and NO-3-N<1.0 mg/L. The NO-3-N wastewater used was provided by NaNO3 on demand.
Chemical analytical methods
The mixed liquor samples were filtered through 0.45 μm filter paper before measurement. The NH+4-N, NO-2-N, NO-3-N, COD and MLSS (mixed liquor suspended solids) were analyzed according to the Standard Methods[6].
Calculations
The NO-2-N accumulation rate (NAR) was calculated[7] as Eq. (1).
NAR (%)=( ρ(NO-2 t )-ρ(NO-2 initial )/( ρ(NO-3 initial )-ρ(NO-3 t ) ×100%(1)
Where ρ(NO-2 initial ) and ρ(NO-2 t ) were the concentration of NO-2-N at the beginning ( t =0) and at time t , mg/L; and ρ(NO-3 initial ) and ρ(NO-3 t ) were the concentration of NO-3-N at the beginning ( t =0) and at time t , mg/L.
The specific NO-3-N reduction and NO-2-N production rates were determined through the NO-3-N reduction and NO-2-N production rates dividing the MLVSS[8].
Results and Discussion
Carbon and nitrogen removal characteristics during long-term operation
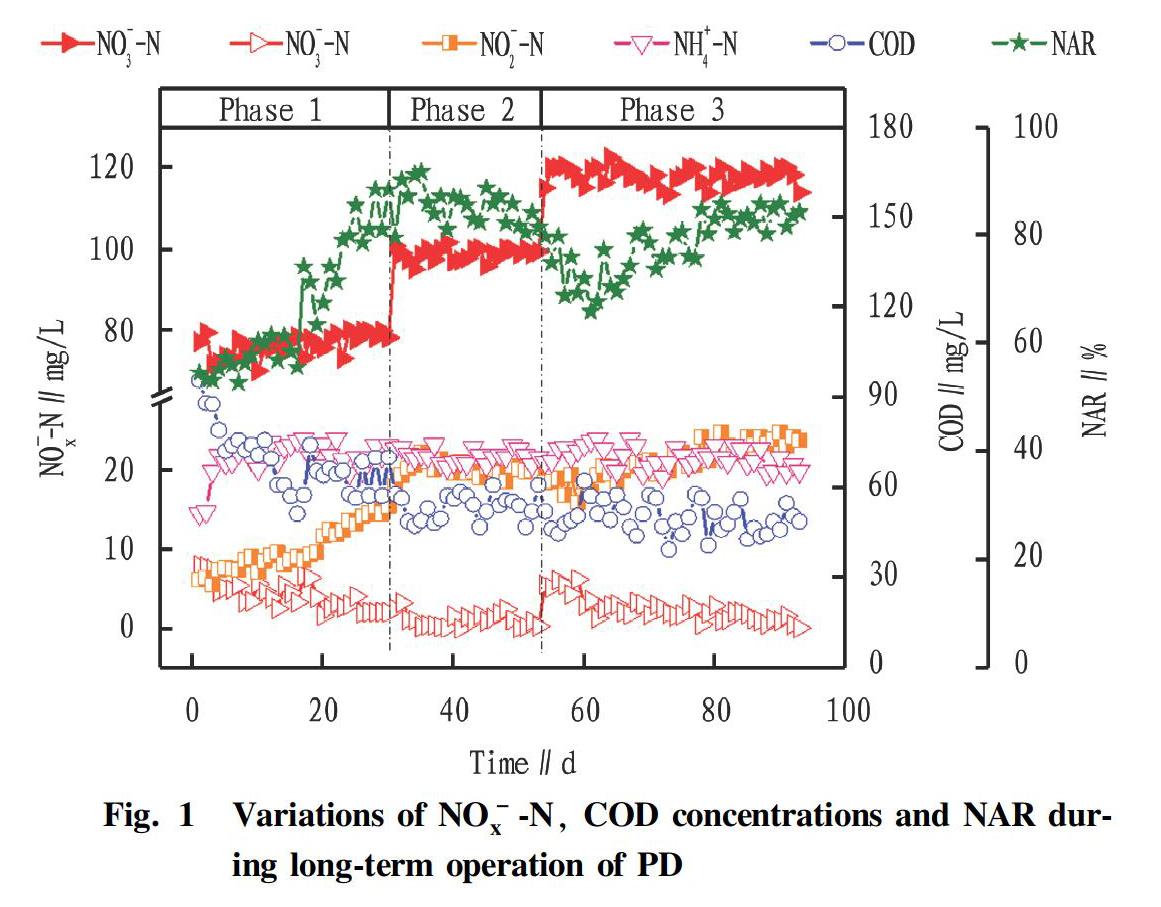
During the long-term operation, the whole process was divided into 3 phases according to the different influent substrate concentrations and NO-2-N accumulation performance. The variations of NO-3-N, NO-2-N, NH+4-N, COD concentrations and NAR were shown in Fig. 1.
In phase 1 (1-30 d), the influent NO-3-N concentration was 76.62 mg/L, and the organic matters in municipal wastewater used was about 234.20 mg/L, which made the initial COD/NO-3-N around 3.05. In 1-19 d, the effluent NO-2-N concentration and system NAR increased slowly, but they were lower than the average values of 10.13 mg/L and 66.45%, respectively. It could be seen that the NO-2-N accumulation performance in the partial denitrification process of the system is not ideal. The reason might be that denitrifying bacteria were difficult to degrade organic matters in municipal wastewater compared with the single carbon source[9]. In 20-30 d, with the enhanced adaptability to various organic matters in municipal wastewater, the NO-2-N accumulation performance was significantly improved, and the effluent NO-2-N concentration and NAR increased to 14.55 mg/L and 81.06%, respectively. Correspondingly, the effluent NO-3-N and COD concentrations decreased to 2.05mg/L and 57.31 mg/L respectively (69 d). It indicated that organic matters in municipal wastewater can be used as carbon source for partial denitrification process.
In phase 2 (31-53 d), in order to improve the carbon removal rate of PD, the influent average NO-3-N concentration was increased to 98.86 mg/L, and the initial COD/NO-3-N was about 3.0 when municipal wastewater was added. The concentration of NO-2-N in the effluent increased significantly, with an average of 20.16 mg/L. And the system NAR fluctuated slightly but remained at a high level, with an average of 85.19%. It might be due to sufficient carbon sources in the system and increased adaptability to municipal wastewater.
In phase 3 (54-93 d), the influent NO-3-N concentration continued to increase to 117.93 mg/L. At this time, the COD of the municipal wastewater was reduced (~137.2 mg/L) , so adding a small amount of sodium acetate made the initial COD/NO-3-N about 2.5. In 54-73 d, the effluent NO-3-N, COD concentrations increased slightly, and the NAR also fluctuated greatly. The reason may be the shorter anoxic reaction time under the condition of lower COD/NO-3-N. Subsequently, the anoxic reaction time was extended to 70 min from 50 min. With the effluent NO-3-N concentration slowly decreased, the effluent NO-2-N concentration and NAR slowly increased and stabilized, and the average values were 23.10 mg/L and 82.26%, respectively. Correspondingly, nitrogen and carbon removal efficiency also reached 91.76% and 65.70%, respectively. In addition, for the system effluent NO-2-N/NH+4-N, it could reach 1.17-1.22, which could basically meet the substrate requirements of Anammox (74-93 d).
Characteristic parameters of partial denitrification in the PD
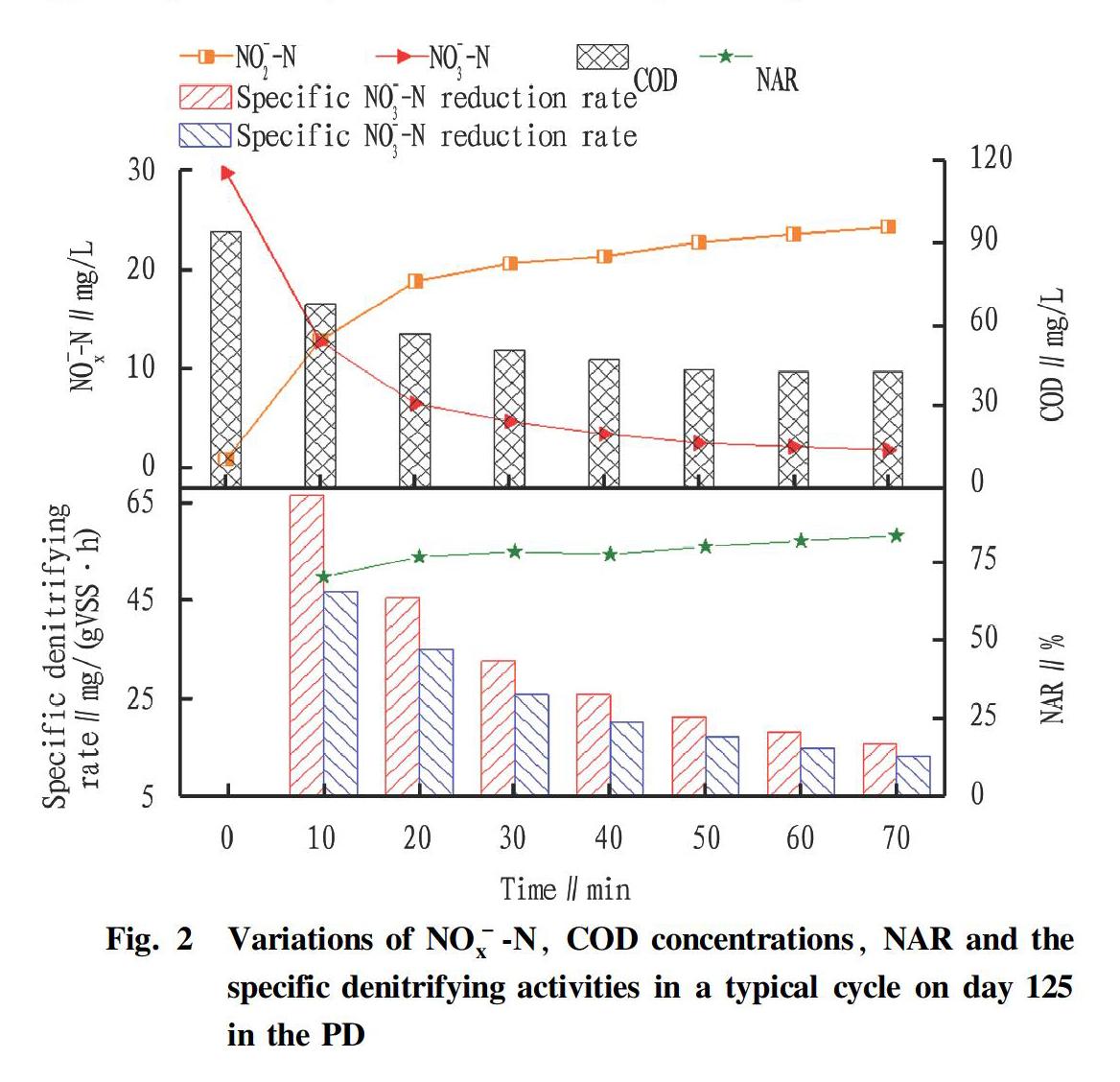
In order to further analyze the mechanism of carbon and nitrogen removal with simultaneously treating low C/N municipal and high NO-3-N wastewaters, and the variations of NO-3-N, NO-2-N, COD concentrations, NAR and specific denitrifying activities in a typical cycle on day 125 of PD was analyzed (Fig. 2).
As shown in Fig. 2, the initial COD, NO-3-N and NO-2-N concentrations were 94.11, 29.8, and 0.89 mg/L, respectively. In 0-20 min, NO-3-N and COD were sufficient in the PD, NO-3-N could be quickly converted into NO-2-N. Specifically, the concentrations of NO-3-N and COD decreased rapidly to 6.49 and 56.51 mg/L respectively, while the NO-2-N concentration increased to 18.83 mg/L. During the period, the system showed efficient specific NO-3-N reduction rate and a specific NO-2-N production rate, specifically 45.41 and 34.95 mg/(gVSS·h) within 20min, respectively. It might be because that high concentration of NO-3-N could provide sufficient nitrate reductase binding sites.
During 20-70 min, the changes of NO-3-N, NO-2-N and COD concentrations were relatively gentle compared to the previous 20 min, and the NO-2-N concentration and NAR reached their maximum values at 70 min (24.31 mg/L, 83.7%). At the end of the reaction, the specific NO-3-N reduction rate and a specific NO-2-N production rate of PD reached their lowest points, 15.57 and 13.03 mg/(gVSS·h), respectively. It was worth noting that the specific NO-3-N reduction rate was higher than the specific NO-2-N production rate. It might be caused by the denitrification process and the inhibitory effect of higher concentrations of NO-2-N. In addition, the production of NO-2-N had a certain lag compared with the reduction of NO-3-N.
Conclusions
The PD showed stable characteristics of nitrogen and carbon removal and NO-2-N accumulation after an adaptation period of about 20 d when municipal wastewater was used. The anoxic reaction time was extended from 50 to 70 min with the initial COD/NO-3-N decreased from 3.0 to about 2.5. And when the influent NO-3-N concentration was 117.93 mg/L, the average effluent NO-2-N concentration and NAR were 23.10 mg/L and 82.26% , respectively, and the nitrogen and carbon removal efficiency reached 91.76% and 65.70%, respectively. In addition, the PD system effluent NO-2-N/NH+4-N at this phase reached 1.17-1.22. Moreover, the cumulative concentration of NO-2-N and the system NAR increased linearly with the consumption of NO-3-N and COD, and the change trend was highly significant within 0-20 min, and gradually flattened. Correspondingly, the specific NO-3-N reduction rate and a specific NO-2-N production rate of PD system were significantly reduced from 45.41 and 34.95 mg/(gVSS·h) to 15.57 and 13.03 mg/(gVSS·h), and the production of NO-2-N had a certain lag compared with the reduction of NO-3-N.
Chunxue BI et al. Optimize Operation of Partial Denitrification System for Simultaneous Treatment of Low C/N Municipal and Nitrate Wastewaters
References
[1] WAKI M, YASUDA T, FUKUMOTO Y, et al. Effect of electron donors on anammox coupling with nitrate reduction for removing nitrogen from nitrate and ammonium[J]. Bioresource Technology, 2013, 130(130C): 592-598.
[2] GE S, PENG YZ, WANG SY, et al. Nitrite accumulation under constant temperature in anoxic denitrification process: The effects of carbon sources and COD/NO-3-N[J]. Bioresource Technology, 2012(114): 137-143.
[3] CAO S, LI B, DU R, et al. Nitrite production in a partial denitrifying upflow sludge bed (USB) reactor equipped with gas automatic circulation (GAC)[J]. Water Research, 2015(90): 309-316.
[4] SHI L, DU R, PENG Y. Achieving partial denitrification using carbon sources in domestic wastewater with waste-activated sludge as inoculum[J]. Bioresource Technology, 2019(283): 18-27.
[5] DU R, PENG Y, CAO S, et al. Advanced nitrogen removal from wastewater by combining anammox with partial denitrification[J]. Bioresource Technology, 2015(179): 497-504.
[6] APHA, AWWA, WEF, et al. ISBN 9780875530130 Standard Methods for the Examination of Water and Wastewater[S]. Washington, DC, USA: Amer Public Health Assn, 2012.
[7] DU R, PENG Y, CAO S, et al. Mechanisms and microbial structure of partial denitrification with high nitrite accumulation [J]. Applied Microbiology & Biotechnology, 2016, 100(4): 2011-2021.
[8] JI J, PENG Y, WANG B, et al. Effects of salinity build-up on the performance and microbial community of partial-denitrification granular sludge with high nitrite accumulation[J]. Chemsphere, 2018(209): 53-60.
[9] WANG X, ZHAO J, YU D, et al. Stable nitrite accumulation and phosphorous removal from nitrate and municipal wastewaters in a combined process of endogenous partial denitrifification and denitrifying phosphorus removal (EPDPR)[J]. Chemical Engineering Journal, 2019(355): 560-571.
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Effects of Different Planting Densities on the Yield of Rice Developed from Seedlings Dry Raised in Plug Trays
- Effects of NaCl and NaHCO3 Stress
- Variety Characteristics and Cultivation Techniques of High-yield and High-resistance Wheat Variety Xindong No.48
- Effects of Different Line Spacing and Seedling Belt Width on Yield Formation of Broad-Width Fine Sowing Wheat
- Efficacy Tests of Nine Insecticides on Tetranychus truncatus Ehara
- Research Progress and Prospect of Sugarcane Smut

