Residual Effect of Fipronil in Eriocheir sinensis
Xi CHEN Cong ZHANG Chao SONG Longxiang FANG Xinyue DONG Jiazhang CHEN
Abstract The residual effects of fipronil aqueous solutions (at mass concentrations of 10, 30 and 50 μg/L, respectively) on Eriocheir sinensis were investigated by the semi-static experiment method. The results showed that among the treatment groups with different mass concentrations, the high mass concentration group contained fipronil at a significantly higher content in the crab legs than the low-mass concentration group, and also a significantly higher content in the body than the low mass concentration group. There were no significant differences in the content of fipronil between different parts of E. sinensis. The trend of fipronil-enriched parts was from the legs to the body to the gonads. Fipronil residue in E. sinensis had no sex selectivity, but it enriched faster in female E. sinensis than males. This study provides a certain reference for dealing with related fishery pollution accidents.
Key words Fipronil; Eriocheir sinensis; Residual effect
In June 2018, Germany discovered that Dutch "poisonous eggs" contaminated with fipronil flowed into supermarkets, and food safety issues attract peoples attention. Fipronil is a broad-spectrum pesticide with an action of stomach poisoning on pests mainly, as well as a contact and systemic action. It is used to kill aphids, lepidopteran larvae, flies, and coleoptera[1-3]. According to pesticide acute toxicity grading standards in China, fipronil is a moderately toxic drug[4]. Since fipronil has a high risk to crustacean aquatic organisms and bees and degrades slowly in water and soil, China has stated in the document Announcement No. 1157 of the Ministry of Agriculture of the Peoples Republic of China that since October 1, 2009, except for some dry field seed coating agents for hygienic use and corn, the sale and use of fipronil-containing pesticide formulations in China are stopped[5].
Eriocheir sinensis flesh is delicious, nutritious, and has high economic value. It is popular among consumers. Corn is one of the main feed ingredients of E. sinensis, and fipronil-containing coating agents may be introduced into the growth environment of E. sinensis. Studies have shown that fipronil is highly toxic to crustaceans[6]. The study of Wang et al.[7] showed that fipronil had a median lethal mass concentration of 0.28 mg/L at 96 h for juvenile Trionyx sinensis (Wiegmann). Through the paddy field-fish pond simulation test, Shan et al.[8] showed that fipronil had an LC50 of 0.008 6 mg/L for E. sinensis (body weight 1 g) at 96 h. At present, there are few studies on the residual content of fipronil in E. sinensis. Meanwhile, no studies have been reported on the differences in fipronil residue between different parts and different genders of E. sinensis. To this end, in this study, a laboratory simulation experiment was carried out to investigate fipronil residue in E. sinensis, aiming to provide a scientific basis for the safety of E. sinensis food and the handling of related fishery pollution accidents.
Materials and Methods
Experimental materials
Tested organisms
The experimental species was E. sinensis (before the last molting), which was purchased from an E. sinensis farm in Jiangyin, Jiangsu Province. The crabs had the same size and a body weight of (91.79±12.21) g. They were raised indoor to allow them to adapt to the environment. Healthy and energetic ones were chosen for testing.
Experimental water
Tap water was aerated outdoor for more than 3 d, and the water temperature was 20-24 ℃.
Instruments and reagents
The instruments included BL-22MS high-speed freezing centrifuge (Shanghai Lu Xiangyi Centrifuge Instrument Co., Ltd.), sartorius centrifuge (Sigma), Acquity UPLC I-Class, Xevo TQD liquid-mass spectrometer (Waters), and Abilent Bond Elut EMR Lipid Polish reverse extraction tube. The reagents included methanol, formic acid, acetonitrile and ethyl acetate, all chromatographically pure, and fipronil standard solution; and the water used was ultrapure water.
Experimental methods
Fipronil exposure treatment
Using the semi-static test method, the indoor temperature was set to 23 ℃. The tested E. sinensis were put into glass tanks (length 39.5 cm×width 28.5 cm×depth 29.5 cm). The tanks were filled with 6 L of different concentrations of diluent, which was aerated with an oxygen pump to increase oxygen. Each tank was input with five individuals. A total of 6 treatments and 2 blank controls were set, and each treatment was repeated 3 times. During the test period, no feed was input, and the drug solution was changed every 48 h. The test period was 4 weeks, and samples were taken every other week. According to the pre-test carried out by our research group to determine the mass concentration of the drug in this test, we not only ensured that fipronil residue could be detected in poisoned E. sinensis, but also kept some E. sinensis alive at the end of the test. The mass concentrations of the treatment groups were 10, 30, and 50 μg/L, and each mass concentration was divided into two treatments by gender.
Pretreatment method
During sampling, one E. sinensis was randomly collected from each tank, and the edible parts including the body (B), gonad (B), and crab legs (L) were taken for detection. A certain amount of each sample (5 g) was weighed into a 50 ml centrifuge tube, and added with 10 ml extracting solution (ethyl acetate, acetonitrile and formic acid with a volume ratio of 99.5∶99.5∶1.0). The mixture was vortex-oscillated at 2 000 r/min for 15 min, and centrifuged at 12 000 r/min for 5 min. Then, 5 ml of the supernatant was added into the Abilent Bond Elut EMR-Lipid enhanced lipid removal purification tube, which had been activated with 5 ml of water. The supernatant was vortex-oscillated at 2 000 r/min for 5 min, and centrifuged at 4 000 r/min for 5 min. All the supernatant centrifuged was transferred into a centrifuge tube containing 1.7 g of EMR Lipid Polish MgSO4, vortex-oscillated at 2 000 r/min for 5 min, and centrifuged at 4 000 r/min for 5 min. The organic phase (upper layer) was filtered with a 0.22 μm organic phase filter membrane, for later UPLC-MS/MS analysis.
Instrumental analysis conditions
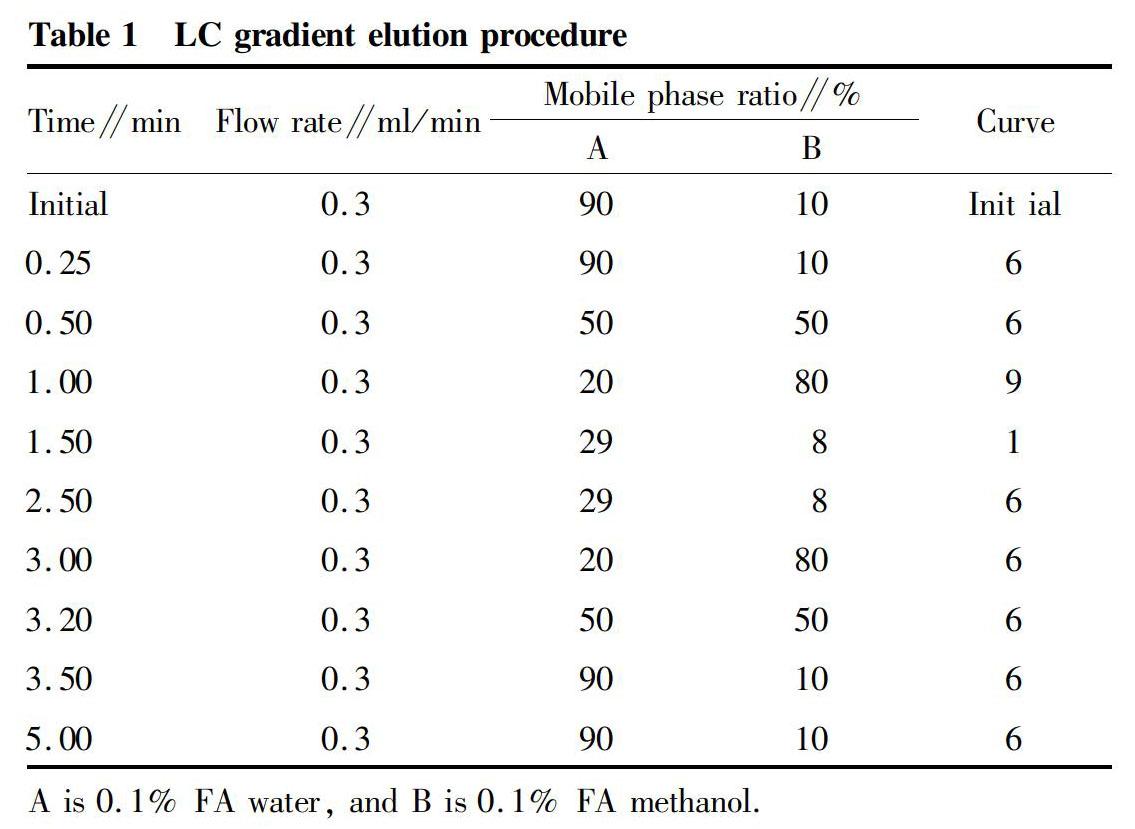
Mass spectrometry conditions were as follows: ion acquisition mode ES-, capillary voltage 2.6 kV, cone voltage 36 V, ion source temperature 150 ℃, desolvent gas temperature 350℃, desolvent gas flow rate 650 L/h, and cone gas flow rate 30 L/h. In the MRM monitoring mode, the fipronil parent ion mass-to-charge ratio(m/z) was 434.844, and the quantitative ion mass-to-charge ratio (m/z) was 317.930; and the retention time was 2.13 min. The chromatography conditions were as follows: chromatography column Acquity UPLC BEH C18 1.7 μm, 2.1×100 mm column, column temperature 30 ℃, autosampler temperature 10 ℃, and injection volume 5 μl. The gradient elution procedure is shown in Table 1.
Results and Analysis
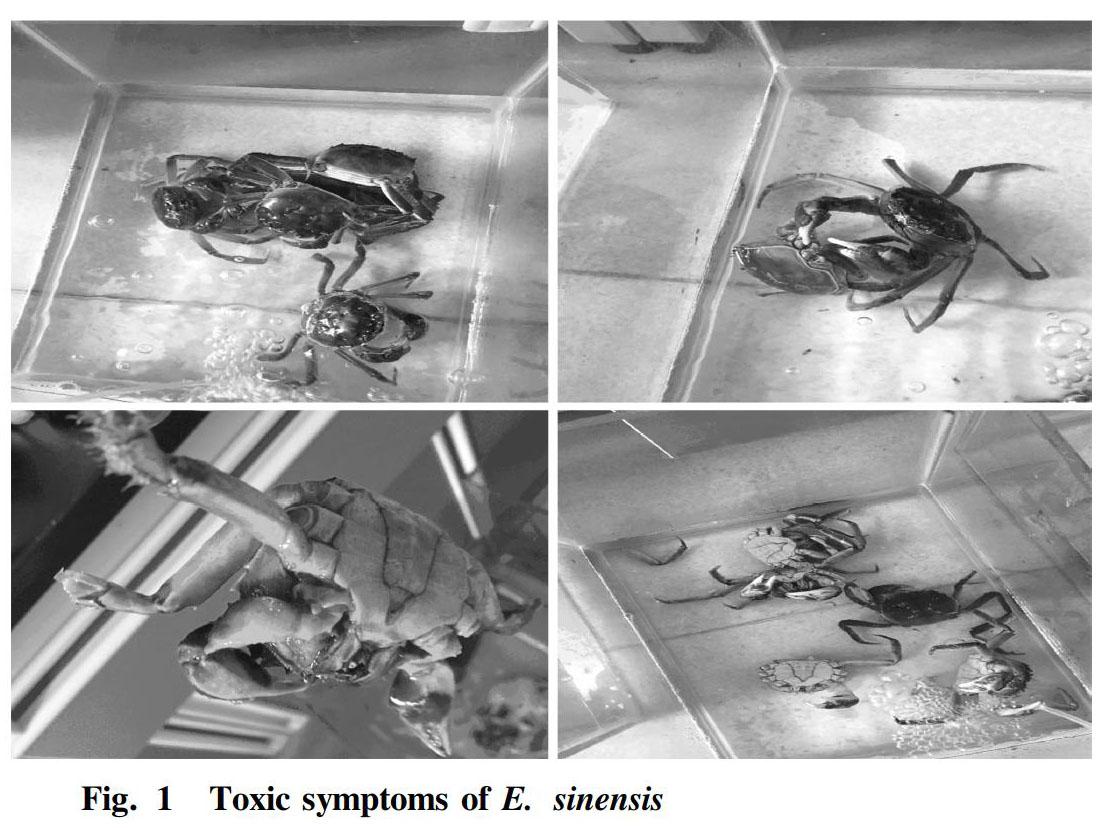
Toxic symptoms of E. sinensis
At the beginning of the test, the tested E. sinensis were more excited and chasing around after being put into the drug solutions, and then rested at the bottom of the containers. They survived in the glass containers and showed no significant difference from the control. In the three treatment groups, within 96 h, there were no obvious toxic symptoms, and some E. sinensis died. In the high mass concentration group, E. sinensis had 8 feet domed, and there was a grouping behavior. The E. sinensis fought each other, using their claws to constantly pinch off other individuals walking legs. After 6 d, the E. sinensis in the high mass concentration group spitted yellow water one after the other and died. With the extension of the test time, there were different numbers of dead individuals in each test gradient. The poisoning behavior of E. sinensis was manifested as clumping, unresponsiveness, and foaming in the mouth. During the dissection, it was found that the body and limbs of the poisoned E. sinensis were soft and had no elasticity, and the carapace and the chest and abdomen were not strongly bonded.
Fipronil belongs to phenylpyrazole compounds. Its mechanism of action is to block the opening of chloride channels mediated by γ-aminobutyric acid, thereby causing excessive excitement of the central nervous system[9]. In comparison with the study of Wang et al.[7], it was found that in the acute toxicity test of fipronil to juvenile T. sinensis, the neurological symptoms of poisoned juvenile T. sinensis are similar to the neurological symptoms of E. sinensis in this test. We believe that fipronil causes poisoning behaviors co-caused by nerve conduction, respiratory obstruction and metabolic disturbance. Pathological features such as glial cell vacuolation and slight expansion of the nuclear intermembrane space were observed in neurons of mice acutely poisoned by fipronil[10]. Carla M. Stehr et al.[11] explored the effects of fipronil on zebrafish embryos and juveniles and found that fipronil could impair the spinal cord movement of fish by inhibiting the function of glycine receptor subtypes. Neurotoxic symptoms such as dorsal arching and clumping behavior of E. sinensis may also be related to the damage of glycine receptors. Acetylcholinesterase can ensure the normal transmission of nerve impulses between synapses. Yan et al.[12] found that fipronil inhibited the acetylcholinesterase of Carassius auratus brain tissue up to 35.21%, and the neurological symptoms of E. sinensis were related to the inhibition of fipronil on acetylcholinesterase.
The contents of fipronil in the same part of E. sinensis at the same time in different mass concentrations
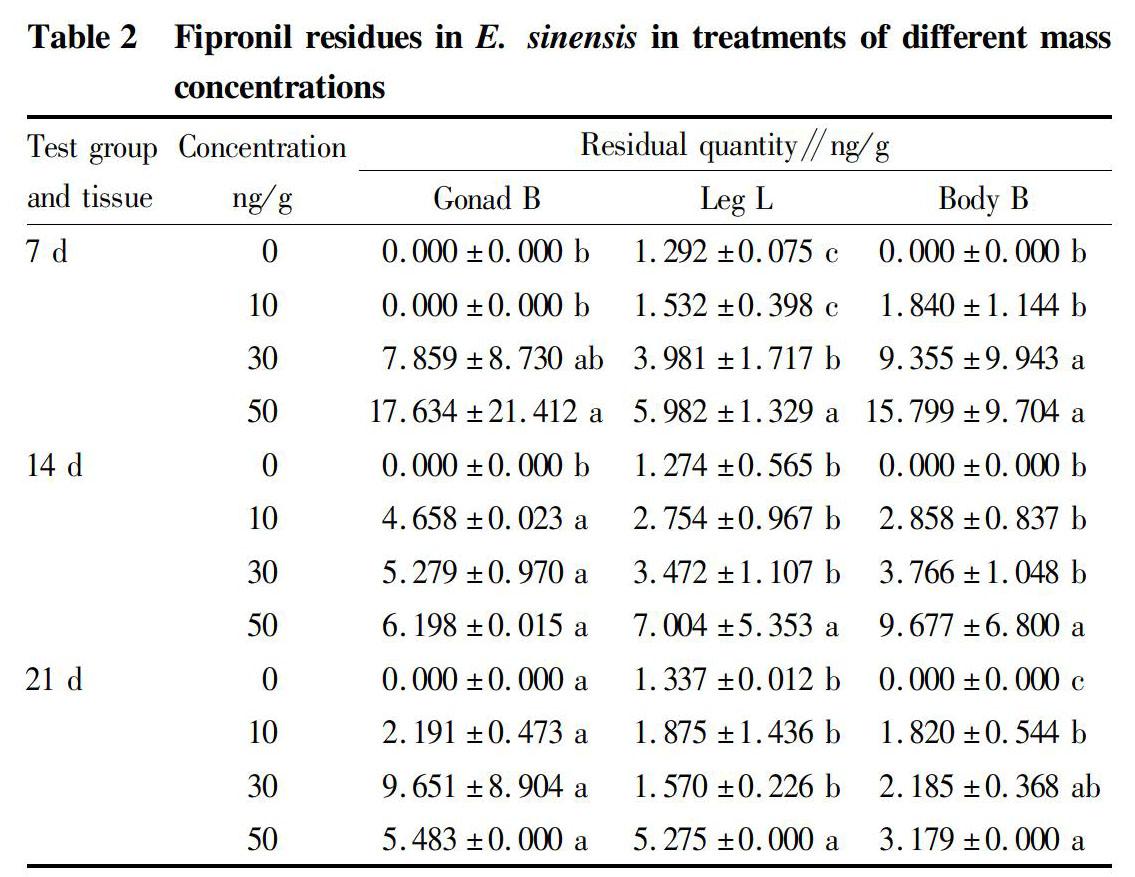
It could be seen from Table 2 that the contents of fipronil in E. sinensis of different treatment groups varied with time. Among them, fipronil was detected in the legs of E. sinensis in the blank group with a content of about 1.3 ng/g. Meanwhile, the residue of fipronil in E. sinensis in the high mass concentration group was higher than that of the medium mass concentration group, which was higher than the residue in the low mass concentration group (Fig. 2). Through data analysis, it was found that the content of fipronil in the crab legs of the high mass concentration group was significantly higher than that of the low mass concentration group (P<0.05), and the content in the body of E. sinensis in the high mass concentration group was also significantly higher than that in the low mass concentration group (P<0.05). The residue of fipronil in the gonads was not significantly different. The content in the gonads of E. sinensis in the high mass concentration group at the first sampling was quite different and unevenly distributed. It might be that under the high mass concentration, the ability of the gonads to absorb fipronil was different or the accumulation of fipronil in the gonads was related to individual differences.
The contents of fipronil in different parts of E. sinensis at the same time in the same mass concentration
According to the analysis of JMP software, there were no differences in the content of fipronil in different parts of E. sinensis. However, under the same exposure time and the same massconcentration, the residual amount of the legs was relatively low. It has been reported that fipronil is higher in adipose tissue[8], and relatively less concentrated in the legs because there is less fat in leg tissues. During the test, the treatment groups all showed the phenomenon of broken legs. It might be that the bony eye area was destroyed by the effect of fipronil, and fipronil damaged the nervous system of E. sinensis, causing the individuals to kill each other.
At 7 d, no residual fipronil was detected in the gonads of the low mass concentration group. In the blank group, the content of fipronil was detected only in the legs. It might be that the enrichment of fipronil begun from the legs, then to the body and finally to the gonads, which might be related to the protection of E. sinensis by the external carapace.
Agricultural Biotechnology2020
The contents of fipronil in different genders of E. sinensis at the same time in the same mass concentration
It could be seen from Fig. 3 that at 7 d, the contents of fipronil in the female and male E. sinensis in the low mass concentration treatment group were not significantly different, and the average detection value was about 2 ng/g. Both the medium mass concentration and high mass concentration treatment groups had significantly higher content of fipronil in females than that in males, and the content in females was twice that in males. At days 14 and 21, only males survived in the high concentration group, and the content of fipronil in males was higher than that in females in the low mass concentration group. Relatively speaking, over time, the enrichment of fipronil in female E. sinensis was faster than that in males. Although only male E. sinensis survived in the high mass concentration group at the end of the test, the content of fipronil in the body was not significantly different from those of other groups, which could not indicate that male E. sinensis was highly tolerated. According to reports, male estuarine benthic copepods are more sensitive to fipronil, which could disrupt the very important neurohormonal cascade in the development of male gonads, thereby impairing their reproductive function and reducing the number of offspring[13]. Fipronil interferes with the endocrine of both female and male rats. It could decrease the thyroid hormone in male rats[4] and also affect the plasma progesterone and estradiol levels in female rats[14]. It could be seen that fipronil has a certain damaging effect on the hormones of female and male animals, but the residue of fipronil in E. sinensis has no gender selectivity. At the same mass concentration, fipronil accumulated faster in female E. sinensis than males, and fipronil had a certain effect on the reproductive function of E. sinensis.
Conclusions
The residual effect of fipronil in E. sinensis: At the same time, the residue of fipronil in E. sinensis was higher in the high mass concentration group than in the medium mass concentration group, and the lowest in the low mass concentration group. There were no significant differences in the content of fipronil in different parts of E. sinensis. The trend of the fipronil-enriched parts begun from the legs, then to the body and finally to the gonads. Fipronil residue in E. sinensis was not gender-selective, but it enriched faster in the female individuals than in the males.
Fipronil is a high-risk drug for E. sinensis. The mass concentration of 10 μg/L in water will not cause the death of E. sinensis, but over time, it could cause a series of toxic symptoms in E. sinensis. Fipronil at very low mass concentrations could not be detected in E. sinensis, but could harm the physiological system of E. sinensis already, and the supervision of fipronil in the water of breeding ponds and the surrounding environment should still be strengthened.
References
[1] CHENG YP, DONG FS, LIU XA, et al. Simultaneous determination of fiproniland its major metabolites in corn and soil by ultra performance liquid chromatography-tandem mass spectrometry[J]. Analytical Methods, 2014, 6(6): 1788-1795.
[2] ZHANG FF, HONG YQ, ZHANG Y. Advance in toxicological study on fipronil[J]. Occupation and Health, 2008, 24(20): 2211-2213. (in Chinese)
[3] HU XF, WEI FX, LI XL, et al. Research progress on the harm of fluonitrile and its detection technology[J]. Food Safety and Quality Detection Technology, 2018, 9(6): 1226-1233. (in Chinese)
[4] ZHOU T, XIAO HX, ZHOU ZJ. Overview of safety evaluation data of fipronil[J]. Journal of Environmental and Occupational Medicine, 2017, 34(8): 745-748. (in Chinese)
[5] Ministry of Agriculture of the Peoples Republic of China. Announcement of the Ministry of Agriculture of the Peoples Republic of China[J]. Chinese Journal of Veterinary Drug, 2014(3): 69. (in Chinese)
[6] XU GC, GU ZY, YANG YQ, et al. Progress in research on risks of pesticide fipronil and its application[J]. Modern Agrochemicals, 2008, 7(2): 1-5, 11. (in Chinese)
[7] WANG ZZ, SHI JJ, LYU GT, et al. Acute toxicity of three common pesticides to juveniles Trionyx sinensis (Wiegmann)[J]. Journal of Zhejiang Ocean University: Natural Science Edition ,2010,29(3): 199-205. (in Chinese)
[8] SHAN ZJ, WANG LS, CAI DJ, et al. Impacts of fipronil on aquatic organisms in paddy-fish pond ecosystem[J]. Scientia Agricultura Sinica, 2002, 35(8): 949-952. (in Chinese)
[9] MERKOWSKY K, SETHI RS, BILL JP, et al. Fipronil induces lung inflammation in vivo and cell death in vitro[J]. Journal of Occupational Medicine and Toxicology, 2016, 11(1): 10.
[10] HE GX, LIN XF, LIU X, et al. Experimental study on toxicity and pathological morphology in mice subjected to fipronil poisoning[J]. Journal of Wenzhou Medical College, 2005, 35(3): 179-181. (in Chinese)
[11] STEHR CM, LINBO TL, INCARDONA JP, et al. The developmental neuro toxicity offipronil: Notochord degeneration and locomot or defects in zebra fish embryos and larvae[J]. Toxicolobical Sciences, 2006, 92(1): 270-278.
[12] YAN HJ, YU L, XIA JY, et al. Acute and chronic toxic effects of abamectin and fipronil on brocaded crucian[J]. Jiangsu Agricultural Sciences, 2011, 39(4): 363-365. (in Chinese)
[13] CARY TL, CHANDLER BT, VOLZ DC, et al. Phenyl pyrazoleinsecticide fipronil induces male infertility in the estuarine meiobenthic crustacean Amphiascust enuiremis[J]. Environmental Science&Technoloby, 2004, 38(2): 522-528.
[14] OHI M, DALSENTER PR, ANDRADE AJ, et al. Reproductive adverse effects of tipronilin Wistar rats[J]. Toxicoloby Letters, 2004, 146(2): 121-127.
- 农业生物技术(英文版)的其它文章
- Investigation on Agronomic Characters of Dwarf Mutant 778 in Broomcorn Millet (Panicum miliaceum L.) and Analysis of Its Sensitivity to GA
- Construction of Technology System on Development and Repropagation in Vitro of Several Cultivars in Pear
- Construction of Camellia oleifera Cultivation Standardization System
- Nutrients Determination in Nuts from Different Torreya grandis Cultivars
- Photosynthetic Physiological Response to Drought Stress of Populus euphratica at Different Ages in Minqin
- Effects of UV-B Radiation on the Activity of Antioxidants in Flue-cured Tobacco Leaves

