Multi-modality imaging of cardiac amyloidosis:Contemporary update
Tom Kai Ming Wang,Ossama K Abou Hassan,Wael Jaber,Bo Xu
Tom Kai Ming Wang,Ossama K Abou Hassan,Wael Jaber,Bo Xu,Section of Cardiovascular Imaging,Robert and Suzanne Tomsich Department of Cardiovascular Medicine,Sydell and Arnold Miller Family Heart and Vascular Institute,Cleveland Clinic,Cleveland,OH 44195,United States
Abstract
Key words:Cardiac amyloidosis;Echocardiography;Cardiac magnetic resonance imaging;Nuclear imaging
INTRODUCTION
Cardiac amyloidosis is the abnormal extracellular deposition of pathological misfolded proteins,forming amyloid fibrils that accumulate in the heart causing progressive disease from myocardial toxicity,cellular dysfunction and death.Over 30 different precursor proteins have been found to cause amyloidosis,with transthyretin (ATTR) and light chain (AL) amyloidosis being the most common.Transthyretin,also known as pre-albumin,is made in the liver transporting thyroid hormone and vitamin A.This is further divided into wild-type (senile-ATTRwt) and variant (hereditary-ATTRv) ATTR amyloidosis.AL or primary amyloidosis on the other hand is a plasma cell clonal disorder with overproduction of immunoglobulin light chain fragments affecting multiple organs.Although cardiac amyloidosis traditionally had poor prognosis with few treatment options,recent advances in multi-modality imaging have enabled earlier diagnosis and improved outcomes in conjunction with initiation of novel therapies.This review aims to discuss the clinical utility of multi-modality cardiac imaging in the contemporary evaluation and management of cardiac amyloidosis.A specific focus is given to the nuclear medicine imaging techniques in cardiac amyloidosis.
CLINICAL CONSIDERATIONS
Epidemiology
Traditionally considered a rare condition,cardiac amyloidosis has become increasingly recognized due to increased awareness and improved cardiac imaging,and this is especially true for ATTR cardiac amyloidosis in selected elderly cohorts.One study found ATTRwt amyloidosis in 13% of 120 inpatients over 60 years of age with heart failure with preserved ejection fraction on nuclear imaging.Another study found 12% of 43 aortic stenosis patients undergoing transcatheter aortic valve replacement having imaging findings suggestive of ATTRwt amyloidosis.In addition,one study of 7733 mitral valve surgery patients also found 0.2% of unrecognized cardiac amyloidosis.ATTRv is relatively less common,despite over 100 point mutations identified for the gene,and V1221I is the most common with 3%-4% of African Americans being heterozygous carriers.AL cardiac amyloidosis is also very rare,with an incidence of approximately 1/100000 per year.Whereas ATTR cardiac amyloidosis usually presents in patients 60 years of age or older and continues to increase in prevalence with aging populations,AL cardiac amyloidosis may present in younger patients.
Presentation
Diastolic heart failure (or heart failure with preserved ejection fraction) is the main manifestation for all forms of cardiac amyloidosis,which includes both left heart failure symptoms of dyspnea on exertion but also right heart failure symptoms of leg edema and ascites.Notably,in a significant proportion of patients,the left ventricular systolic function and ejection fraction may be impaired in addition to diastolic dysfunction.Light-headedness and syncope can occur as a result of bradyarrhythmias or advanced atrioventricular block,while atrial fibrillation is also common.For ATTRv amyloidosis,two patterns have been observed,the first in the endemic foci of Portugal and Japan that manifests as early-onset disease with cardiac conduction disturbances often needing pacemaker implantation;the second pattern occurs in non-endemic areas,associated with later disease onset,and marked amyloid deposition and diastolic dysfunction.These manifestations correlate with subendocardial long amyloid fibrils deposition leading to atrophy and degeneration in the first group,but diffuse myocardial short amyloid fibril deposition in the second group on histopathology.Fatigue and weakness may be related to low cardiac output,while angina due to coronary involvement is rare.
More importantly,there are many extracardiac manifestations associated with cardiac amyloidosis,such as bilateral carpal tunnel syndrome (in all subtypes although most common in ATTRwt),low or normalization of previously high bloodpressure,spinal stenosis (especially ATTR subtypes),peripheral and autonomic neuropathy (especially AL and ATTRv),macroglossia and/or periorbital purpura(AL),proteinuria including nephrotic range (especially AL),jaw claudication,diarrhea and weight loss.
Signs on physical examination include pulmonary rales,elevated jugular venous pressure,leg edema,ascites,hepatic congestion and other extracardiac manifestations listed earlier.ATTR cardiac amyloidosis should also be considered in elderly patients with a new diagnosis of “hypertrophic cardiomyopathy” or low flow low gradient aortic stenosis especially with preserved EF and low stroke volume index.
Differential diagnoses
One of the challenges when evaluating patients for cardiac amyloidosis is the wide range of differential diagnoses that needs to be considered with similar clinical and imaging manifestations of increased left ventricular wall thickness.Possibilities include hypertensive cardiomyopathy,aortic stenosis,hypertrophic cardiomyopathy,dilated cardiomyopathy,left ventricular non-compactionand other reasons for heart failure with preserved ejection fraction.Other infiltrative diseases that can mimic cardiac amyloidosis include Anderson Fabry disease,Danon disease,sarcoidosis,hemochromatosis and Loeffler's endocarditis.Differential diagnoses should be evaluated thoroughly,as part of the assessment for cardiac amyloidosis.
Non-imaging investigations
The three main non-imaging investigations for cardiac amyloidosis are electrocardiogram (ECG),blood tests and myocardial biopsy.Important ECG features include low to normal QRS voltage (less than what is expected from the degree of left ventricular wall thickness may be seen,and associated with adverse prognosis -Figure 1),and QS waves in two consecutive leads with pseudoinfarction pattern may also be present,although these typical findings may not be present in at least 50% of patients.Both cardiac biomarkers N-terminal pro-b-type natriuretic peptide (NTpro BNP) and troponin I and T are often chronically elevated in cardiac amyloidosis,so this diagnosis needs to be considered when these biomarkers are elevated after ruling out an acute coronary syndrome.Serum free light chain assay is preferred as a more sensitive test to detect AL amyloidosis compared with serum or urine protein electrophoresis,with abnormal kappa-lambda ratio (low suggesting monoclonal lambda and high suggesting monoclonal kappa light chain excess),as opposed to elevated levels with normal ratio in renal failure.Endomyocardial biopsy,albeit rarely utilized nowadays,remains the traditional gold standard for diagnostic confirmation of cardiac amyloidosis with near 100% sensitivity,identifying amyloid deposition extracellularly throughout the myocardium,though mainly subendocardial and diffuse for AL and patchy and transmural for ATTR.Biopsies from other affected sites (for example:Fat pads,kidney,liver and bone marrow) can also be performed to evaluate for amyloidosis in these organs.
MULTIMODALITY IMAGING EVALUATION
Echocardiography
The echocardiographic protocol for evaluating cardiac amyloidosis is described in Table 1.Based on this,it is mandatory to evaluate for left ventricular wall thickness,myocardial echogenicity,atrial size (and function),interatrial septum and valvular thickness,pericardial effusion,diastolic function including mitral inflow E and A wave ratio and deceleration times,tissue Doppler s',e' and a' velocities,and estimate pulmonary arterial systolic and right atrial pressures.Characterization of left ventricular longitudinal strain including the Bulls's eye plot by speckle-tracking echocardiography is also recommended.Imaging views and frame rates (at least 20 Hertz) should be optimized for two-dimensional and Doppler echocardiography.Overall interpretation of echocardiographic findings needs to be categorized as not suggestive,strongly suggestive and equivocal,and the latter two categories supporting further investigations.Integration of clinical presentation with electrocardiography,biomarker and other imaging findings is prudent,and comparison with prior echocardiographic findings is encouraged.
Figures 2 illustrates some characteristic echocardiographic findings.In the presence of increased left ventricular thickness with small or normal left ventricular size,and the absence of other clear causes,such as hypertension,advanced renal disease,and hypertrophic cardiomyopathy,cardiac amyloidosis needs to be considered.The increased thickness can also affect the right ventricle,interatrial septum and heart valves,and pericardial effusion may also be present.Low voltage ECG in this settingis favors the presence of amyloid cardiac involvement.Left and right atrial dilation and dysfunction is common,as well as stasis and even thrombi in the left atrium and appendage.Increased myocardial echogenicity may be seen,although this is nonspecific,particularly with harmonic imaging and improved echocardiographic technology.A range of diastolic dysfunction from mild to severe,with evidence of elevated left ventricular filling pressures is often seen.Reduced left ventricular global longitudinal strain with normal ejection fraction is very common,and the typical “cherry-on-top” pattern is when there is impairment of basal segments strain compared to the relatively spared apex,quantitatively shown in one study as the relative apical longitudinal strain/average of basal and mid longitudinal strain ratio of at least 1 to be characteristic of cardiac amyloidosis.Transesophageal and threedimensional echocardiography do not generally add significantly to two-dimensional transthoracic echocardiography,unless there is improved spatial resolution.One utility however for transesophageal echocardiography is the exclusion of left atrial appendage thrombus which are not uncommon in patients with suspected amyloidosis with and without atrial fibrillation.
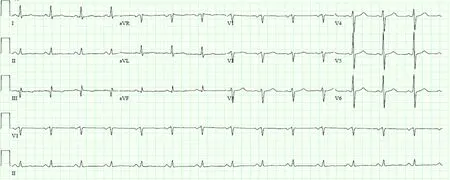
Figure 1 Characteristic electrocardiogram of cardiac amyloidosis demonstrating low QRS voltages.
Beyond diagnosis,echocardiography also plays a role in risk evaluation of cardiac amyloidosis.Strain imaging plays an important role in prognostication,especially for AL cardiac amyloidosis.Reduced left ventricular systolic strain predicts all-cause mortality in patients with preserved left ventricular ejection fraction,as well as a higher relative regional strain ratio.Similarly,impaired right ventricular strain was associated with worse survival in cardiac amyloidosis.Other measures of left ventricular systolic function have also been shown to be associated with mortality including lower myocardial contractile fraction,mid-wall fractional shortening and Tei index.Finally,left atrial size,left-sided valve thickening and pericardial effusions have been reported as poor prognostic markers.Further research is required to integrate echocardiographic with clinical and biomarkers to create a multifactorial risk algorithm for cardiac amyloidosis.
Magnetic resonance imaging
The magnetic resonance imaging (MRI) protocol for assessing cardiac amyloidosis is listed in Table 2.On the steady state free precession cine imaging with 2,3 and 4-chamber and short-axis stack views,left ventricular size,wall thickness,ejection fraction,stroke volume and mass can all be evaluated.Left and right atrial dimensions and function can also be evaluated,as well as the presence of pericardial effusion.Late gadolinium enhancement (LGE) imaging using phase-sensitive inversion recovery is preferred,with the same views as cine imaging.Native T1 precontrast and post-contrast mapping,should also be performed,usually at mid and basal short-axis and apical 4-chamber views.TI (inversion time scout) should be performed,and difficulty to null the myocardium over a range of inversion times may be observed.Similar to echocardiography,an overall interpretation of strongly suggestive,equivocal or not suggestive of cardiac amyloidosis should be made,as well as direct comparison with prior imaging evaluation.
Because of the tissue characterization utility of MRI,it is especially useful in the evaluation of increased left ventricular wall thickness,as well as identifying cardiac involvement of systemic amyloidosis.Left ventricular ejection fraction may be preserved or impaired,but there is long-axis impairment with relative apical functional sparing and stroke volume index is often reduced.The thickened left ventricular wall is usually concentric,but may be asymmetric especially for the ATTR subtype.Traditional LGE protocols have been challenging because of the difficultynulling the myocardium in amyloidosis,which may be used to suggest this diagnosis,and T1 inversion scout is helpful to time delayed imaging.This problem has been overcome by phase-sensitive inversion recovery sequences,and the pattern of distribution is often subendocardial (especially for AL subtype) or transmural(especially for ATTR subtype or advanced disease affecting both ventricles).A recent meta-analysis also reported 85% sensitivity and 92% specificity for LGE for diagnosing cardiac amyloidosis.Another important study assessed the utility of LGE patterns in 46 AL and 51 ATTR cardiac amyloidosis patients.They found that ATTR patients had more extensive LGE,with 90% of ATTR compared to 37% of AL patients having transmural LGE,and 100% of ATTR compared to 72% of AL patients had right ventricular LGE (< 0.001 for both comparisons).More recently,T1 mapping pre-contrast as native T1 (with use of modified look-locker inversion recovery protocol) or post-contrast as extracellular cell volume (ECV representing infiltration) have helped the detection and quantification of infiltration for cardiac amyloidosis with high accuracy.The reference ranges depend on the magnetic field strength,for example native T1 950 ± 21 ms and electrocardiogram 26% ± 4% for 1.5 Tesla scanners,and native T1 1139 ± 37 ms (males 1109 ± 73 ms and females 1139 ±37 ms) for 3.0 Tesla scanners.Notably,MRI cannot distinguish between ATTR and AL cardiac amyloidosis.The cardiac MRI findings of a real-life case of ATTR cardiac amyloidosis are shown in Figure 3.
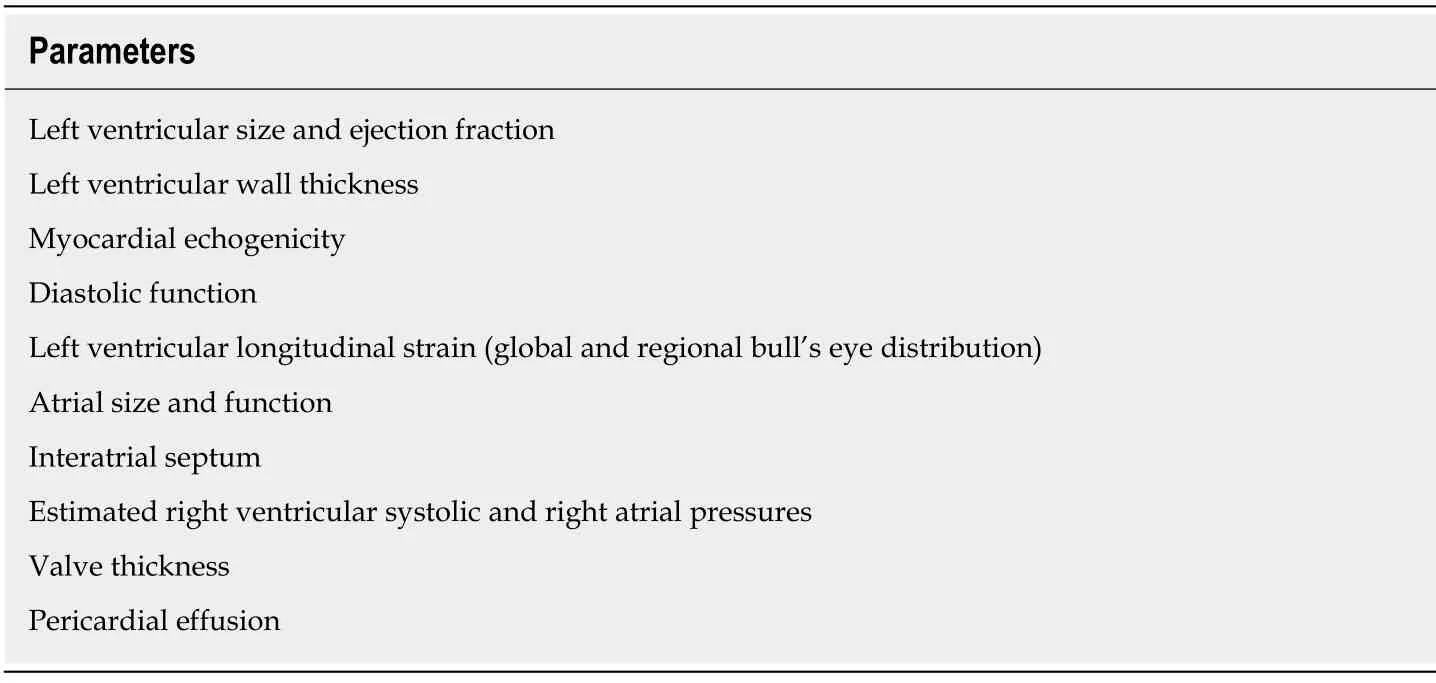
Table 1 Echocardiographic parameters to evaluate for cardiac amyloidosis,modified from expert consensus recommendations[5]
MRI also has important prognostic roles in cardiac amyloidosis.Similar to other cardiovascular diseases,LGE is associated with higher all-cause mortality in a metaanalysis.Methods of systematic quantification of LGE for cardiac amyloidosis have not yet been developed,but this may help assess the degree of infiltration,progression and monitor response to treatment in the future.Higher native T1 mapping and extracellular volume when seen support the diagnosis of cardiac amyloidosis and are both also associated with worse prognosis.T2 mapping for quantitatively assessing cardiac amyloidosis is also under investigation,with one study showing higher T2 representing edema to be associated with higher mortality.
Nuclear imaging
A relatively small number of radiotracers remain relevant in clinical practice today,predominantly 99mTc-phosphate derivatives.Those include 99mTc-DPD(technetium-3,3-diphosphono-1,2-propanodicarboxylic acid),99mTc-PYP (technetium pyrophosphate),and 99mTc-HMDP (technetium hydroxymethylene-diphosphonate).The mechanism of action of 99mTc-phosphate derivatives is hypothesized to be linked to the binding of calcium-rich TTR fibrils to phosphate-based tracers.These radiotracers preferentially bind calcium rich ATTR-type rather than AL-type amyloid deposits,and thus have high sensitivity and specificity for ATTR amyloidosis.It is important to note that the documented sensitivity is dependent on studies of patients with advanced disease;the yield of 99mTc-phosphate derivatives in early stages of the disease is still unknown.The practical acquisition protocols are available by the American Society of Nuclear Cardiology.The patient is positioned supine with no pre-requisites of fasting or any other dietary preparations.The scan is done at rest and a planar image is acquired initially followed by a single-photon emission computerized tomography (SPECT) study;the dosages of radiotracers are 10-20 mCi(370-740 MBq) IV dose forTc-DPD,Tc-PYP,andTc-HMDP.The scan is done at1 hour after theTc-PYP administration (and a 3 hours acquisition is optional),while it is done at 2 or 3 hours afterTc-DPD/HMDP administration.The heart field is scanned mainly (Chest is optional).A 140 keV energy window is used with a low energy,high resolution collimator.The planar matrix is recommended at 256 × 256 while the SPECT is at least 64 × 64 with a 3.5-6.5 pixel size.For the planar parameters,a 90 degree detector configuration is used to acquire anterior and lateral images simultaneously with a magnification of 1.46 and image count duration of 750000.For the SPECT parameters,a 90 degrees detector configuration (with optional 180) is used with a 180 degree angular range (360 degree optional).No ECG gating is required.The magnification is 1.46 at 180 degrees and 1 at 360 degrees.
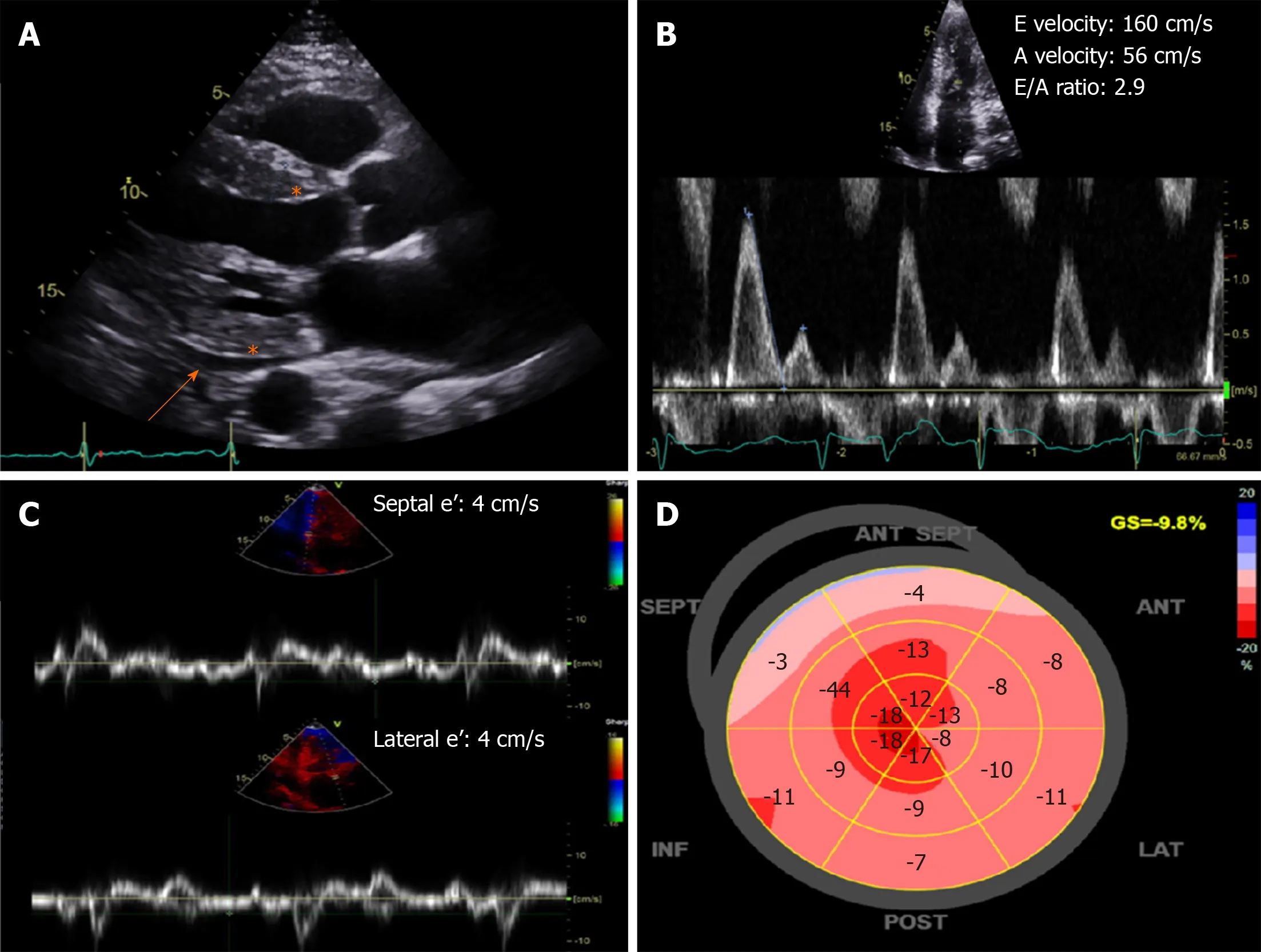
Figure 2 Characteristic echocardiography findings of cardiac amyloidosis.A:Increased left ventricular wall thickness and echogenicity (asterisk) with small effusion (orange arrow);B and C:Severe (restrictive) diastolic dysfunction with mitral inflow E/A ratio > 2.0 and low septal and lateral e' velocities by tissue Doppler;D:Bull's eye plot showing a “cherry-top” pattern of left ventricular peak systolic longitudinal strain,consistent with apical sparing pattern.
The interpretation of each study starts with the evaluation of the images,both planar and SPECT.A diffuse radiotracer uptake in the myocardium confirms the diagnosis of ATTR cardiac amyloidosis.It is important to take simultaneous anterior and lateral planar images,as well compare SPECT images to differentiate tracer uptake in the myocardium from activity from the blood pool that can be misconstrued as cardiac uptake,overlapping activity in sternum or ribs with fractures,and focal recent myocardial infarcts.If the tracer is visually detected in the myocardium on SPECT,the next step entails semi-quantitative grading (Figure 4).For the one-hour approach,an elliptical/circular region-of-interest (ROI) is drawn on the anterior planar images over the heart,avoiding sternal overlap and excluding adjacent lung.This ROI is mirrored over the contralateral lung to set a standard that nulls background and rib uptake.A heart-to-contralateral lung (H/CL) ratio is then calculated.For the three hour approach,a grading scale is used to compare tracer uptake relative to ribs;a grade zero has no myocardial uptake and normal bone uptake,grade 1:Myocardial uptake is less than the ribs,grade 2:Myocardial uptake is equal to the ribs,and grade 3:Myocardial uptake is greater than the ribs.Quantitative measures in identifying high diagnostic accuracy include a H/CL ratio of ≥ 1.5 on 1-h images for 99mTc tracersand ≥ 1.3 on 3-h images in 99mTc-PYP studies.
The degree of cardiac uptake of 99mTc tracers correlates with overall mortality.A heart-to-whole body (H/WB) retention in 99mTc-DPD studies,and H/CL in 99mTc-PYP studies have been linked to patients' outcomes.Combining the degree of uptake with other variables such as NYHA Class,NT-proBNP,and interventricular septal thickness improves the prognostic risk stratification.Current clinical practicesupports the use of 99mTc-derivatives to differentiate between ATTR and AL amyloidosis.The high specificity of 99mTc-PYP a negative blood and urine monoclonal protein is highly predictive of ATTR disease without the need of an endomyocardial biopsy.Furthermore,99mTc-DPD may be able to discriminate the amyloid fibrils by length and distribution that distinguishes early- and late-onset ATTRv.Genetic testing,however,remains imperative to differentiate mutant versus ATTRwt due to its screening implications for family members.
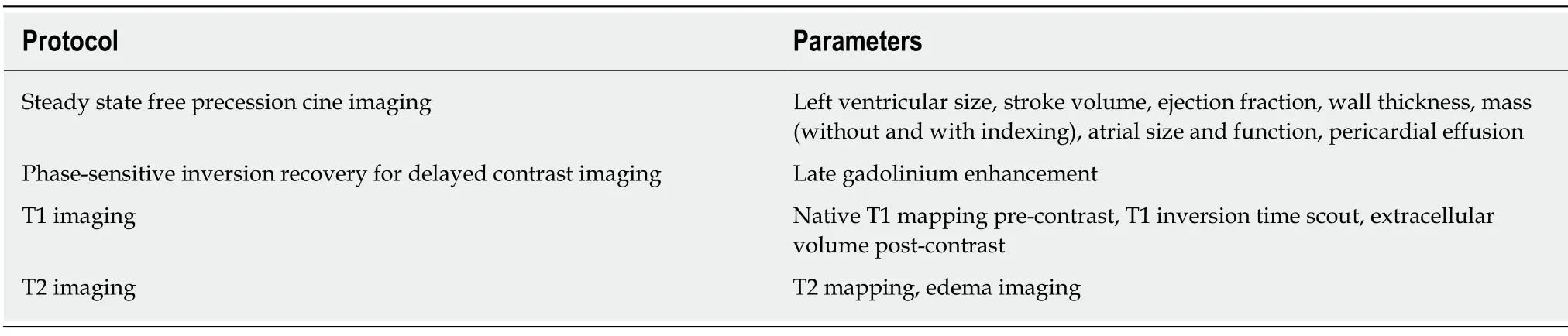
Table 2 Magnetic resonance imaging evaluation of cardiac amyloidosis,modified from expert consensus recommendations[5]
Positron emission tomography (PET) imaging is also showing some promise in the diagnosis of cardiac amyloidosis,but has yet to achieve standardized clinical practice protocols,as most of the experiences are single center-based and for research purposes.In general,targeted amyloid-binding 18F tracers are highly specific to AL and ATTR amyloid deposits with some variation.Commonly used and researched tracers include N-[methyl-(11)C]2-(4′-methylamino-phenyl)-6-hydroxybenzothiazole(11C-Pittsburgh compound B 11C-PiB),18F-florbetapir,and 18F-florbetaben.
There are no standardized practice protocols endorsing the use of PET tracers yet.The assessment of PET images can be visual or through one of several indices of tracer uptake.Static images are reconstructed using data acquired throughout the study on established reconstructions PET software.Qualitative assessment of the images include myocardial standardized uptake value (SUV),target-to-background ratio,and myocardial retention index.The quantitative analysis requires left ventricular (LV)contouring prior to identifying the uptake quantitatively.This is done by drawing base to apex LV contours of 10 mm thickness.A standard uptake value (SUV) of the myocardium can then be extracted from the image.The SUV of the adjacent descending thoracic aorta is also measured and averaged.A ratio of myocardium-toblood SUV is calculated by dividing the maximal myocardial SUV to the averaged SUV of the descending aorta.The target-to-background index is calculated as the ratio of the mean LV myocardial SUV to the blood pool SUV over a set time during the study.Myocardial retention index is measured as mean LV SUV in each dynamic frame of the image divided by the integral of the blood pool 18F-florbetapir time-activity curve at that time point
Most importantly,PET tracers allow for quantitative assessment of amyloid burden.Also,PET images are usually fused with CT imaging or MRI improving reading accuracy.Using 18F-florbetapir and 18F-florbetaben,a target-to-background(myocardium to blood pool) ratio > 1.5 and a retention index of > 0.025/min separates cardiac amyloid patients from controls.Recent studies on 11C-PiB have shown the potential of these tracers as independent predictors of clinical outcomes.Due to their recent use,there are still limited data on the prognostic role of PET imaging.Table 3 summarizes the standard PET nuclear imaging acquisition and protocols for cardiac amyloidosis.
INTEGRATION OF MULTIMODALITY IMAGING ASSESSMENT
Diagnostic criteria
The latest diagnostic criteria for amyloidosis incorporating biopsy results,typical cardiac imaging features,biomarkers and blood tests for cardiac amyloidosis defined by the expert consensus from several international cardiology and cardiac imaging societies are summarized in Table 4.Positive endomyocardial biopsy for cardiac amyloidosis with apple-green birefringence under polarized light with Congo red staining,immunohistochemistry or mass spectrometry typing alone is sufficient for diagnosing all subtypes of cardiac amyloidosis.Positive extracardiac biopsy of ATTR or AL cardiac amyloidosis requires typical cardiac imaging features to be also present to reach a diagnosis.For AL cardiac amyloidosis,abnormally elevated cardiacbiomarkers NT-pro BNP or troponins with other causes excluded may be a substitute to typical cardiac imaging features for diagnosis.In addition,a clinical diagnosis of ATTR cardiac amyloidosis can also be made if nuclear imaging criteria are satisfied after negative serologic markers for AL;absence of clonal plasma cell process by serum free light chains and serum and urine immunofixation.Figure 5 presents a schematic of how multi-modality is used in the assessment and diagnosis of cardiac amyloidosis.
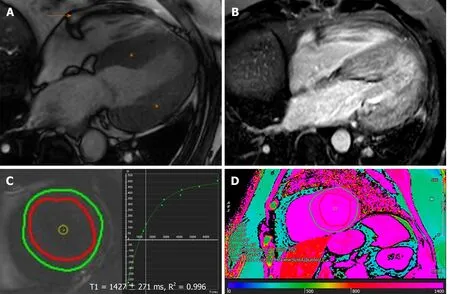
Figure 3 Characteristic magnetic resonance findings of cardiac amyloidosis.A:Increased left ventricular wall thickness (asterisk) with trivial pericardial effusion(orange arrow) on steady state free precession;B:Delayed enhancement imaging (phase sensitive inversion recovery sequence) demonstrating diffusely abnormal,transmural late gadolinium enhancement in the myocardium;C and D:Native T1-mapping (using the modified look-locker inversion recovery sequence) at left ventricular (C) basal level short-axis slice and (D) color map at left ventricular mid level short-axis,showing significantly elevated native T1-values and consistent with interstitial fibrosis.Note that the imaging acquisition in this example was performed using a dedicated 3.0 Tesla machine.
Strengths and weaknesses of imaging modalities
The advantages and disadvantages of echocardiography,MRI and nuclear imaging to evaluate cardiac amyloidosis are summarized in Table 5.Echocardiography remains the first line cardiac imaging investigation because of being readily available,safe,low cost,portable and has the most experience for the evaluation of diastolic function and myocardial deformation,but usually requires further confirmatory investigations.MRI uniquely allows for tissue characterization and is the gold standard for chamber quantification because of high spatial resolution,although access,cost,claustrophobia,contrast use and metallic device may preclude from its use.Nuclear imaging is also useful especially for identifying ATTR cardiac amyloidosis,however limitations include availability,cost,radiation exposure.
Novel therapeutics
The treatment for cardiac amyloidosis includes both treatment of heart failure and other cardiac complications,and treatment of the underlying amyloid infiltrative disease.The latter is markedly different between ATTR and AL cardiac amyloidosis,therefore accurate classification by multi-modality imaging and other investigations are critical.Traditionally,chemotherapy regimens and stem cell transplantation are the mainstay treatments for AL amyloidosis eliminating plasma cell clones central to its pathophysiology to alter the natural history with poor prognosis.The recent novel therapeutics in ATTR cardiac amyloidosis deserve special mention.Tafamidis is a drug that binds transthyretin to reduce amyloidogenesis,and in a randomized trial of 441 patients significantly reduced both all-cause mortality and cardiovascular hospitalization by 30%-32% while slowing functional and quality of life decline,becoming the new preferred therapy for ATTR amyloidosis.Inotersen is a oligonucleotide drug blocking hepatic transthyretin synthesis,and in a randomized controlled trial of 172 patients improved the course ofneurologic disease and quality of life in ATTRv amyloidosis.Patisiran is a RNA interference drug also reducing hepatic transthyretin production which ins another randomized controlled trial of 225 patients similarly improved functional capacity and quality of life.Of note,the management and novel therapies for cardiac amyloidosis is not the focus of this review.
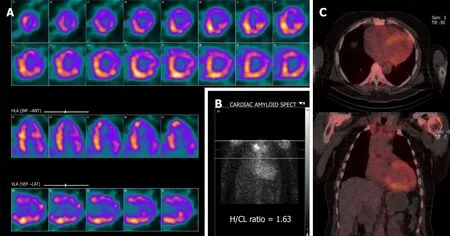
Figure 4 Tc-99m-Pyrophosphate SPECT of the chest with computed tomography attenuation correction at 3 h after intravenous injection of Tc-99m Pyrophosphate.A:Single-photon emission computerized tomography (SPECT) exhibits diffuse uptake pattern;B:Planar image depicts by visual comparison to bone a Grade 2 uptake (equal to rib uptake).The calculated H/CL ratio is measured at 1.63;C:SPECT/Computed tomography fusion showing distribution of the increased cardiac uptake.SPECT:Single-photon emission computerized tomography;H/CL:Heart-to-contralateral lung.
CONCLUSION
Significant advances have occurred in multimodality cardiac imaging for assessing cardiac amyloidosis.Echocardiography,MRI and nuclear imaging all play important and complementary roles in this clinical scenario.Echocardiography is first-line for chamber quantification,assessing diastolic function and myocardial deformation.MRI uniquely offers tissue characterization and quantification of infiltration on top of chamber quantification.Nuclear imaging is the only currently available non-invasive modality able to distinguish ATTR from AL cardiac amyloidosis.Each modality also plays a role in risk stratification for adverse outcomes.Contemporary imaging plays a central role in helping improve the diagnosis and management outcomes of cardiac amyloidosis.It remains to be seen whether we can use these non-invasive imaging tools to follow disease progression/regression after initiation of novels therapies especially with ATTR cardiac amyloidosis.
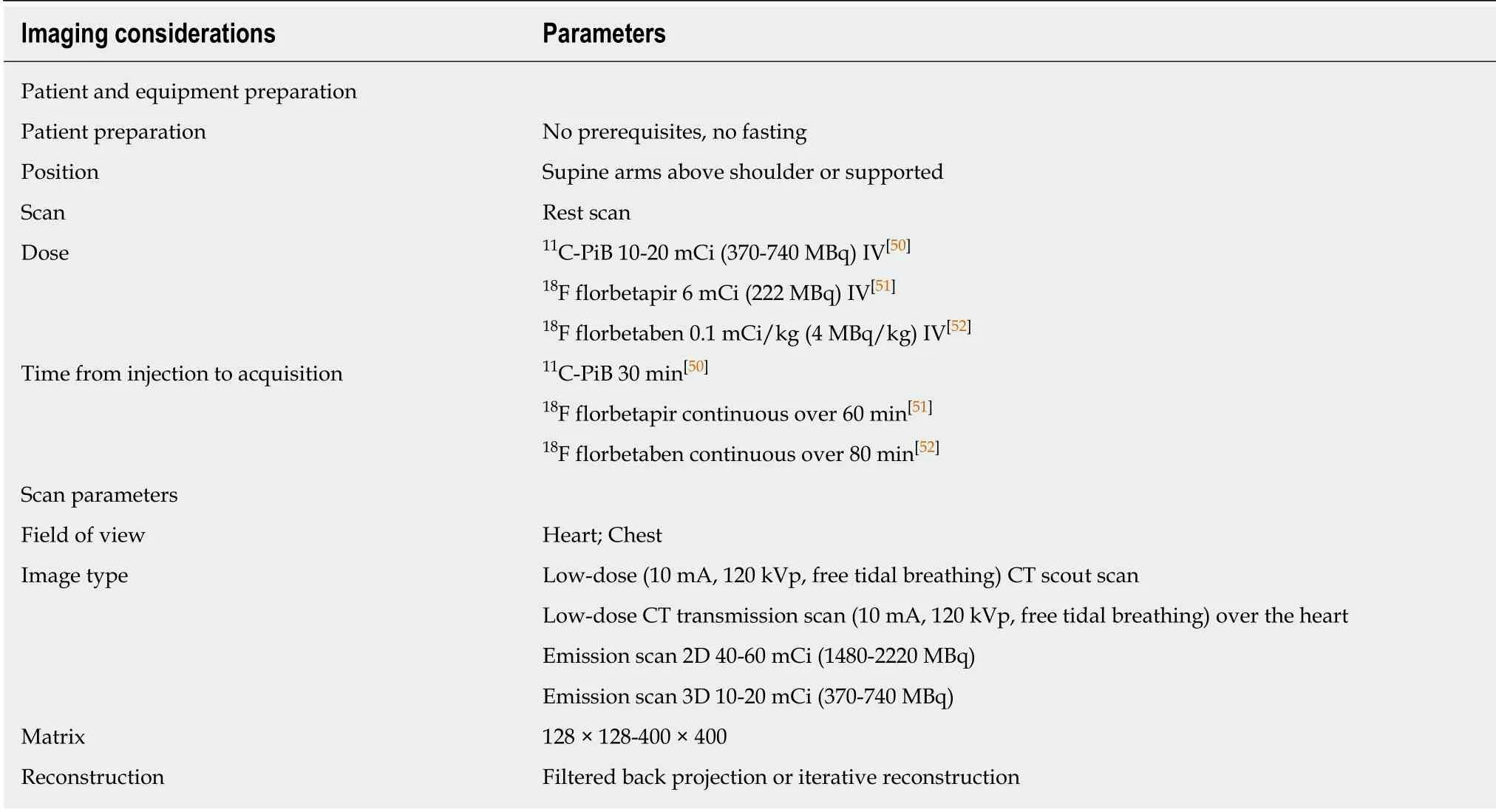
Table 3 Utilized acquisition parameters for 11C-PiB,18F florbetapir,and 18F florbetaben studies for cardiac amyloidosis[50-52]
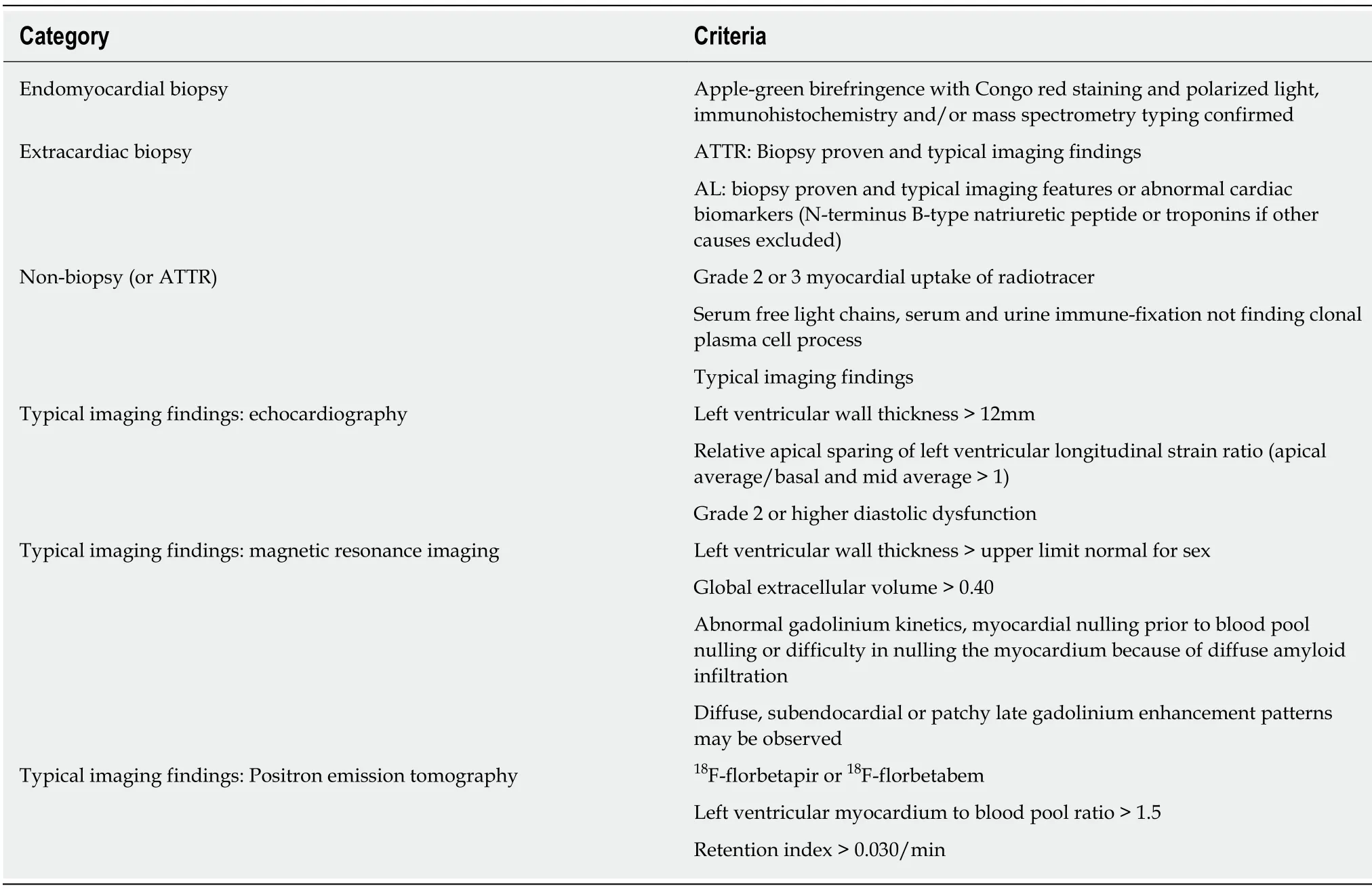
Table 4 Contemporary diagnostic criteria for cardiac amyloidosis,modified from expert consensus recommendations[61]
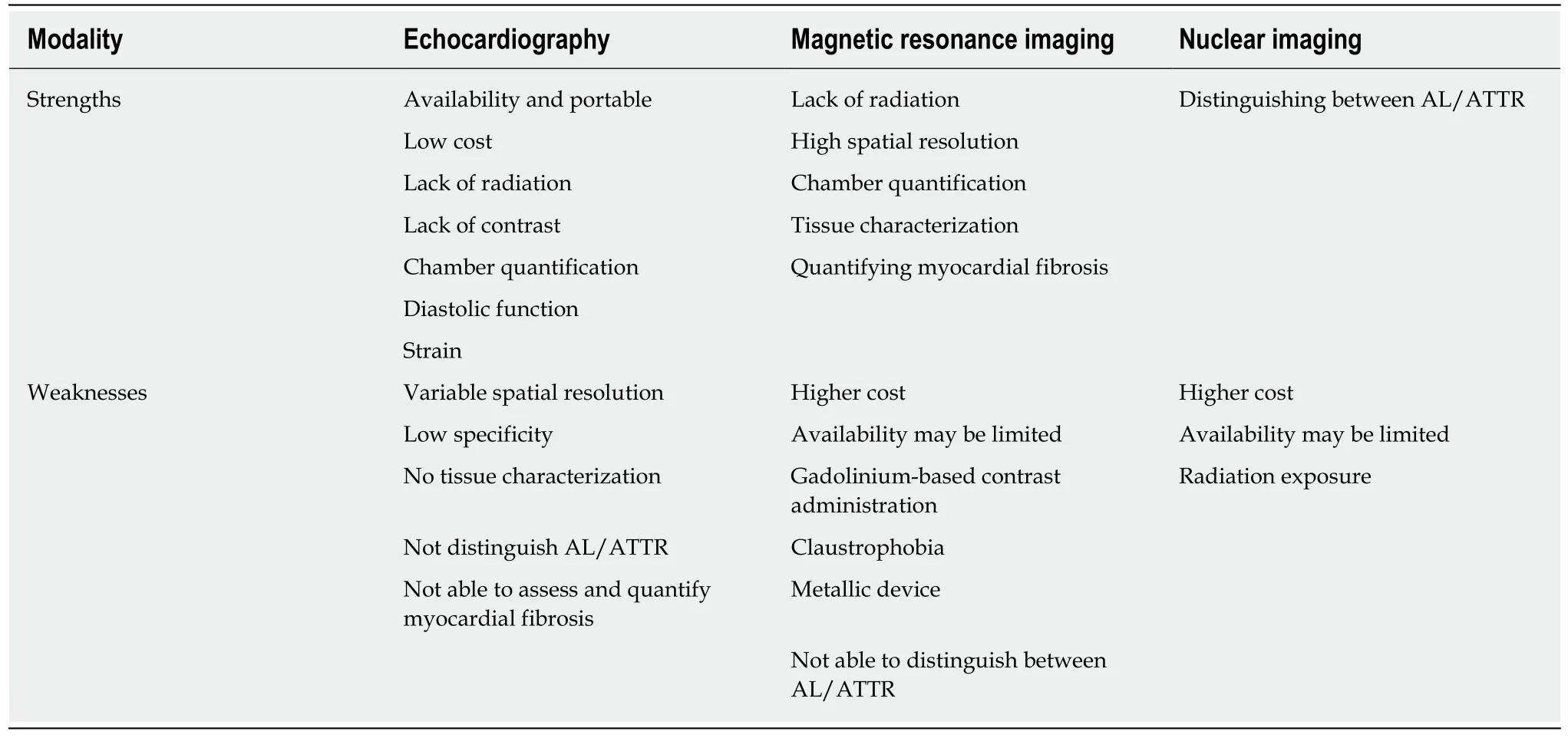
Table 5 Strengths and limitations of each imaging modality for assessing cardiac amyloidosis

Figure 5 Schematic diagram of the stepwise use of electrocardiogram and multi-modality imaging in the diagnosis and evaluation of cardiac amyloidosis.ECG:Electrocardiogram;AL:Light chain amyloidosis;TTR:Transthyretin;ECHO:Echocardiogram;SPECT:Single-photon emission computerized tomography;H/CL:Heart-to-contralateral lung;PET:Positron-emission tomography;SUV:Standardized uptake value;MRI:Magnetic-resonance imaging;ATTR:Transthyretin amyloidosis.
 World Journal of Radiology2020年6期
World Journal of Radiology2020年6期
- World Journal of Radiology的其它文章
- Current and future applications of ultrasound imaging in peripheral nerve disorders
